Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures
Abstract
1. Introduction
2. Results
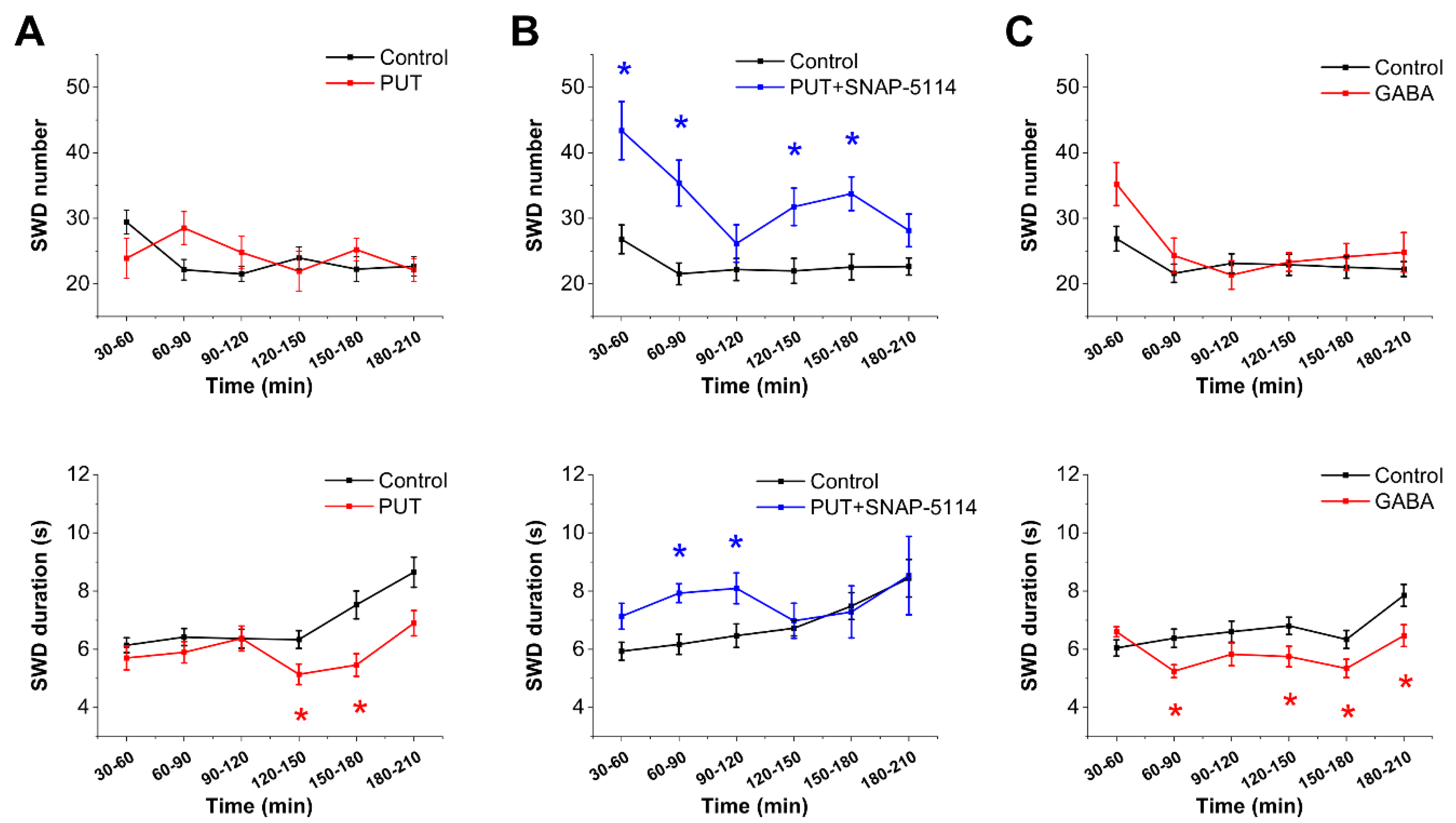
2.1. Exogenously Applied PUT Reduces Seizure Duration in the In Vivo WAG/Rij Rat Model of Absence Epilepsy
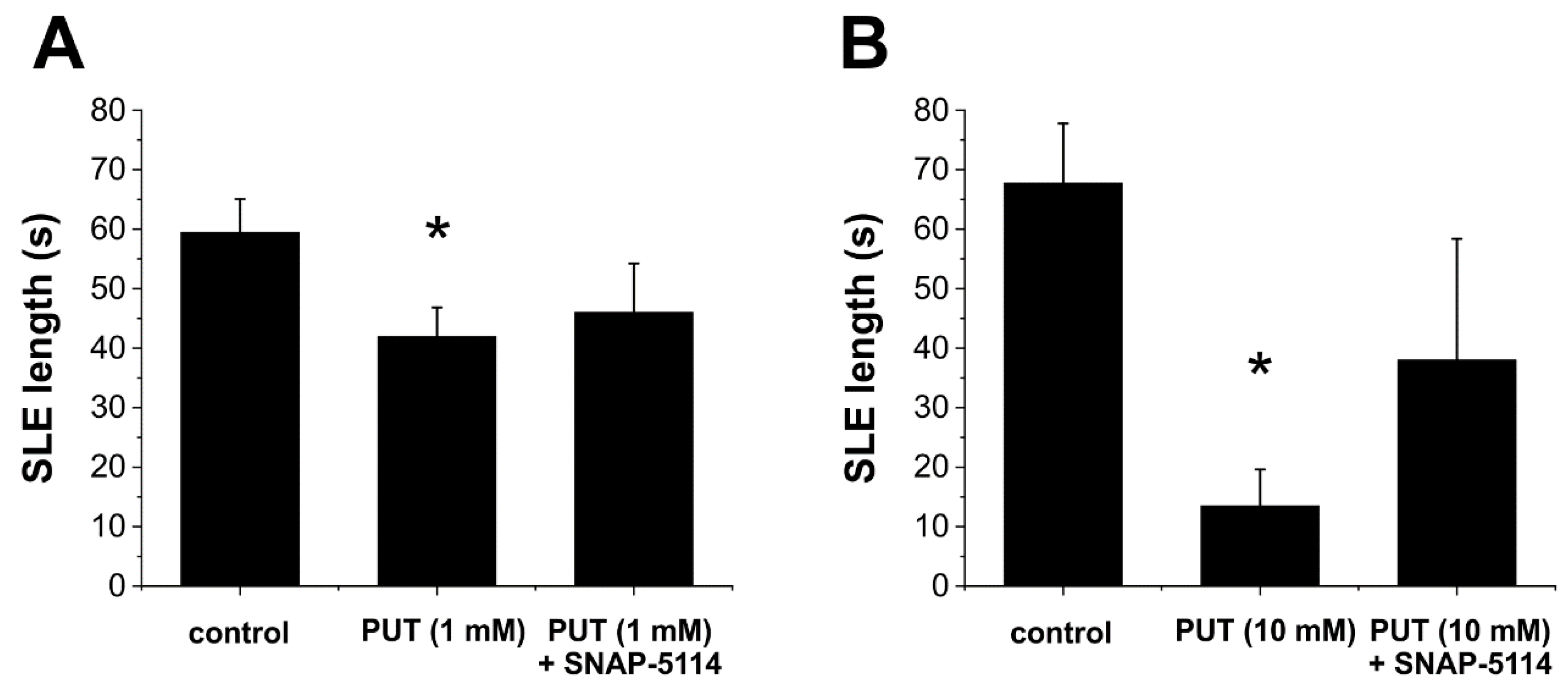
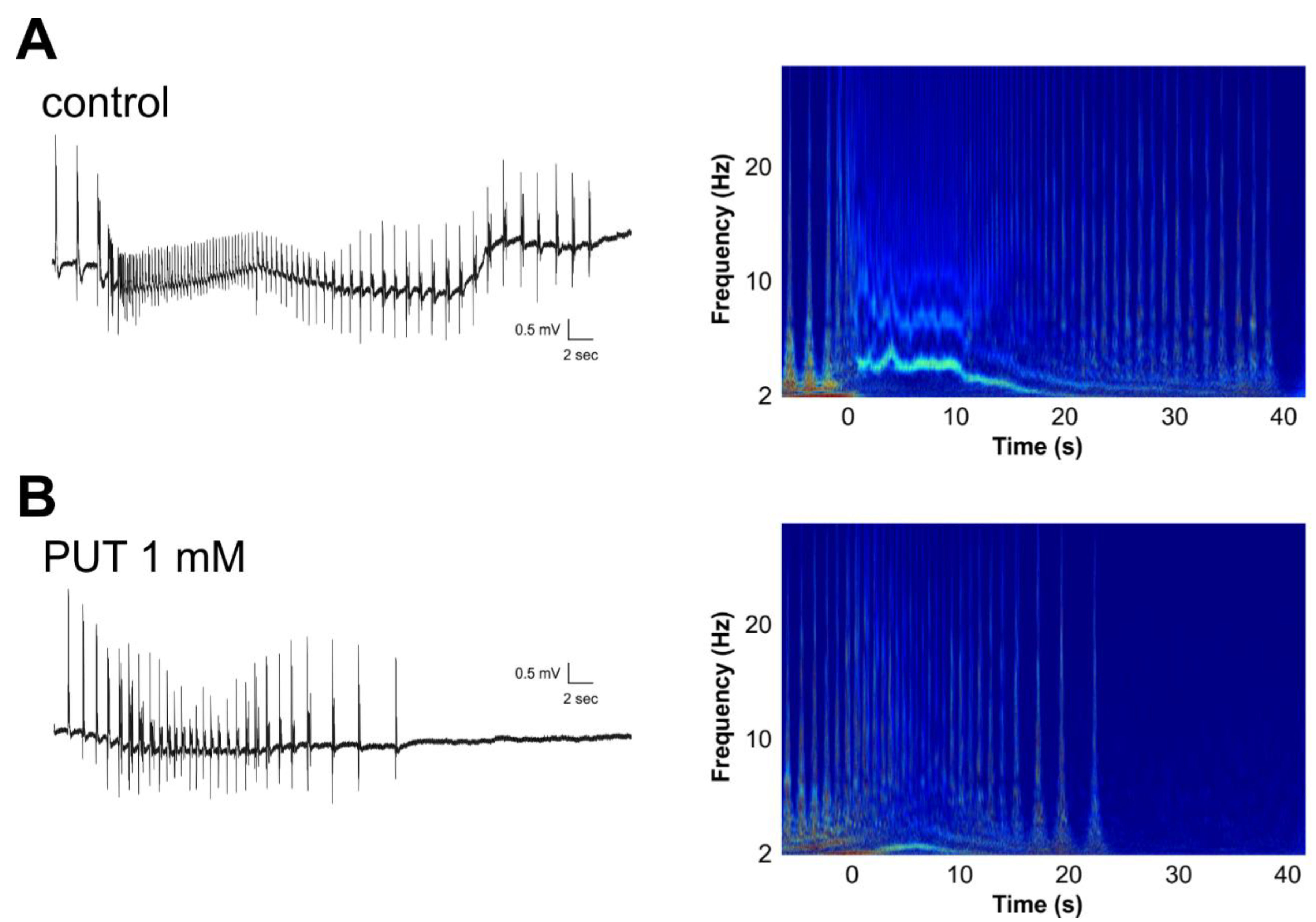
2.2. PUT Dose-Dependently Reduces Seizure Duration in the In Vitro Low-[Mg2+] Model of Temporal Lobe Epilepsy
2.3. Glial GABA Release Following PUT Application Specifically Reduces Excitatory Signals
2.4. PUT Specifically Limits Tonic Phase of SLEs in the In Vitro Low-[Mg2+] Model of Temporal Lobe Epilepsy
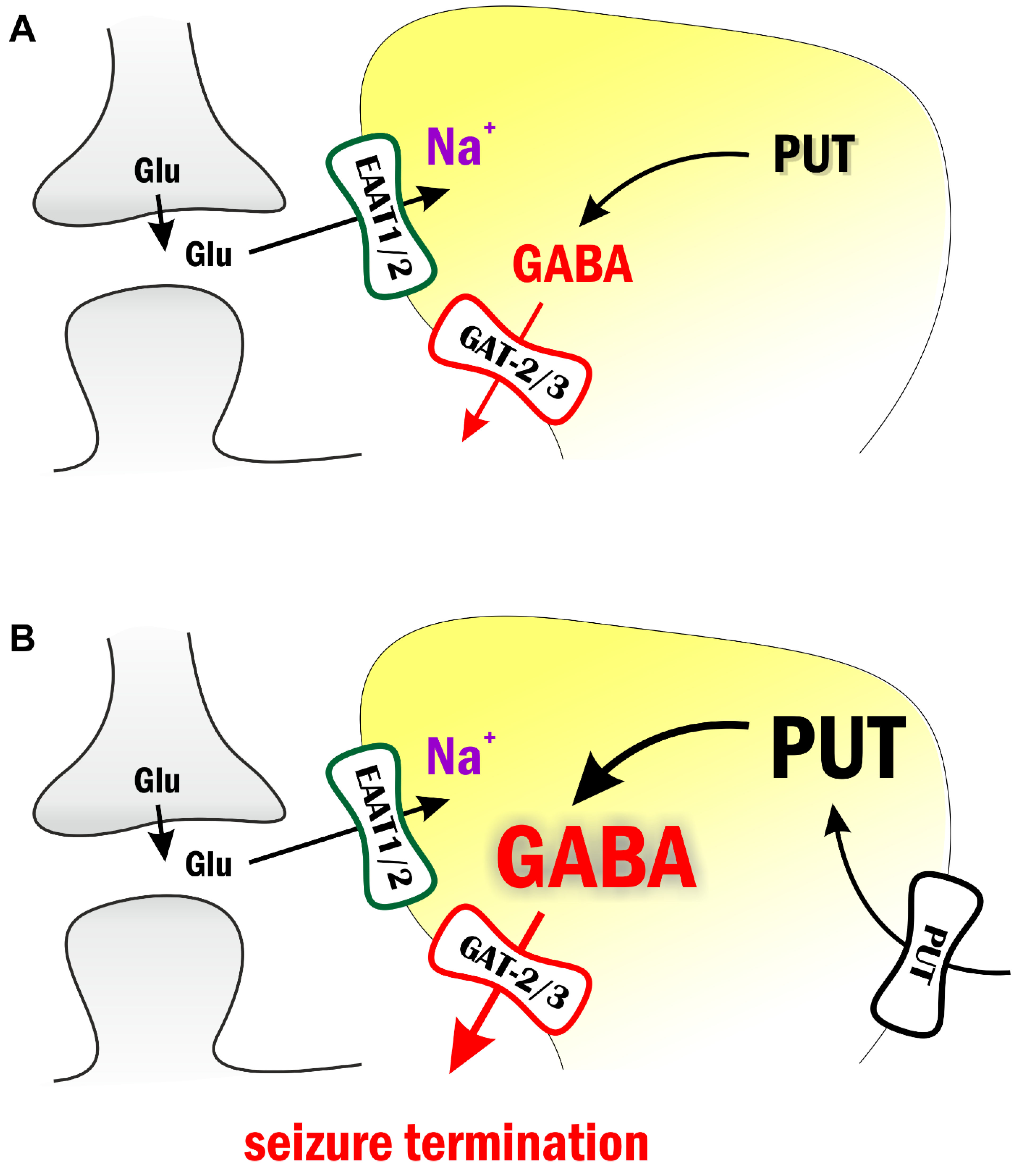
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Solutions
4.3. Slice Preparation
4.4. Electrophysiology
4.5. Data Digitization and Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timofeev, I.; Steriade, M. Neocortical seizures: Initiation, development and cessation. Neuroscience 2004, 123, 299–336. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, S.; Gracely, E.J.; Sperling, M.R. How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia 2006, 47, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Lado, F.A.; Moshé, S.L. How do seizures stop? Epilepsia 2008, 49, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.P.; Bazhenov, M. Ionic dynamics mediate spontaneous termination of seizures and postictal depression state. J. Neurosci. 2011, 31, 8870–8882. [Google Scholar] [CrossRef]
- Kovac, S.; Abramov, A.Y.; Walker, M.C. Energy depletion in seizures: Anaplerosis as a strategy for future therapies. Neuropharmacology 2013, 69, 96–104. [Google Scholar] [CrossRef]
- Proix, T.; Jirsa, V.K.; Bartolomei, F.; Guye, M.; Truccolo, W. Predicting the spatiotemporal diversity of seizure propagation and termination in human focal epilepsy. Nat. Commun. 2018, 9, 1088. [Google Scholar] [CrossRef]
- Lasztóczi, B.; Antal, K.; Nyikos, L.; Emri, Z.; Kardos, J. High-frequency synaptic input contributes to seizure initiation in the low-[Mg2+] model of epilepsy. Eur. J. Neurosci. 2004, 19, 1361–1372. [Google Scholar] [CrossRef]
- Kovács, Z.; Kékesi, K.A.; Dobolyi, Á.; Lakatos, R.; Juhász, G. Absence epileptic activity changing effects of non-adenosine nucleoside inosine, guanosine and uridine in Wistar Albino Glaxo Rijswijk rats. Neuroscience 2015, 300, 593–608. [Google Scholar] [CrossRef]
- Vincze, R.; Péter, M.; Szabó, Z.; Kardos, J.; Héja, L.; Kovács, Z. Connexin 43 differentially regulates epileptiform activity in models of convulsive and non-convulsive epilepsies. Front. Cell. Neurosci. 2019, 13, 173. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, D.; Song, Z. Neuronal networks and energy bursts in epilepsy. Neuroscience 2015, 287, 175–186. [Google Scholar] [CrossRef]
- Tryba, A.K.; Merricks, E.M.; Lee, S.; Pham, T.; Cho, S.J.; Nordli, D.R.; Eissa, T.L.; Goodman, R.R.; McKhann, G.M.; Emerson, R.G.; et al. Role of paroxysmal depolarization in focal seizure activity. J. Neurophysiol. 2019, 122, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Hamm, J.P.; Peterka, D.S.; Yuste, R. Acute focal seizures start as local synchronizations of neuronal ensembles. J. Neurosci. 2019, 39, 8562–8575. [Google Scholar] [CrossRef] [PubMed]
- Héja, L.; Barabás, P.; Nyitrai, G.; Kékesi, K.A.; Lasztóczi, B.; Toke, O.; Tárkányi, G.; Madsen, K.; Schousboe, A.; Dobolyi, A.; et al. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS ONE 2009, 4, e7153. [Google Scholar] [CrossRef] [PubMed]
- Héja, L.; Nyitrai, G.; Kékesi, O.; Dobolyi, A.; Szabó, P.; Fiáth, R.; Ulbert, I.; Pál-Szenthe, B.; Palkovits, M.; Kardos, J. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol. 2012, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Skatchkov, S.N.; Veh, R.W.; Szabó, Z.; Németh, K.; Szabó, P.T.; Kardos, J.; Héja, L. Critical Role of Astrocytic Polyamine and GABA Metabolism in Epileptogenesis. Front. Cell. Neurosci. 2022, 15, 787319. [Google Scholar] [CrossRef]
- Meeren, H.K.M.; Pijn, J.P.M.; Van Luijtelaar, E.L.J.M.; Coenen, A.M.L.; Da Silva, F.H.L. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J. Neurosci. 2002, 22, 1480–1495. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Nagatomo, K.; Tokumaru, J.; Takaki, M.; Willmore, L.J. Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 2007, 1151, 55–61. [Google Scholar] [CrossRef]
- Cope, D.W.; Di Giovanni, G.; Fyson, S.J.; Orban, G.; Errington, A.C.; Lorincz, M.L.; Gould, T.M.; Carter, D.A.; Crunelli, V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 2009, 15, 1392–1398. [Google Scholar] [CrossRef]
- Yoon, B.-E.; Jo, S.; Woo, J.; Lee, J.-H.; Kim, T.; Kim, D.; Lee, C.J. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol. Brain 2011, 4, 42. [Google Scholar] [CrossRef]
- Wójtowicz, A.M.; Dvorzhak, A.; Semtner, M.; Grantyn, R. Reduced tonic inhibition in striatal output neurons from Huntington mice due to loss of astrocytic GABA release through GAT-3. Front. Neural Circuits 2013, 7, 188. [Google Scholar] [CrossRef]
- Unichenko, P.; Dvorzhak, A.; Kirischuk, S. Transporter-mediated replacement of extracellular glutamate for GABA in the developing murine neocortex. Eur. J. Neurosci. 2013, 38, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Kékesi, O.; Ioja, E.; Szabó, Z.; Kardos, J.; Héja, L. Recurrent seizure-like events are associated with coupled astroglial synchronization. Front. Cell. Neurosci. 2015, 9, 215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Héja, L.; Szabó, Z.; Péter, M.; Kardos, J. Spontaneous Ca2+ Fluctuations Arise in Thin Astrocytic Processes With Real 3D Geometry. Front. Cell. Neurosci. 2021, 15, 617989. [Google Scholar] [CrossRef] [PubMed]
- Rieck, J.; Skatchkov, S.N.; Derst, C.; Eaton, M.J.; Veh, R.W. Unique Chemistry, Intake, and Metabolism of Polyamines in the Central Nervous System (CNS) and Its Body. Biomolecules 2022, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Peeters, B.W.M.M.; van Rijn, C.M.; Vossen, J.M.H.; Coenen, A.M.L. Effects of GABA-ergic agents on spontaneous non-convulsive epilepsy, EEG and behaviour, in the WAG/Rij inbred strain of rats. Life Sci. 1989, 45, 1171–1176. [Google Scholar] [CrossRef]
- Coenen, A.M.L.; Blezer, E.H.M.; van Luijtelaar, E.L.J.M. Effects of the GABA-uptake inhibitor tiagabine on electroencephalogram, spike-wave discharges and behaviour of rats. Epilepsy Res. 1995, 21, 89–94. [Google Scholar] [CrossRef]
- Nyikos, L.; Lasztóczi, B.; Antal, K.; Kovács, R.; Kardos, J. Desynchronisation of spontaneously recurrent experimental seizures proceeds with a single rhythm. Neuroscience 2003, 121, 705–717. [Google Scholar] [CrossRef]
- Schevon, C.A.; Weiss, S.A.; McKhann, G.; Goodman, R.R.; Yuste, R.; Emerson, R.G.; Trevelyan, A.J. Evidence of an inhibitory restraint of seizure activity in humans. Nat. Commun. 2012, 3, 1060. [Google Scholar] [CrossRef]
- Trevelyan, A.J.; Schevon, C.A. How inhibition influences seizure propagation. Neuropharmacology 2013, 69, 45–54. [Google Scholar] [CrossRef]
- Liou, J.Y.; Ma, H.; Wenzel, M.; Zhao, M.; Baird-Daniel, E.; Smith, E.H.; Daniel, A.; Emerson, R.; Yuste, R.; Schwartz, T.H.; et al. Role of inhibitory control in modulating focal seizure spread. Brain 2018, 141, 2083–2097. [Google Scholar] [CrossRef]
- Magloire, V.; Mercier, M.S.; Kullmann, D.M.; Pavlov, I. GABAergic Interneurons in Seizures: Investigating Causality With Optogenetics. Neuroscientist 2019, 25, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Kardos, J.; Heinemann, U.; Kann, O. Mitochondrial calcium ion and membrane potential transients follow the pattern of epileptiform discharges in hippocampal slice cultures. J. Neurosci. 2005, 25, 4260–4269. [Google Scholar] [CrossRef]
- Szabó, Z.; Péter, M.; Héja, L.; Kardos, J. Dual role for astroglial copper-assisted polyamine metabolism during intense network activity. Biomolecules 2021, 11, 604. [Google Scholar] [CrossRef]
- Călin, A.; Ilie, A.S.; Akerman, C.J. Disrupting epileptiform activity by preventing parvalbumin interneuron depolarization block. J. Neurosci. 2021, 41, 9452–9465. [Google Scholar] [CrossRef] [PubMed]
- Panthi, S.; Leitch, B. Chemogenetic Activation of Feed-Forward Inhibitory Parvalbumin-Expressing Interneurons in the Cortico-Thalamocortical Network During Absence Seizures. Front. Cell. Neurosci. 2021, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gong, Y.; Ruan, Y.; Chen, Z.; Xu, C. Secondary Epileptogenesis: Common to See, but Possible to Treat? Front. Neurol. 2021, 12, 747372. [Google Scholar] [CrossRef]
- Kovács, Z.; Czurkó, A.; Kékesi, K.A.; Juhász, G. Intracerebroventricularly administered lipopolysaccharide enhances spike-wave discharges in freely moving WAG/Rij rats. Brain Res. Bull. 2011, 85, 410–416. [Google Scholar] [CrossRef]
- Benedikt, J.; Inyushin, M.; Kucheryavykh, Y.V.; Rivera, Y.; Kucheryavykh, L.Y.; Nichols, C.G.; Eaton, M.J.; Skatchkov, S.N. Intracellular polyamines enhance astrocytic coupling. NeuroReport 2012, 23, 1021–1025. [Google Scholar] [CrossRef]
- Méndez-González, M.P.; Rivera-Aponte, D.E.; Benedikt, J.; Maldonado-Martínez, G.; Tejeda-Bayron, F.; Skatchkov, S.N.; Eaton, M.J. Downregulation of astrocytic kir4.1 potassium channels is associated with hippocampal neuronal hyperexcitability in type 2 diabetic mice. Brain Sci. 2020, 10, 72. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780080475134. [Google Scholar]
- Coenen, A.M.L.; Van Luijtelaar, E.L.J.M. Genetic animal models for absence epilepsy: A review of the WAG/Rij strain of rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, Z.; Skatchkov, S.N.; Szabó, Z.; Qahtan, S.; Méndez-González, M.P.; Malpica-Nieves, C.J.; Eaton, M.J.; Kardos, J.; Héja, L. Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures. Int. J. Mol. Sci. 2022, 23, 8191. https://doi.org/10.3390/ijms23158191
Kovács Z, Skatchkov SN, Szabó Z, Qahtan S, Méndez-González MP, Malpica-Nieves CJ, Eaton MJ, Kardos J, Héja L. Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures. International Journal of Molecular Sciences. 2022; 23(15):8191. https://doi.org/10.3390/ijms23158191
Chicago/Turabian StyleKovács, Zsolt, Serguei N. Skatchkov, Zsolt Szabó, Saif Qahtan, Miguel P. Méndez-González, Christian J. Malpica-Nieves, Misty J. Eaton, Julianna Kardos, and László Héja. 2022. "Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures" International Journal of Molecular Sciences 23, no. 15: 8191. https://doi.org/10.3390/ijms23158191
APA StyleKovács, Z., Skatchkov, S. N., Szabó, Z., Qahtan, S., Méndez-González, M. P., Malpica-Nieves, C. J., Eaton, M. J., Kardos, J., & Héja, L. (2022). Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures. International Journal of Molecular Sciences, 23(15), 8191. https://doi.org/10.3390/ijms23158191







