Transcriptome Profile of Thyroid Glands in Bile Duct Ligation Mouse Model
Abstract
1. Introduction
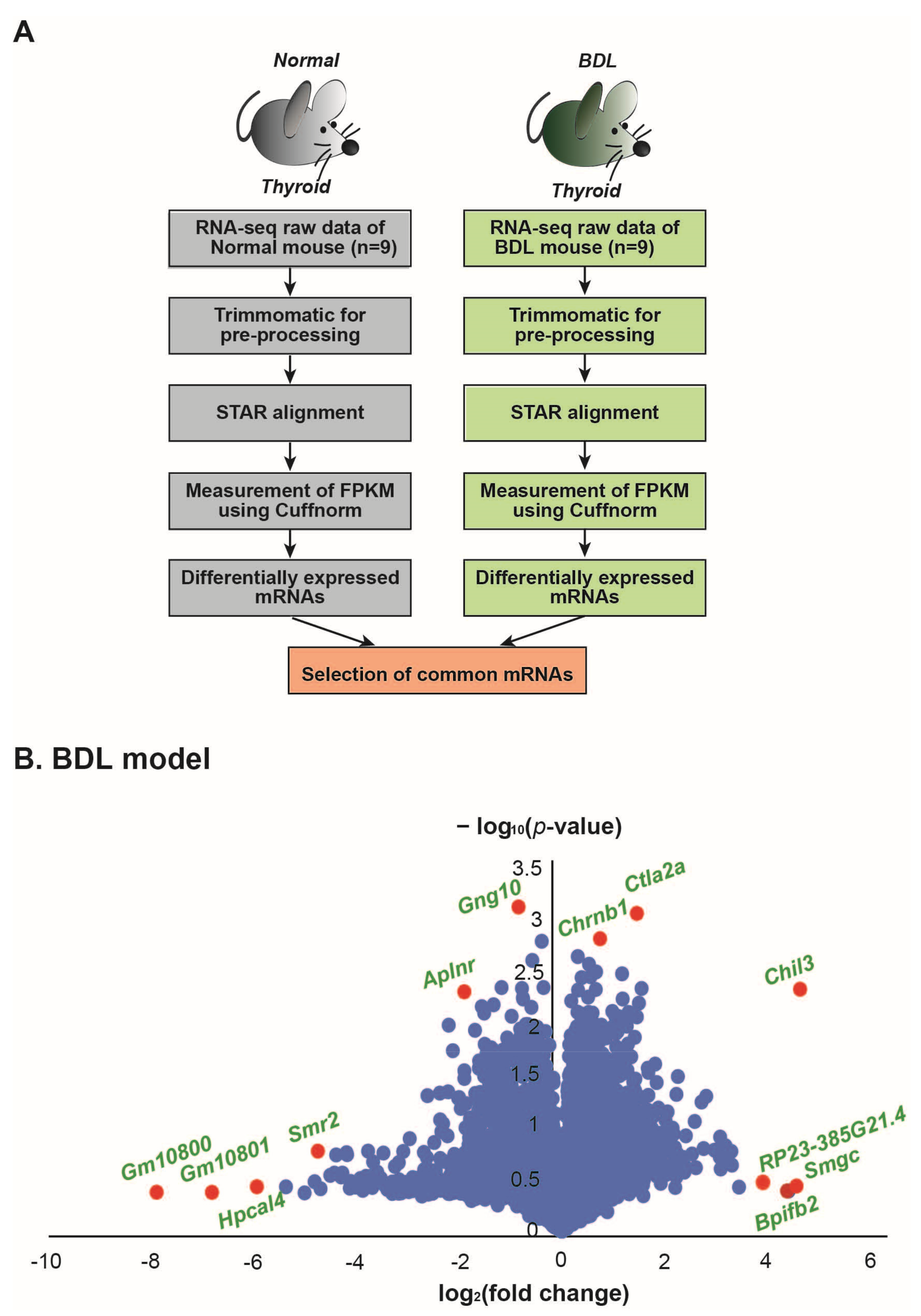
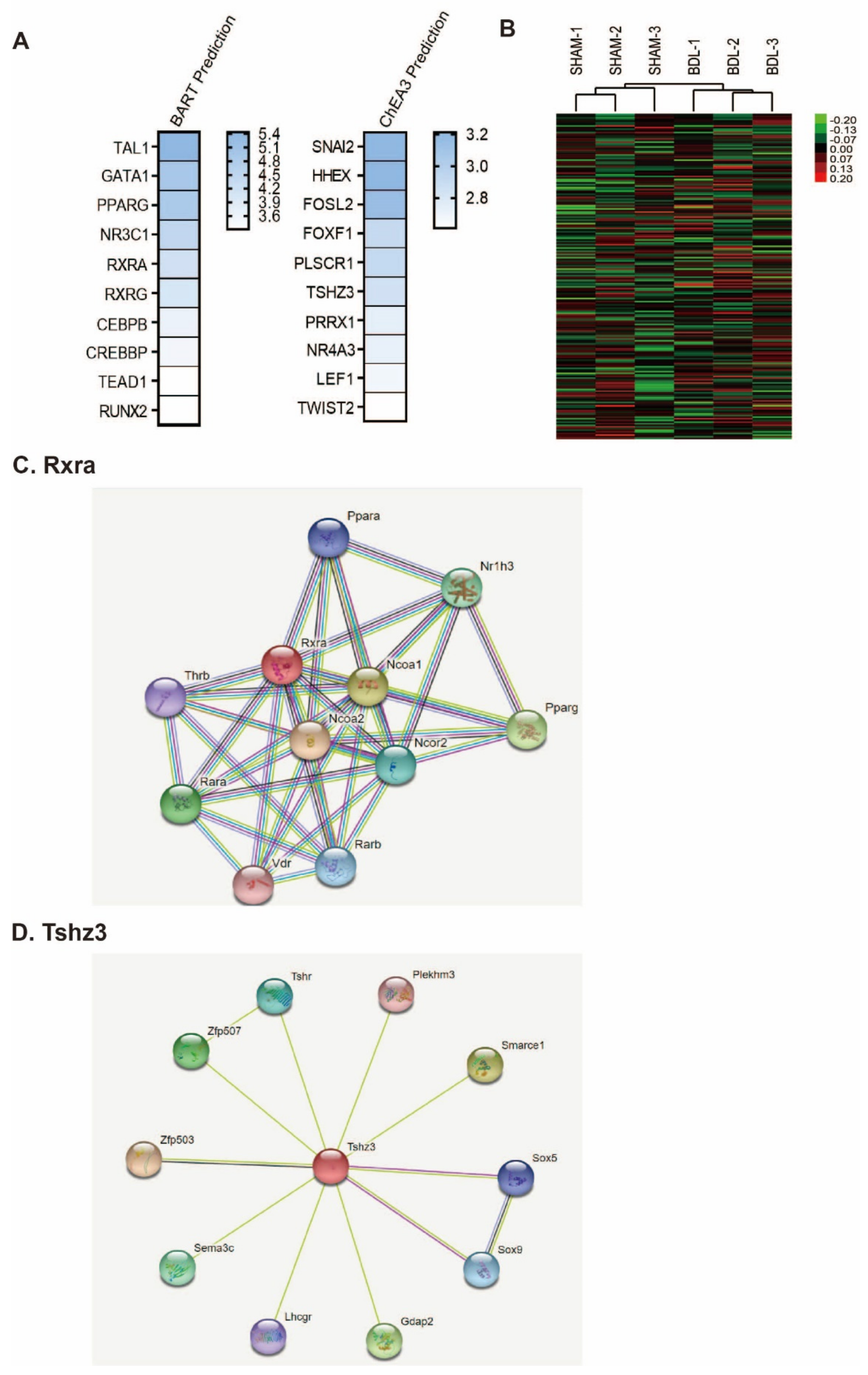
2. Results
3. Discussion
4. Materials and Methods
4.1. BDL Surgery and Thyroid Gland Sampling
4.2. Analysis of RNA Sequencing Data
4.3. Functional Analysis of mRNAs
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St. Germain, D.L.; Galton, V.A.; Hernandez, A. Minireview: Defining the roles of the iodothyronine deiodinases: Current concepts and challenges. Endocrinology 2009, 150, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Gereben, B.; Zeold, A.; Dentice, M.; Salvatore, D.; Bianco, A.C. Activation and inactivation of thyroid hormone by deiodinases: Local action with general consequences. Cell. Mol. Life Sci. 2008, 65, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef]
- Thompson, C.C.; Potter, G.B. Thyroid hormone action in neural development. Cereb. Cortex 2000, 10, 939–945. [Google Scholar] [CrossRef]
- Quignodon, L.; Legrand, C.; Allioli, N.; Guadano-Ferraz, A.; Bernal, J.; Samarut, J.; Flamant, F. Thyroid hormone signaling is highly heterogeneous during pre- and postnatal brain development. J. Mol. Endocrinol. 2004, 33, 467–476. [Google Scholar] [CrossRef]
- Morreale de Escobar, G.; Obregon, M.J.; Escobar del Rey, F. Role of thyroid hormone during early brain development. Eur. J. Endocrinol. 2004, 151 (Suppl. S3), U25–U37. [Google Scholar] [CrossRef]
- Feely, J.; Isles, T.E. Screening for thyroid dysfunction in diabetics. Br. Med. J. 1979, 1, 1678. [Google Scholar] [CrossRef]
- Delitala, A.P.; Fanciulli, G.; Maioli, M.; Delitala, G. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. Eur. J. Intern. Med. 2017, 38, 17–24. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bulow, I.; Perrild, H.; Ovesen, L.; Jorgensen, T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef]
- Tatar, E.; Kircelli, F.; Asci, G.; Carrero, J.J.; Gungor, O.; Demirci, M.S.; Ozbek, S.S.; Ceylan, N.; Ozkahya, M.; Toz, H.; et al. Associations of triiodothyronine levels with carotid atherosclerosis and arterial stiffness in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Tatar, E.; Sezis Demirci, M.; Kircelli, F.; Gungor, O.; Yaprak, M.; Asci, G.; Basci, A.; Ozkahya, M.; Ok, E. The association between thyroid hormones and arterial stiffness in peritoneal dialysis patients. Int. Urol. Nephrol. 2012, 44, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Chen, C.Y.; Tsai, M.M.; Tsai, C.Y.; Lin, K.H. Molecular functions of thyroid hormones and their clinical significance in liver-related diseases. BioMed Res. Int. 2013, 2013, 601361. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef]
- Qiu, S.; Cao, P.; Guo, Y.; Lu, H.; Hu, Y. Exploring the Causality Between Hypothyroidism and Non-alcoholic Fatty Liver: A Mendelian Randomization Study. Front. Cell Dev. Biol. 2021, 9, 643582. [Google Scholar] [CrossRef]
- Calsolaro, V.; Bottari, M.; Coppini, G.; Lemmi, B.; Monzani, F. Endocrine dysfunction and cognitive impairment. Minerva Endocrinol. 2021, 46, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Sorvillo, F.; Mazziotti, G.; Carbone, A.; Morisco, F.; Cioffi, M.; Rotondi, M.; Stornaiuolo, G.; Amato, G.; Gaeta, G.B.; Caporaso, N.; et al. Increased serum reverse triiodothyronine levels at diagnosis of hepatocellular carcinoma in patients with compensated HCV-related liver cirrhosis. Clin. Endocrinol. 2003, 58, 207–212. [Google Scholar] [CrossRef]
- Kayacetin, E.; Kisakol, G.; Kaya, A. Low serum total thyroxine and free triiodothyronine in patients with hepatic encephalopathy due to non-alcoholic cirrhosis. Swiss Med. Wkly. 2003, 133, 210–213. [Google Scholar]
- Malik, R.; Hodgson, H. The relationship between the thyroid gland and the liver. QJM 2002, 95, 559–569. [Google Scholar] [CrossRef]
- Bal, C.; Chawla, M. Hyperthyroidism and jaundice. Indian J. Nucl. Med. 2010, 25, 131–134. [Google Scholar] [CrossRef]
- Silveira, M.G.; Mendes, F.D.; Diehl, N.N.; Enders, F.T.; Lindor, K.D. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int. 2009, 29, 1094–1100. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Mehrdad, M.; Mortazavi, S.; Joukar, F.; Khak, M.; Atrkar-Roushan, Z. Decreased serum total T3 level in hepatitis B and C related cirrhosis by severity of liver damage. Ann. Hepatol. 2012, 11, 667–671. [Google Scholar] [CrossRef]
- Agiasotelli, D.; Alexopoulou, A.; Vasilieva, L.; Dourakis, S.P. Low free T3 levels are related to early mortality in patients with decompensated cirrhosis and acute-on chronic liver failure. J. Hepatol. 2014, 61, 1446–1447. [Google Scholar] [CrossRef]
- Lin, T.Y.; Shekar, A.O.; Li, N.; Yeh, M.W.; Saab, S.; Wilson, M.; Leung, A.M. Incidence of abnormal liver biochemical tests in hyperthyroidism. Clin. Endocrinol. 2017, 86, 755–759. [Google Scholar] [CrossRef]
- Punekar, P.; Sharma, A.K.; Jain, A. A Study of Thyroid Dysfunction in Cirrhosis of Liver and Correlation with Severity of Liver Disease. Indian J. Endocrinol. Metab. 2018, 22, 645–650. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, M. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int. J. Mol. Sci. 2020, 21, 5927. [Google Scholar] [CrossRef]
- Mantovani, A.; Nascimbeni, F.; Lonardo, A.; Zoppini, G.; Bonora, E.; Mantzoros, C.S.; Targher, G. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1270–1284. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Curro, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Manka, P.; Bechmann, L.; Best, J.; Sydor, S.; Claridge, L.C.; Coombes, J.D.; Canbay, A.; Moeller, L.; Gerken, G.; Wedemeyer, H.; et al. Low Free Triiodothyronine Is Associated with Advanced Fibrosis in Patients at High Risk for Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2019, 64, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoo, E.R.; Li, A.A.; Fernandes, C.T.; Tighe, S.P.; Cholankeril, G.; Hameed, B.; Ahmed, A. Low-Normal Thyroid Function Is Associated With Advanced Fibrosis Among Adults in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 2379–2381. [Google Scholar] [CrossRef] [PubMed]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Lee, W.M.; Wendon, J.; Larsen, F.S.; Williams, R. Acute liver failure: A curable disease by 2024? J. Hepatol. 2015, 62, S112–S120. [Google Scholar] [CrossRef]
- Reuben, A.; Tillman, H.; Fontana, R.J.; Davern, T.; McGuire, B.; Stravitz, R.T.; Durkalski, V.; Larson, A.M.; Liou, I.; Fix, O.; et al. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann. Intern. Med. 2016, 164, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Guven, K.; Kelestimur, F.; Yucesoy, M. Thyroid function tests in non-alcoholic cirrhotic patients with hepatic encephalopathy. Eur. J. Med. 1993, 2, 83–85. [Google Scholar] [PubMed]
- Wang, L.; Yu, W.; Cao, W.; Lu, W. The abnormality of thyroid hormones in patients with type A hepatic encephalopathy. Oncotarget 2017, 8, 67821–67828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz-Fontenla, F.; Castillo-Pradillo, M.; Diaz-Gomez, A.; Ibanez-Samaniego, L.; Gancedo, P.; Guzman-de-Villoria, J.A.; Fernandez-Garcia, P.; Banares-Canizares, R.; Garcia-Martinez, R. Refractory hepatic encephalopathy in a patient with hypothyroidism: Another element in ammonia metabolism. World J. Gastroenterol. 2017, 23, 5246–5252. [Google Scholar] [CrossRef]
- Cho, I.; Koo, B.N.; Kam, E.H.; Lee, S.K.; Oh, H.; Kim, S.Y. Bile duct ligation of C57BL/6 mice as a model of hepatic encephalopathy. Anesth. Pain Med. 2020, 15, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.P.; Lee, J.J.; Chang, Y.C.; Lin, C.H.; Li, Y.S.; Liu, C.L. Overexpression of chitinase-3-like protein 1 is associated with structural recurrence in patients with differentiated thyroid cancer. J. Pathol. 2020, 252, 114–124. [Google Scholar] [CrossRef]
- Greenberg, J.; Zarnegar, R. Exploring the role of chitinase-3-like protein 1 in recurrence patterns among patients with differentiated thyroid cancer(dagger). J. Pathol. 2020, 252, 343–345. [Google Scholar] [CrossRef]
- Nikolova, D.N.; Zembutsu, H.; Sechanov, T.; Vidinov, K.; Kee, L.S.; Ivanova, R.; Becheva, E.; Kocova, M.; Toncheva, D.; Nakamura, Y. Genome-wide gene expression profiles of thyroid carcinoma: Identification of molecular targets for treatment of thyroid carcinoma. Oncol. Rep. 2008, 20, 105–121. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, F.; Jia, H.; Ye, Z.; Yao, S. FK506-binding protein 5 promotes the progression of papillary thyroid carcinoma. J. Int. Med. Res. 2021, 49, 3000605211008325. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.I.; Lagadari, M.; Piwien-Pilipuk, G.; Galigniana, M.D. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J. Biol. Chem. 2011, 286, 30152–30160. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.; Sanchez-Madrid, F.; Acevedo, A.; Larranaga, E.; de Landazuri, M.O. Expression of vascular adhesion molecules on human endothelia in autoimmune thyroid disorders. Clin. Exp. Immunol. 1995, 102, 328–334. [Google Scholar] [CrossRef]
- Hara, H.; Sugita, E.; Sato, R.; Ban, Y. Plasma selectin levels in patients with Graves’ disease. Endocr. J. 1996, 43, 709–713. [Google Scholar] [CrossRef]
- Wenisch, C.; Myskiw, D.; Gessl, A.; Graninger, W. Circulating selectins, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in hyperthyroidism. J. Clin. Endocrinol. Metab. 1995, 80, 2122–2126. [Google Scholar] [CrossRef]
- Bal, N.; Kocer, N.E.; Ertorer, M.E.; Canpolat, E.T.; Kayaselcuk, F. Maspin, E-selectin, and P-selectin expressions in papillary thyroid carcinomas and their correlation with prognostic parameters. Pathol. Res. Pract. 2008, 204, 743–750. [Google Scholar] [CrossRef]
- Garcia, J.; Callewaert, N.; Borsig, L. P-selectin mediates metastatic progression through binding to sulfatides on tumor cells. Glycobiology 2007, 17, 185–196. [Google Scholar] [CrossRef]
- Chen, J.L.; Chen, W.X.; Zhu, J.S.; Chen, N.W.; Zhou, T.; Yao, M.; Zhang, D.Q.; Wu, Y.L. Effect of P-selectin monoclonal antibody on metastasis of gastric cancer and immune function. World J. Gastroenterol. 2003, 9, 1607–1610. [Google Scholar] [CrossRef]
- Ferroni, P.; Roselli, M.; Martini, F.; D’Alessandro, R.; Mariotti, S.; Basili, S.; Spila, A.; Aloe, S.; Palmirotta, R.; Maggini, A.; et al. Prognostic value of soluble P-selectin levels in colorectal cancer. Int. J. Cancer 2004, 111, 404–408. [Google Scholar] [CrossRef]
- Dohan, O.; De la Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The sodium/iodide Symporter (NIS): Characterization, regulation, and medical significance. Endocr. Rev. 2003, 24, 48–77. [Google Scholar] [CrossRef]
- Targovnik, H.M.; Citterio, C.E.; Rivolta, C.M. Iodide handling disorders (NIS, TPO, TG, IYD). Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef]
- Spitzweg, C.; Morris, J.C. Genetics and phenomics of hypothyroidism and goiter due to NIS mutations. Mol. Cell. Endocrinol. 2010, 322, 56–63. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ebrhim, R.S.; Abdullah, M.A.; Weiss, R.E. A Novel Missense Mutation in the SLC5A5 Gene in a Sudanese Family with Congenital Hypothyroidism. Thyroid 2018, 28, 1068–1070. [Google Scholar] [CrossRef]
- Zhang, C.X.; Zhang, J.X.; Yang, L.; Zhang, C.R.; Cheng, F.; Zhang, R.J.; Fang, Y.; Wang, Z.; Wu, F.Y.; Li, P.Z.; et al. Novel Compound Heterozygous Pathogenic Mutations of SLC5A5 in a Chinese Patient With Congenital Hypothyroidism. Front. Endocrinol. 2021, 12, 620117. [Google Scholar] [CrossRef]
- de Morais, R.M.; Sobrinho, A.B.; de Souza Silva, C.M.; de Oliveira, J.R.; da Silva, I.C.R.; de Toledo Nobrega, O. The Role of the NIS (SLC5A5) Gene in Papillary Thyroid Cancer: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 9128754. [Google Scholar] [CrossRef]
- Dey, S.; Curtis, D.J.; Jane, S.M.; Brandt, S.J. The TAL1/SCL transcription factor regulates cell cycle progression and proliferation in differentiating murine bone marrow monocyte precursors. Mol. Cell. Biol. 2010, 30, 2181–2192. [Google Scholar] [CrossRef]
- Blackwell, J.; Harries, L.W.; Pilling, L.C.; Ferrucci, L.; Jones, A.; Melzer, D. Changes in CEBPB expression in circulating leukocytes following eccentric elbow-flexion exercise. J. Physiol. Sci. 2015, 65, 145–150. [Google Scholar] [CrossRef]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef]
- Klecha, A.J.; Barreiro Arcos, M.L.; Frick, L.; Genaro, A.M.; Cremaschi, G. Immune-endocrine interactions in autoimmune thyroid diseases. Neuroimmunomodulation 2008, 15, 68–75. [Google Scholar] [CrossRef]
- Shih, A.; Zhang, S.; Cao, H.J.; Tang, H.Y.; Davis, F.B.; Davis, P.J.; Lin, H.Y. Disparate effects of thyroid hormone on actions of epidermal growth factor and transforming growth factor-alpha are mediated by 3′,5′-cyclic adenosine 5′-monophosphate-dependent protein kinase II. Endocrinology 2004, 145, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, C.F.; Simpson, E.E.; Beattie, J.H.; O’Connor, J.M.; Campbell, D.J.; Strain, J.J.; Wallace, J.M. Preliminary evidence of immune function modulation by thyroid hormones in healthy men and women aged 55-70 years. J. Endocrinol. 2009, 202, 55–63. [Google Scholar] [CrossRef]
- Alamino, V.A.; Montesinos, M.D.M.; Soler, M.F.; Giusiano, L.; Gigena, N.; Fozzatti, L.; Maller, S.M.; Mendez-Huergo, S.P.; Rabinovich, G.A.; Pellizas, C.G. Dendritic Cells Exposed to Triiodothyronine Deliver Pro-Inflammatory Signals and Amplify IL-17-Driven Immune Responses. Cell. Physiol. Biochem. 2019, 52, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Guasti, L.; Cosentino, M.; De Piazza, D.; Simoni, C.; Piantanida, E.; Cimpanelli, M.; Klersy, C.; Bartalena, L.; Venco, A.; et al. Thyroid hormone regulation of cell migration and oxidative metabolism in polymorphonuclear leukocytes: Clinical evidence in thyroidectomized subjects on thyroxine replacement therapy. Life Sci. 2006, 78, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Rotondi, M.; Ferrari, S.M.; Fallahi, P.; Romagnani, P.; Franceschini, S.S.; Serio, M.; Ferrannini, E. Interferon-gamma-inducible alpha-chemokine CXCL10 involvement in Graves’ ophthalmopathy: Modulation by peroxisome proliferator-activated receptor-gamma agonists. J. Clin. Endocrinol. Metab. 2006, 91, 614–620. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Berti, P.; Materazzi, G.; Marchetti, I.; Ugolini, C.; Basolo, F.; Miccoli, P.; Ferrannini, E. Evaluation of the sensitivity to chemotherapeutics or thiazolidinediones of primary anaplastic thyroid cancer cells obtained by fine-needle aspiration. Eur. J. Endocrinol. 2008, 159, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, E.C.; Badamas, J. Acute liver failure secondary to metastatic medullary thyroid cancer. Case Rep. Hepatol. 2011, 2011, 603757. [Google Scholar] [CrossRef]
- Borzio, M.; Caldara, R.; Borzio, F.; Piepoli, V.; Rampini, P.; Ferrari, C. Thyroid function tests in chronic liver disease: Evidence for multiple abnormalities despite clinical euthyroidism. Gut 1983, 24, 631–636. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, D.; Kim, H.K.; Kim, Y.-K.; Song, J. Transcriptome Profile of Thyroid Glands in Bile Duct Ligation Mouse Model. Int. J. Mol. Sci. 2022, 23, 8244. https://doi.org/10.3390/ijms23158244
Jo D, Kim HK, Kim Y-K, Song J. Transcriptome Profile of Thyroid Glands in Bile Duct Ligation Mouse Model. International Journal of Molecular Sciences. 2022; 23(15):8244. https://doi.org/10.3390/ijms23158244
Chicago/Turabian StyleJo, Danbi, Hee Kyung Kim, Young-Kook Kim, and Juhyun Song. 2022. "Transcriptome Profile of Thyroid Glands in Bile Duct Ligation Mouse Model" International Journal of Molecular Sciences 23, no. 15: 8244. https://doi.org/10.3390/ijms23158244
APA StyleJo, D., Kim, H. K., Kim, Y.-K., & Song, J. (2022). Transcriptome Profile of Thyroid Glands in Bile Duct Ligation Mouse Model. International Journal of Molecular Sciences, 23(15), 8244. https://doi.org/10.3390/ijms23158244





