Effect of Chemically Modified Carbon-Coated Iron Nanoparticles on the Structure of Human Atherosclerotic Plaques Ex Vivo and on Adipose Tissue in Chronic Experiment In Vivo
Abstract
:1. Introduction
2. Results
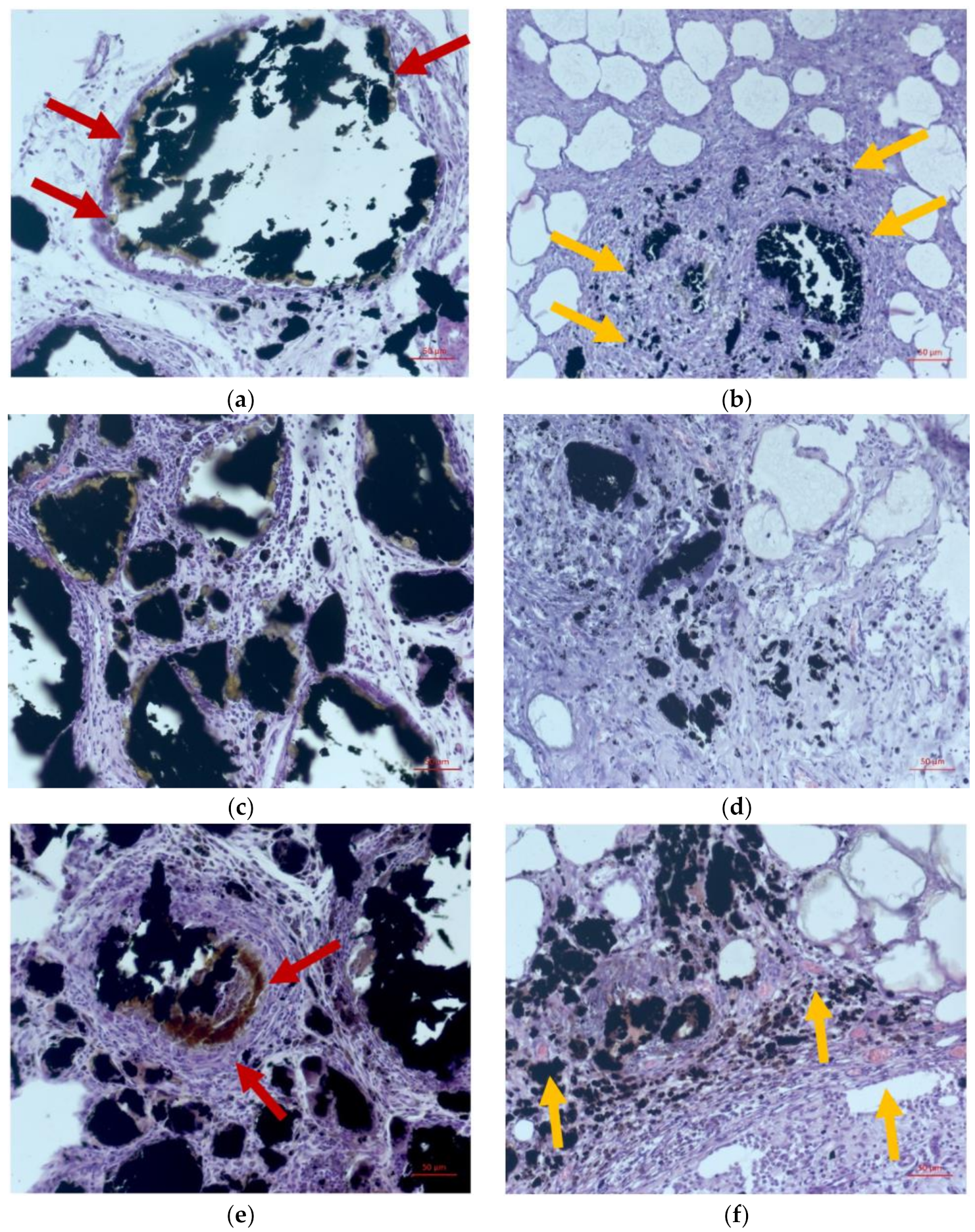
2.1. Morphological Study
2.2. Study of the Interaction of Atherosclerotic Plaques with Fe@C Nanocomposites
3. Discussion
4. Materials and Methods
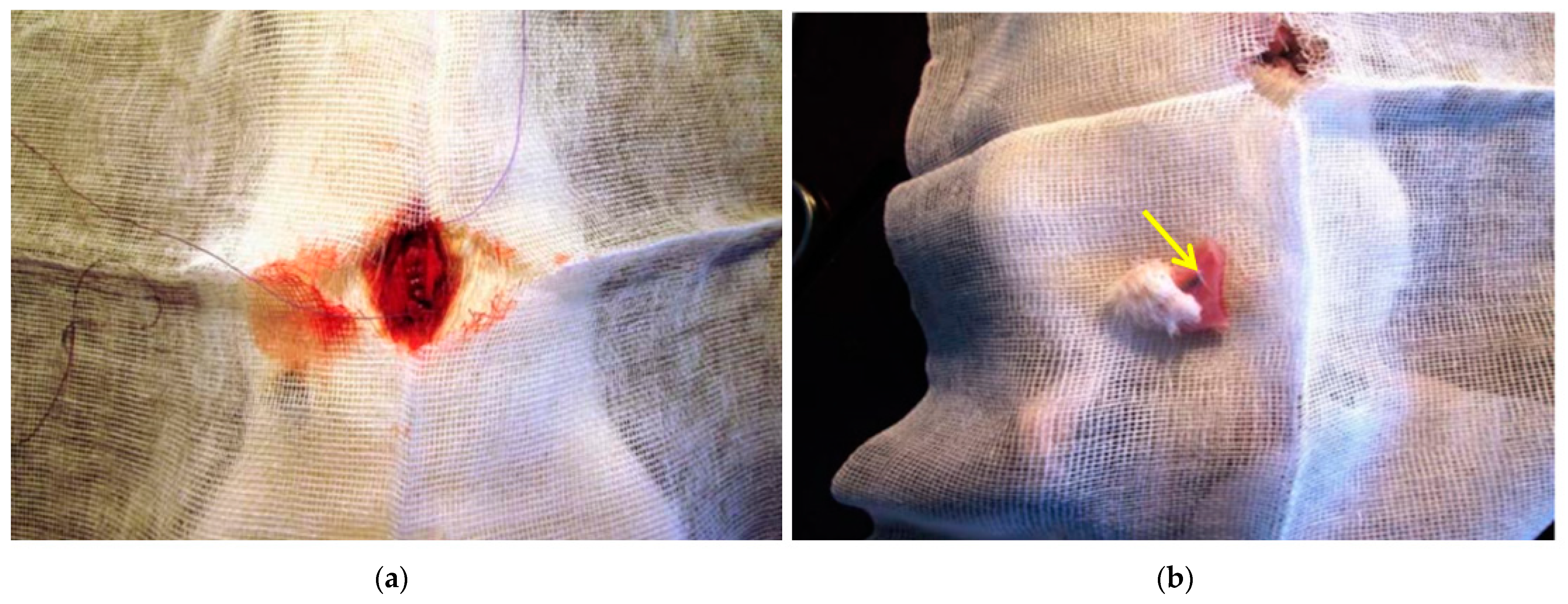
4.1. Samples for Chronic Experiment In Vivo
4.2. Research on Laboratory Animals
4.3. Morphological Studies
4.4. Ex Vivo Experiment with Human Atherosclerotic Plaques
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ala-Korpela, M. The culprit is the carrier, not the loads: Cholesterol, triglycerides and apolipoprotein B in atherosclerosis and coronary heart disease. Int. J. Epidemiol. 2019, 48, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, T.F.; Landmesser, U.; von Eckardstein, A.; Fogelman, A.M. High-Density Lipoprotein: Vascular Protective Effects, Dysfunction, and Potential as Therapeutic Target. Circ. Res. 2014, 114, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frias, J.C.; Williams, K.J.; Fisher, E.A.; Fayad, Z.A. Recombinant HDL-Like Nanoparticles: A Specific Contrast Agent for MRI of Atherosclerotic Plaques. J. Am. Chem. Soc. 2004, 126, 16316–16317. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gaytan, B.L.; Fay, F.; Lobatto, M.E.; Tang, J.; Ouimet, M.; Kim, Y.; van der Staay, S.E.M.; van Rijs, S.M.; Priem, B.; Zhang, L.; et al. HDL-Mimetic PLGA Nanoparticle to Target Atherosclerosis Plaque Macrophages. Bioconjug. Chem. 2015, 26, 443–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herranz, F.; Salinas, B.; Groult, H.; Pellico, J.; Lechuga-Vieco, A.; Bhavesh, R.; Ruiz-Cabello, J. Superparamagnetic Nanoparticles for Atherosclerosis Imaging. Nanomaterials 2014, 4, 408–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandramouli, S.; Sanjana, S.; Swathi, S. Use of Super Paramagnetic Iron-Oxide Nanoparticles in the Treatment of Atherosclerosis. In Proceedings of the 5th International Conference on Biomedical Engineering in Vietnam, Ho Chi Minh, Vietnam, 16–18 June 2014; Toi, V., Lien Phuong, T., Eds.; Springer: Cham, Switzerland, 2014; Volume 46. [Google Scholar] [CrossRef]
- Matuszak, J.; Lutz, B.; Sekita, A.; Zaloga, J.; Alexiou, C.; Lyer, S.; Cicha, I. Drug delivery to atherosclerotic plaques using superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 8443–8460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, S.; Zhang, J.; Martinez-Zaguilan, R.; Sennoune, S.; Hossen, M.N.; Wang, S. Detecting atherosclerotic lesions and intimal macrophages using targeted nanovesicles. J. Control. Release 2015, 220, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zu, Y.; Dhanasekara, C.S.; Li, J.; Wu, D.; Fan, Z.; Wang, S. Detection and treatment of atherosclerosis using nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9.1, e1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuschner, F.; Dutta, P.; Gorbatov, R.; Novobrantseva, T.I.; Donahoe, J.S.; Courties, G.; Lee, K.M.; Kim, J.I.; Markmann, J.F.; Marinelli, B. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011, 29, 1005–1010. [Google Scholar] [CrossRef]
- Iverson, N.M.; Plourde, N.M.; Sparks, S.M.; Wang, J.; Patel, E.N.; Shah, P.S.; Lewis, D.R.; Zablocki, K.R.; Nackman, G.B.; Uhrich, K.E. Dual use of amphiphilic macromolecules as cholesterol efflux triggers and inhibitors of macrophage athero-inflammation. Biomaterials 2011, 32, 8319–8327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakov, A.E.; Uimin, M.A.; Lokteva, E.S.; Mysik, A.A.; Kachevskii, S.A.; Turakulova, A.O.; Lunin, V.V. The synthesis, structure and properties of carbon-containing nano-composites based on nickel, palladium, and iron. Russ. J. Phys. Chem. A 2009, 83, 1187–1193. [Google Scholar] [CrossRef]
- Akhmedov, S.; Afanasyev, S.; Trusova, M.; Postnikov, P.; Rogovskaya, Y.; Grakova, E.; Kopeva, K.; Carreon Paz, R.K.; Balakin, S.; Wiesmann, H.-P.; et al. Chemically Modified Biomimetic Carbon-Coated Iron Nanoparticles for Stent Coatings: In Vitro Cytocompatibility and In Vivo Structural Changes in Human Atherosclerotic Plaques. Biomedicines 2021, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, S.D.; Afanasiev, S.A.; Filimonov, V.D.; Postnikov, P.S.; Trusova, M.E.; Karpov, R.S. Agent for Selective Adjustment of Blood Lipids. U.S. Patent US9,789,134,B2, 17 October 2017. [Google Scholar]
- Postnikov, P.S.; Trusova, M.E.; Fedushchak, T.A.; Uimin, M.A.; Ermakov, A.E.; Filimonov, V.D. Aryldiazonium tosylates as new efficient agents for covalent grafting of aromatic groups on carbon coatings of metal nanoparticles. Nanotechnol. Russ. 2010, 5, 446–449. [Google Scholar] [CrossRef]
- Pershina, A.G.; Lytkina, O.A.; Postnikov, P.S.; Trusova, M.E.; Svitich, D.Y.; Filimonov, V.D.; Sazonov, A.E. Development of HRP-functionalized carbon-coated iron nanoparticles using arenediazonium tosylates. In Biochemistry and Biotechnology; Nova Science Publishers: New York, NY, USA, 2012; Volume 39. [Google Scholar]
- Morozova, M.A.; Trusova, M.E.; Kutonova, K.V.; Filimonov, V.D. New effective and environmental friendly method for the hydrodediazoniation of arenediazonium tosylates using Fe-core/carbon-shell nanoparticles. Adv. Mater. Res. 2014, 1040, 263–267. [Google Scholar] [CrossRef]




| Duration of Implantation, Days | Area of Fringing Phenomenon, µm2 | |
|---|---|---|
| Non-Modified Fe@C NPs | Modified Fe@C NPs | |
| 7 | - * | 3.38 ± 1.19 |
| 14 | - * | 6.31 ± 0.10 |
| 21 | - * | 9.62 ± 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmedov, S.; Afanasyev, S.; Beshchasna, N.; Trusova, M.; Stepanov, I.; Rebenkova, M.; Poletykina, E.; Vecherskiy, Y.; Tverdokhlebov, S.; Bolbasov, E.; et al. Effect of Chemically Modified Carbon-Coated Iron Nanoparticles on the Structure of Human Atherosclerotic Plaques Ex Vivo and on Adipose Tissue in Chronic Experiment In Vivo. Int. J. Mol. Sci. 2022, 23, 8241. https://doi.org/10.3390/ijms23158241
Akhmedov S, Afanasyev S, Beshchasna N, Trusova M, Stepanov I, Rebenkova M, Poletykina E, Vecherskiy Y, Tverdokhlebov S, Bolbasov E, et al. Effect of Chemically Modified Carbon-Coated Iron Nanoparticles on the Structure of Human Atherosclerotic Plaques Ex Vivo and on Adipose Tissue in Chronic Experiment In Vivo. International Journal of Molecular Sciences. 2022; 23(15):8241. https://doi.org/10.3390/ijms23158241
Chicago/Turabian StyleAkhmedov, Shamil, Sergey Afanasyev, Natalia Beshchasna, Marina Trusova, Ivan Stepanov, Mariya Rebenkova, Ekaterina Poletykina, Yuri Vecherskiy, Sergei Tverdokhlebov, Evgeny Bolbasov, and et al. 2022. "Effect of Chemically Modified Carbon-Coated Iron Nanoparticles on the Structure of Human Atherosclerotic Plaques Ex Vivo and on Adipose Tissue in Chronic Experiment In Vivo" International Journal of Molecular Sciences 23, no. 15: 8241. https://doi.org/10.3390/ijms23158241
APA StyleAkhmedov, S., Afanasyev, S., Beshchasna, N., Trusova, M., Stepanov, I., Rebenkova, M., Poletykina, E., Vecherskiy, Y., Tverdokhlebov, S., Bolbasov, E., Balakin, S., Opitz, J., Yermakov, A., & Kozlov, B. (2022). Effect of Chemically Modified Carbon-Coated Iron Nanoparticles on the Structure of Human Atherosclerotic Plaques Ex Vivo and on Adipose Tissue in Chronic Experiment In Vivo. International Journal of Molecular Sciences, 23(15), 8241. https://doi.org/10.3390/ijms23158241












