Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis
Abstract
:1. Introduction
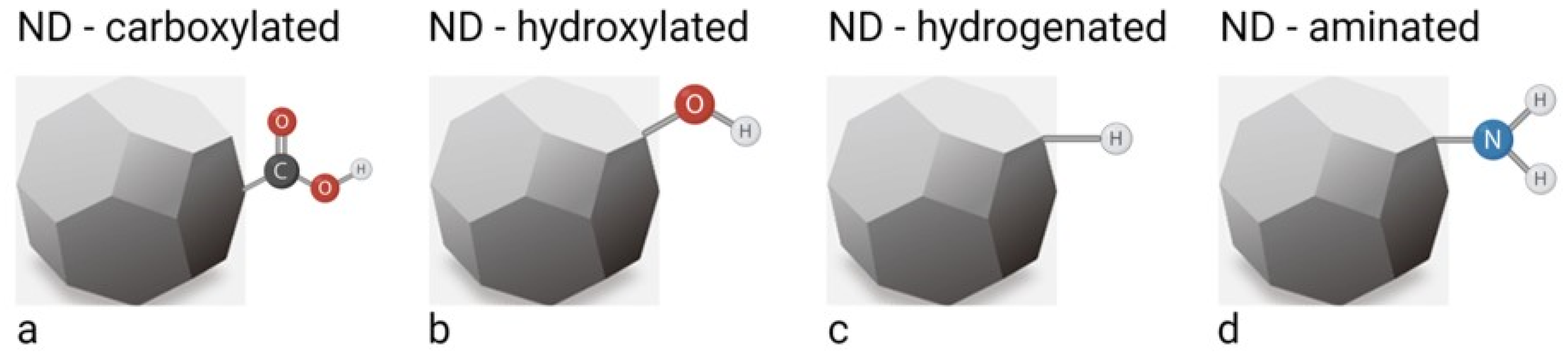
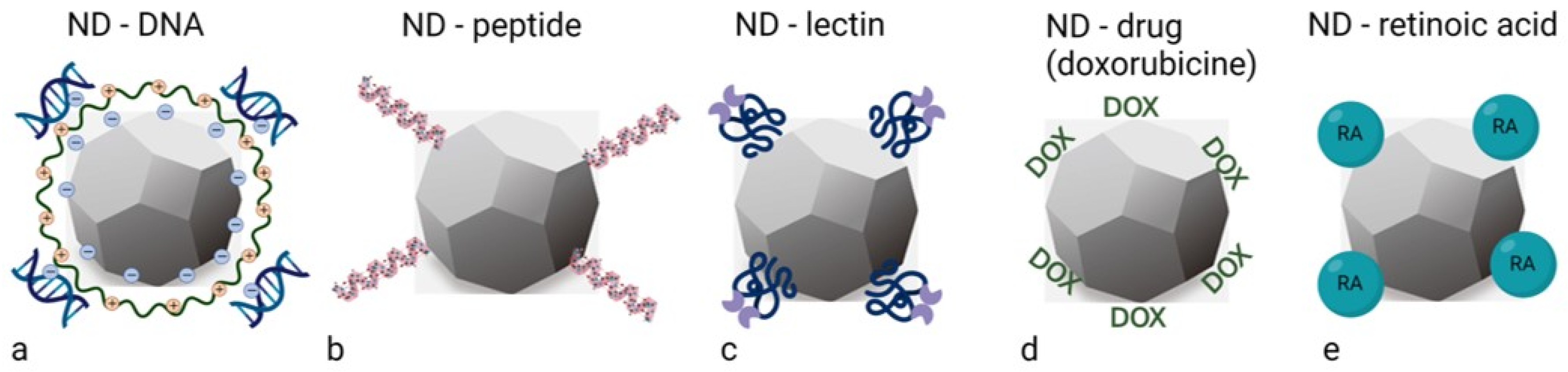
2. Synthesis, Properties and Functionalisation of Nanodiamonds
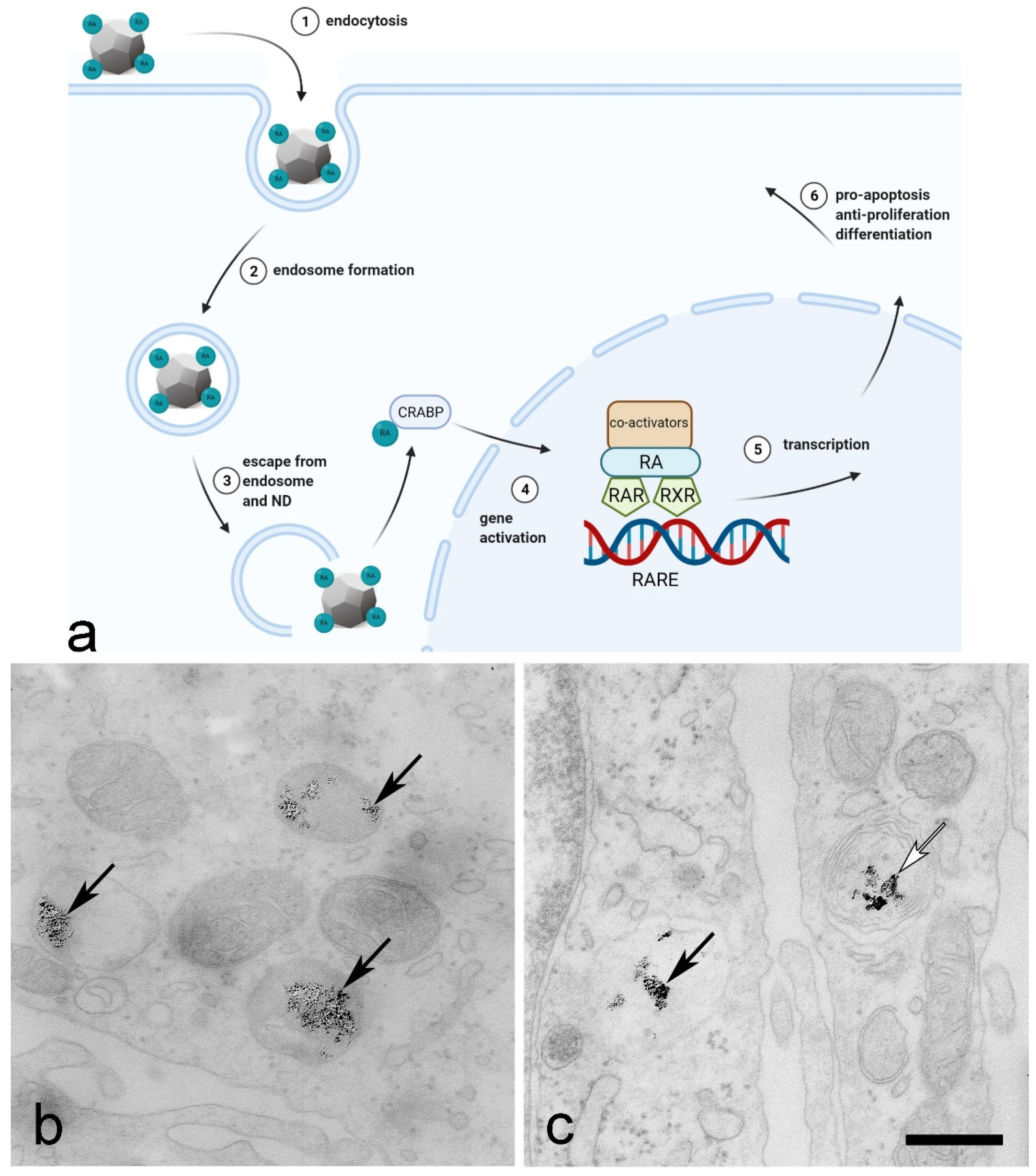
3. Prospective of Nanodiamonds in Bladder Cancer Management
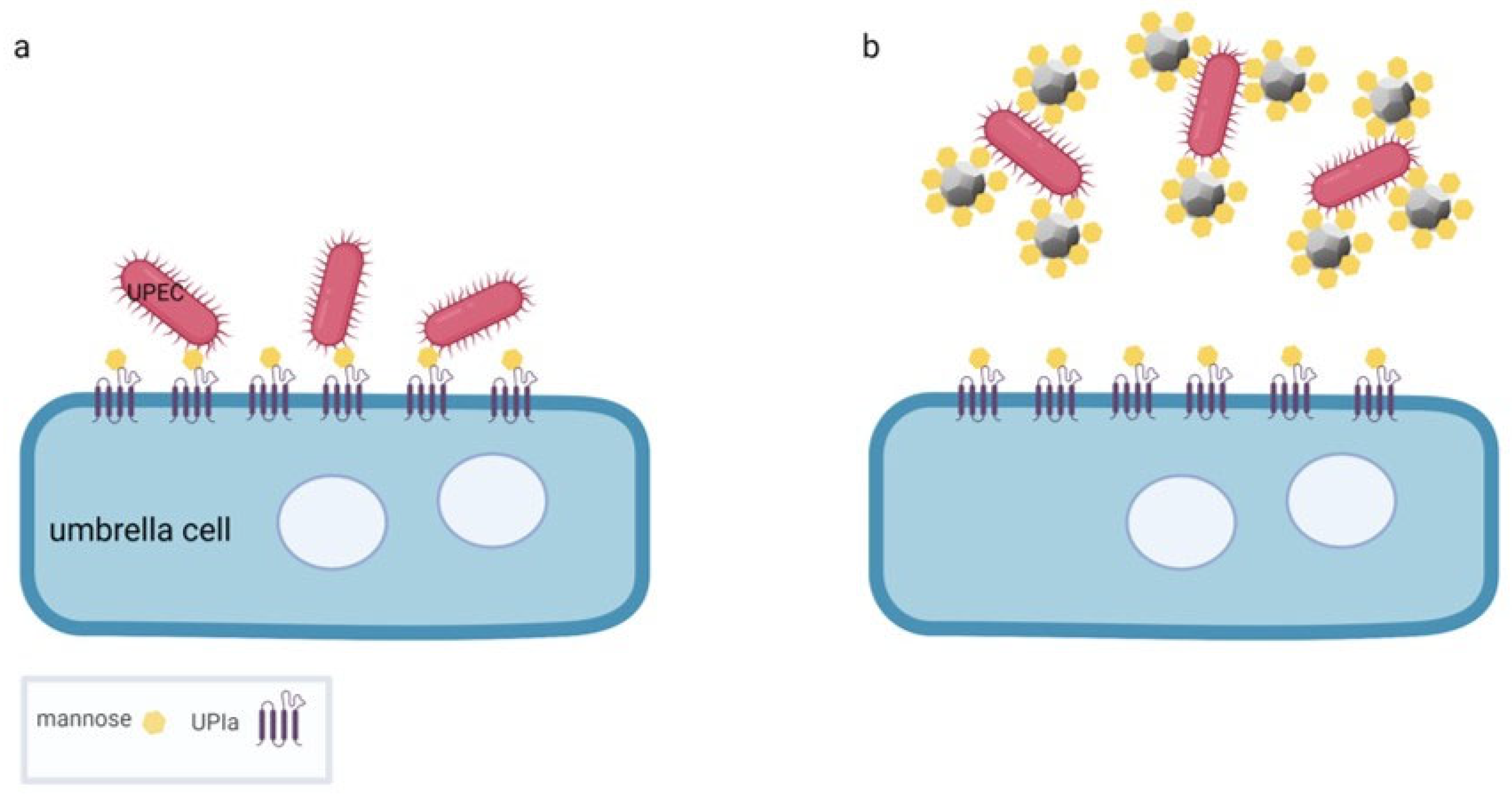
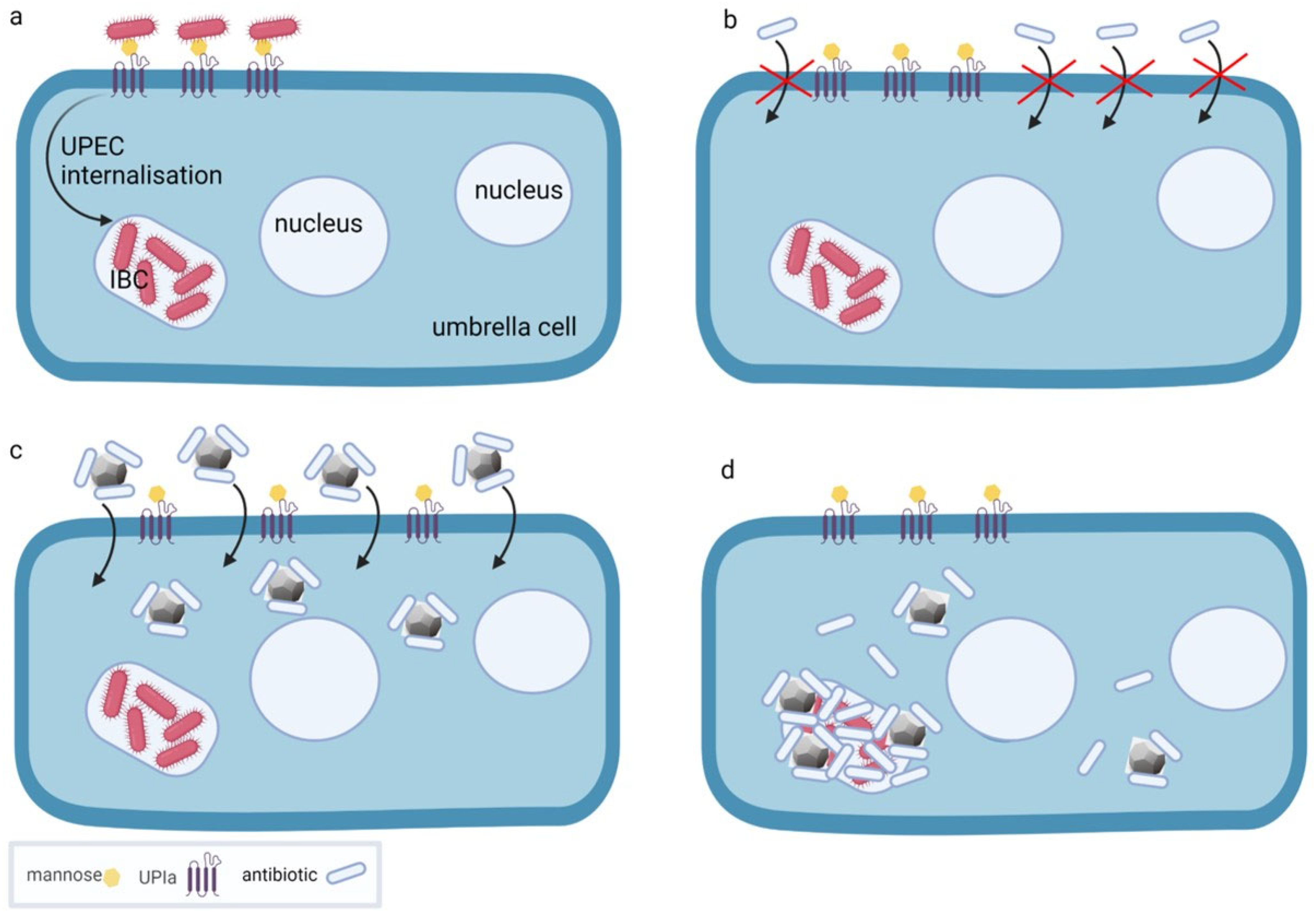
4. Nanodiamond Platforms for the Treatment of Bacterial Cystitis
5. Diagnostics, Therapy and Theranostics Using Nanodiamonds in Diseases of the Urinary Bladder
6. Comparison of Nanodiamonds with Other Nanoparticles
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Volkov, K.V.; Danilenko, V.V.; Elin, V.I. Synthesis of diamond from the carbon in the detonation products of explosives. Combust. Explos. Shock. Waves 1990, 26, 366–368. [Google Scholar] [CrossRef]
- Holt, K.B. Diamond at the nanoscale: Applications of diamond nanoparticles from cellular biomarkers to quantum computing. Philos. Trans. A Math. Phys. Eng. Sci. 2007, 365, 2845–2861. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Yu, S.J.; Kang, M.W.; Chang, H.C.; Chen, K.M.; Yu, Y.C. Bright fluorescent nanodiamonds: No photobleaching and low cytotoxicity. J. Am. Chem. Soc. 2005, 127, 17604–17605. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, D.; Kreft, M.E.; Grdadolnik, M.; Mitev, D.; Iglič, A.; Veranič, P. Detonation nanodiamonds are promising nontoxic delivery system for urothelial cells. Protoplasma 2018, 255, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Neuman, K.C. Surface Modification of Fluorescent Nanodiamonds for Biological Applications. Nanomaterials 2021, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Ashfold, M.N.R.; Goss, J.P.; Green, B.L.; May, P.W.; Newton, M.E.; Peaker, C.V. Nitrogen in Diamond. Chem. Rev. 2020, 120, 5745–5794. [Google Scholar] [CrossRef]
- Vaijayanthimala, V.; Lee, D.K.; Kim, S.V.; Yen, A.; Tsai, N.; Ho, D.; Chang, H.C.; Shenderova, O. Nanodiamond-mediated drug delivery and imaging: Challenges and opportunities. Expert Opin. Drug Deliv. 2015, 12, 735–749. [Google Scholar] [CrossRef]
- Chipaux, M.; van der Laan, K.J.; Hemelaar, S.R.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds and Their Applications in Cells. Small 2018, 14, e1704263. [Google Scholar] [CrossRef]
- Vlasov, I.I.; Shiryaev, A.A.; Rendler, T.; Steinert, S.; Lee, S.Y.; Antonov, D.; Voros, M.; Jelezko, F.; Fisenko, A.V.; Semjonova, L.F.; et al. Molecular-sized fluorescent nanodiamonds. Nat. Nanotechnol. 2014, 9, 54–58. [Google Scholar] [CrossRef]
- Havlik, J.; Petrakova, V.; Rehor, I.; Petrak, V.; Gulka, M.; Stursa, J.; Kucka, J.; Ralis, J.; Rendler, T.; Lee, S.-Y.; et al. Boosting nanodiamond fluorescence: Towards development of brighter probes. Nanoscale 2013, 5, 3208–3211. [Google Scholar] [CrossRef]
- Boudou, J.P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thorel, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef]
- Chang, Y.R.; Lee, H.Y.; Chen, K.; Chang, C.C.; Tsai, D.S.; Fu, C.C.; Lim, T.S.; Tzeng, Y.K.; Fang, C.Y.; Han, C.C.; et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat. Nanotechnol. 2008, 3, 284–288. [Google Scholar] [CrossRef]
- Fu, C.C.; Lee, H.Y.; Chen, K.; Lim, T.S.; Wu, H.Y.; Lin, P.K.; Wei, P.K.; Tsao, P.H.; Chang, H.C.; Fann, W. Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc. Natl. Acad. Sci. USA 2007, 104, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Shenderova, O.A.; Shames, A.I.; Nunn, N.A.; Torelli, M.D.; Vlasov, I.; Zaitsev, A. Review Article: Synthesis, properties, and applications of fluorescent diamond particles. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2019, 37, 030802. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.; Lawson, S.C.; Collins, A.T.; Mainwood, A.; Sharp, S.J. Vacancy-related centers in diamond. Phys. Rev. B Condens Matter 1992, 46, 13157–13170. [Google Scholar] [CrossRef]
- Fang, C.Y.; Chang, C.C.; Mou, C.Y.; Chang, H.C. Preparation and Characterization of Ion-Irradiated Nanodiamonds as Photoacoustic Contrast Agents. J. Nanosci. Nanotechnol. 2015, 15, 1037–1044. [Google Scholar] [CrossRef]
- Tzeng, Y.K.; Zhang, J.L.; Lu, H.; Ishiwata, H.; Dahl, J.; Carlson, R.M.; Yan, H.; Schreiner, P.R.; Vuckovic, J.; Shen, Z.X.; et al. Vertical-Substrate MPCVD Epitaxial Nanodiamond Growth. Nano Lett. 2017, 17, 1489–1495. [Google Scholar] [CrossRef]
- Dahl, J.E.; Moldowan, J.M.; Wei, Z.; Lipton, P.A.; Denisevich, P.; Gat, R.; Liu, S.; Schreiner, P.R.; Carlson, R.M. Synthesis of higher diamondoids and implications for their formation in petroleum. Angew. Chem. Int. Ed. Engl. 2010, 49, 9881–9885. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Kruger, A. Hard and soft: Biofunctionalized diamond. Angew. Chem. Int. Ed. Engl. 2006, 45, 6426–6427. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, K. FTIR study of ultradispersed diamond powder synthesized by explosive detonation. Carbon 1995, 33, 1663–1671. [Google Scholar] [CrossRef]
- Krüger, A.; Kataoka, F.; Ozawa, M.; Fujino, T.; Suzuki, Y.; Aleksenskii, A.E.; Vul’, A.Y.; Ōsawa, E. Unusually tight aggregation in detonation nanodiamond: Identification and disintegration. Carbon 2005, 43, 1722–1730. [Google Scholar] [CrossRef]
- Krüger, A.; Liang, Y.; Jarre, G.; Stegk, J. Surface functionalisation of detonation diamond suitable for biological applications. J. Mater. Chem. 2006, 16, 2322–2328. [Google Scholar] [CrossRef]
- Hens, S.C.; Cunningham, G.; Tyler, T.; Moseenkov, S.; Kuznetsov, V.; Shenderova, O. Nanodiamond bioconjugate probes and their collection by electrophoresis. Diam. Relat. Mater. 2008, 17, 1858–1866. [Google Scholar] [CrossRef]
- Martín, R.; Heydorn, P.C.; Alvaro, M.; Garcia, H. General Strategy for High-Density Covalent Functionalization of Diamond Nanoparticles Using Fenton Chemistry. Chem. Mater. 2009, 21, 4505–4514. [Google Scholar] [CrossRef]
- Girard, H.A.; Petit, T.; Perruchas, S.; Gacoin, T.; Gesset, C.; Arnault, J.C.; Bergonzo, P. Surface properties of hydrogenated nanodiamonds: A chemical investigation. Phys. Chem. Chem. Phys. 2011, 13, 11517–11523. [Google Scholar] [CrossRef]
- Ando, T.; Ishii, M.; Kamo, M.; Sato, Y. Thermal hydrogenation of diamond surfaces studied by diffuse reflectance Fourier-transform infrared, temperature-programmed desorption and laser Raman spectroscopy. J. Chem. Soc. Faraday Trans. 1993, 89, 1783–1789. [Google Scholar] [CrossRef]
- Arnault, J.C.; Girard, H.A. Hydrogenated nanodiamonds: Synthesis and surface properties. Curr. Opin. Solid State Mater. Sci. 2017, 21, 10–16. [Google Scholar] [CrossRef]
- Sotowa, K.-I.; Amamoto, T.; Sobana, A.; Kusakabe, K.; Imato, T. Effect of treatment temperature on the amination of chlorinated diamond. Diam Relat Mater 2004, 13, 145–150. [Google Scholar] [CrossRef]
- Wolcott, A.; Schiros, T.; Trusheim, M.E.; Chen, E.H.; Nordlund, D.; Diaz, R.E.; Gaathon, O.; Englund, D.; Owen, J.S. Surface Structure of Aerobically Oxidized Diamond Nanocrystals. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 26695–26702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradac, C.; Rastogi, I.D.; Cordina, N.M.; Garcia-Bennett, A.; Brown, L.J. Influence of surface composition on the colloidal stability of ultra-small detonation nanodiamonds in biological media. Diam. Relat. Mater. 2018, 83, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent Progress on Silica Coating of Nanoparticles and Related Nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, C.; Feng, L.; Ji, Y.; Tao, L.; Huang, Q.; Li, S.; Wei, Y. PEGylation and polyPEGylation of nanodiamond. Polymer 2012, 53, 3178–3184. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, M.; Wang, K.; Huang, H.; Wan, Q.; Tao, L.; Fu, L.; Zhang, X.; Wei, Y. Direct surface PEGylation of nanodiamond via RAFT polymerization. Appl. Surf. Sci. 2015, 357, 2147–2153. [Google Scholar] [CrossRef]
- Neburkova, J.; Vavra, J.; Cigler, P. Coating nanodiamonds with biocompatible shells for applications in biology and medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 43–53. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Chen, M.; Lam, R.; Xu, X.; Osawa, E.; Ho, D. Polymer-Functionalized Nanodiamond Platforms as Vehicles for Gene Delivery. ACS Nano 2009, 3, 2609–2616. [Google Scholar] [CrossRef]
- Alhaddad, A.; Adam, M.-P.; Botsoa, J.; Dantelle, G.; Perruchas, S.; Gacoin, T.; Mansuy, C.; Lavielle, S.; Malvy, C.; Treussart, F.; et al. Nanodiamond as a Vector for siRNA Delivery to Ewing Sarcoma Cells. Small 2011, 7, 3087–3095. [Google Scholar] [CrossRef] [Green Version]
- Torelli, M.D.; Rickard, A.G.; Backer, M.V.; Filonov, D.S.; Nunn, N.A.; Kinev, A.V.; Backer, J.M.; Palmer, G.M.; Shenderova, O.A. Targeting Fluorescent Nanodiamonds to Vascular Endothelial Growth Factor Receptors in Tumor. Bioconjug. Chem. 2019, 30, 604–613. [Google Scholar] [CrossRef]
- Ali, M.S.; Metwally, A.A.; Fahmy, R.H.; Osman, R. Chitosan-coated nanodiamonds: Mucoadhesive platform for intravesical delivery of doxorubicin. Carbohydr. Polym. 2020, 245, 116528. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins: Cell-agglutinating and sugar-specific proteins. Science 1972, 177, 949–959. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [Green Version]
- Visnjar, T.; Romih, R.; Zupancic, D. Lectins as possible tools for improved urinary bladder cancer management. Glycobiology 2019, 29, 355–365. [Google Scholar] [CrossRef]
- Zupancic, D.; Kreft, M.E.; Romih, R. Selective binding of lectins to normal and neoplastic urothelium in rat and mouse bladder carcinogenesis models. Protoplasma 2014, 251, 49–59. [Google Scholar] [CrossRef]
- Neutsch, L.; Plattner, V.E.; Polster-Wildhofen, S.; Zidar, A.; Chott, A.; Borchard, G.; Zechner, O.; Gabor, F.; Wirth, M. Lectin mediated biorecognition as a novel strategy for targeted delivery to bladder cancer. J. Urol. 2011, 186, 1481–1488. [Google Scholar] [CrossRef]
- Plattner, V.E.; Wagner, M.; Ratzinger, G.; Gabor, F.; Wirth, M. Targeted drug delivery: Binding and uptake of plant lectins using human 5637 bladder cancer cells. Eur. J. Pharm. Biopharm. 2008, 70, 572–576. [Google Scholar] [CrossRef]
- Terada, D.; Genjo, T.; Segawa, T.F.; Igarashi, R.; Shirakawa, M. Nanodiamonds for bioapplications-specific targeting strategies. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129354. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [Green Version]
- Global Cancer Observatory (International Agency for Research on Cancer WHO). Cancer Fact Sheets (Bladder). 2020. Available online: https://gco.iarc.fr/ (accessed on 7 April 2022).
- Magers, M.J.; Lopez-Beltran, A.; Montironi, R.; Williamson, S.R.; Kaimakliotis, H.Z.; Cheng, L. Staging of bladder cancer. Histopathology 2019, 74, 112–134. [Google Scholar] [CrossRef] [Green Version]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmstrom, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Ritch, C.R.; Velasquez, M.C.; Kwon, D.; Becerra, M.F.; Soodana-Prakash, N.; Atluri, V.S.; Almengo, K.; Alameddine, M.; Kineish, O.; Kava, B.R.; et al. Use and Validation of the AUA/SUO Risk Grouping for Nonmuscle Invasive Bladder Cancer in a Contemporary Cohort. J. Urol. 2020, 203, 505–510. [Google Scholar] [CrossRef]
- Lisik, K.; Krokosz, A. Application of Carbon Nanoparticles in Oncology and Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8341. [Google Scholar] [CrossRef]
- Ji, Z.; Lin, G.; Lu, Q.; Meng, L.; Shen, X.; Dong, L.; Fu, C.; Zhang, X. Targeted therapy of SMMC-7721 liver cancer in vitro and in vivo with carbon nanotubes based drug delivery system. J. Colloid Interface Sci. 2012, 365, 143–149. [Google Scholar] [CrossRef]
- Xu, S.; Cui, F.; Huang, D.; Zhang, D.; Zhu, A.; Sun, X.; Cao, Y.; Ding, S.; Wang, Y.; Gao, E.; et al. PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. Int. J. Nanomed. 2019, 14, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Neutsch, L.; Eggenreich, B.; Herwig, E.; Marchetti-Deschmann, M.; Allmaier, G.; Gabor, F.; Wirth, M. Biomimetic delivery strategies at the urothelium: Targeted cytoinvasion in bladder cancer cells via lectin bioconjugates. Pharm. Res. 2014, 31, 819–832. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.K.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, 216rv214. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Patel, S.G.; Cohen, A.; Weiner, A.B.; Steinberg, G.D. Intravesical therapy for bladder cancer. Expert Opin. Pharm. 2015, 16, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.W. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit. Rev. Ther. Drug Carr. Syst. 1992, 9, 135–187. [Google Scholar]
- Setyawati, M.I.; Mochalin, V.N.; Leong, D.T. Tuning Endothelial Permeability with Functionalized Nanodiamonds. ACS Nano 2016, 10, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Lojk, J.; Bregar, V.B.; Strojan, K.; Hudoklin, S.; Veranic, P.; Pavlin, M.; Kreft, M.E. Increased endocytosis of magnetic nanoparticles into cancerous urothelial cells versus normal urothelial cells. Histochem. Cell Biol. 2018, 149, 45–59. [Google Scholar] [CrossRef]
- Hussein, N.A.; Malla, S.; Pasternak, M.A.; Terrero, D.; Brown, N.G.; Ashby, C.R.; Assaraf, Y.G.; Chen, Z.-S.; Tiwari, A.K. The role of endolysosomal trafficking in anticancer drug resistance. Drug Resist. Updates 2021, 57, 100769. [Google Scholar] [CrossRef]
- Prabhakar, N.; Khan, M.H.; Peurla, M.; Chang, H.-C.; Hänninen, P.E.; Rosenholm, J.M. Intracellular Trafficking of Fluorescent Nanodiamonds and Regulation of Their Cellular Toxicity. ACS Omega 2017, 2, 2689–2693. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.W.; Waalkes, S.; Serth, J.; Hennenlotter, J.; Tezval, H.; Stenzl, A.; Kuczyk, M.A.; Merseburger, A.S. Decreased galectin-8 is a strong marker for recurrence in urothelial carcinoma of the bladder. Urol. Int. 2011, 87, 143–150. [Google Scholar] [CrossRef]
- Przybylo, M.; Hoja-Lukowicz, D.; Litynska, A.; Laidler, P. Different glycosylation of cadherins from human bladder non-malignant and cancer cell lines. Cancer Cell Int. 2002, 2, 6. [Google Scholar] [CrossRef]
- Przybylo, M.; Litynska, A.; Pochec, E. Different adhesion and migration properties of human HCV29 non-malignant urothelial and T24 bladder cancer cells: Role of glycosylation. Biochimie 2005, 87, 133–142. [Google Scholar] [CrossRef]
- Apfelthaler, C.; Gassenbauer, P.; Weisse, S.; Gabor, F.; Wirth, M. A lectin mediated delivery system for the intravesical treatment of bladder diseases using poly-(L)-glutamic acid as polymeric backbone. Eur. J. Pharm. Sci. 2018, 111, 376–382. [Google Scholar] [CrossRef]
- Neutsch, L.; Eggenreich, B.; Herwig, E.; Marchetti-Deschmann, M.; Allmaier, G.; Gabor, F.; Wirth, M. Lectin bioconjugates trigger urothelial cytoinvasion—A glycotargeted approach for improved intravesical drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 367–375. [Google Scholar] [CrossRef]
- Neutsch, L.; Wirth, E.M.; Spijker, S.; Pichl, C.; Kählig, H.; Gabor, F.; Wirth, M. Synergistic targeting/prodrug strategies for intravesical drug delivery–lectin-modified PLGA microparticles enhance cytotoxicity of stearoyl gemcitabine by contact-dependent transfer. J. Control Release 2013, 169, 62–72. [Google Scholar] [CrossRef]
- Tratnjek, L.; Jeruc, J.; Romih, R.; Zupančič, D. Vitamin A and Retinoids in Bladder Cancer Chemoprevention and Treatment: A Narrative Review of Current Evidence, Challenges and Future Prospects. Int. J. Mol. Sci. 2021, 22, 3510. [Google Scholar] [CrossRef]
- Chow, E.K.; Zhang, X.Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci. Transl. Med. 2011, 3, 73ra21. [Google Scholar] [CrossRef]
- Man, H.B.; Kim, H.; Kim, H.-J.; Robinson, E.; Liu, W.K.; Chow, E.K.-H.; Ho, D. Synthesis of nanodiamond–daunorubicin conjugates to overcome multidrug chemoresistance in leukemia. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G. Vitamin A and its Derivatives-Retinoic Acid and Retinoid Pharmacology. Am. J. Biomed. Sci. Res. 2019, 3, 162–177. [Google Scholar] [CrossRef]
- Shimkunas, R.A.; Robinson, E.; Lam, R.; Lu, S.; Xu, X.; Zhang, X.Q.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-insulin complexes as pH-dependent protein delivery vehicles. Biomaterials 2009, 30, 5720–5728. [Google Scholar] [CrossRef]
- Xi, G.; Robinson, E.; Mania-Farnell, B.; Vanin, E.F.; Shim, K.W.; Takao, T.; Allender, E.V.; Mayanil, C.S.; Soares, M.B.; Ho, D.; et al. Convection-enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatment. Nanomedicine 2014, 10, 381–391. [Google Scholar] [CrossRef]
- Li, Y.; Tong, Y.; Cao, R.; Tian, Z.; Yang, B.; Yang, P. In vivo enhancement of anticancer therapy using bare or chemotherapeutic drug-bearing nanodiamond particles. Int. J. Nanomed. 2014, 9, 1065–1082. [Google Scholar] [CrossRef] [Green Version]
- Locharoenrat, K. Efficacy of nanodiamond-doxorubicin complexes on human breast adenocarcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4053–4058. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Pierstorff, E.; Osawa, E.; Ho, D. Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 2007, 7, 3305–3314. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Lin, Y.C.; Cheng, C.L. A review of recent advances in nanodiamond-mediated drug delivery in cancer. Expert Opin. Drug Deliv. 2021, 18, 369–382. [Google Scholar] [CrossRef]
- Li, T.F.; Li, K.; Zhang, Q.; Wang, C.; Yue, Y.; Chen, Z.; Yuan, S.J.; Liu, X.; Wen, Y.; Han, M.; et al. Dendritic cell-mediated delivery of doxorubicin-polyglycerol-nanodiamond composites elicits enhanced anti-cancer immune response in glioblastoma. Biomaterials 2018, 181, 35–52. [Google Scholar] [CrossRef]
- Alawdi, S.H.; El-Denshary, E.S.; Safar, M.M.; Eidi, H.; David, M.O.; Abdel-Wahhab, M.A. Neuroprotective Effect of Nanodiamond in Alzheimer’s Disease Rat Model: A Pivotal Role for Modulating NF-κB and STAT3 Signaling. Mol. Neurobiol. 2017, 54, 1906–1918. [Google Scholar] [CrossRef]
- Dielubanza, E.J.; Schaeffer, A.J. Urinary tract infections in women. Med. Clin. N. Am. 2011, 95, 27–41. [Google Scholar] [CrossRef]
- Bower, J.M.; Eto, D.S.; Mulvey, M.A. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic 2005, 6, 18–31. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, G.; Chan, S.Y.; Shapiro, E.; Kong, X.P.; Wu, X.R.; Sun, T.T.; Costello, C.E. Distinct glycan structures of uroplakins Ia and Ib: Structural basis for the selective binding of FimH adhesin to uroplakin Ia. J. Biol. Chem. 2006, 281, 14644–14653. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J. Cell Sci. 2001, 114, 4095–4103. [Google Scholar] [CrossRef]
- Wu, X.R.; Sun, T.T.; Medina, J.J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: Relation to urinary tract infections. Proc. Natl. Acad. Sci. USA 1996, 93, 9630–9635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemm, P.; Hjerrild, L.; Gjermansen, M.; Schembri, M.A. Structure-function analysis of the self-recognizing Antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 2004, 51, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eto, D.S.; Sundsbak, J.L.; Mulvey, M.A. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol. 2006, 8, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, A.E.; Norton, J.P.; Spivak, A.M.; Mulvey, M.A. Urinary tract infections: Current and emerging management strategies. Clin. Infect. Dis. 2013, 57, 719–724. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Lindhorst, T.K. The Bacterial Lectin FimH, a Target for Drug Discovery—Carbohydrate Inhibitors of Type 1 Fimbriae-Mediated Bacterial Adhesion. Eur. J. Org. Chem. 2011, 2011, 3583–3609. [Google Scholar] [CrossRef]
- Barras, A.; Martin, F.A.; Bande, O.; Baumann, J.S.; Ghigo, J.M.; Boukherroub, R.; Beloin, C.; Siriwardena, A.; Szunerits, S. Glycan-functionalized diamond nanoparticles as potent E. coli anti-adhesives. Nanoscale 2013, 5, 2307–2316. [Google Scholar] [CrossRef]
- Iyer, J.K.; Dickey, A.; Rouhani, P.; Kaul, A.; Govindaraju, N.; Singh, R.N.; Kaul, R. Nanodiamonds facilitate killing of intracellular uropathogenic E. coli in an in vitro model of urinary tract infection pathogenesis. PLoS ONE 2018, 13, e0191020. [Google Scholar] [CrossRef] [Green Version]
- Min, G.; Zhou, G.; Schapira, M.; Sun, T.T.; Kong, X.P. Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J. Cell Sci. 2003, 116, 4087–4094. [Google Scholar] [CrossRef] [Green Version]
- Zaak, D.; Hungerhuber, E.; Schneede, P.; Stepp, H.; Frimberger, D.; Corvin, S.; Schmeller, N.; Kriegmair, M.; Hofstetter, A.; Knuechel, R. Role of 5-aminolevulinic acid in the detection of urothelial premalignant lesions. Cancer 2002, 95, 1234–1238. [Google Scholar] [CrossRef]
- Mowatt, G.; N’Dow, J.; Vale, L.; Nabi, G.; Boachie, C.; Cook, J.A.; Fraser, C.; Griffiths, T.R. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: Systematic review and meta-analysis. Int. J. Technol. Assess. Health Care 2011, 27, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Draga, R.O.; Grimbergen, M.C.; Kok, E.T.; Jonges, T.N.; Bosch, J.L. Predictors of false positives in 5-aminolevulinic acid-induced photodynamic diagnosis of bladder carcinoma: Identification of patient groups that may benefit most from highly specific optical diagnostics. Urology 2009, 74, 851–856. [Google Scholar] [CrossRef]
- Khanal, M.; Larsonneur, F.; Raks, V.; Barras, A.; Baumann, J.-S.; Martin, F.A.; Boukherroub, R.; Ghigo, J.-M.; Ortiz Mellet, C.; Zaitsev, V.; et al. Inhibition of type 1 fimbriae-mediated Escherichia coli adhesion and biofilm formation by trimeric cluster thiomannosides conjugated to diamond nanoparticles. Nanoscale 2015, 7, 2325–2335. [Google Scholar] [CrossRef] [Green Version]
- Lien, Z.Y.; Hsu, T.C.; Liu, K.K.; Liao, W.S.; Hwang, K.C.; Chao, J.I. Cancer cell labeling and tracking using fluorescent and magnetic nanodiamond. Biomaterials 2012, 33, 6172–6185. [Google Scholar] [CrossRef]
- Ni, J.S.; Li, Y.; Yue, W.; Liu, B.; Li, K. Nanoparticle-based Cell Trackers for Biomedical Applications. Theranostics 2020, 10, 1923–1947. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Li, C.; Huang, D.; Wang, Q.; Wang, Q. Recent Advances in Tracking the Transplanted Stem Cells Using Near-Infrared Fluorescent Nanoprobes: Turning from the First to the Second Near-Infrared Window. Adv. Healthc. Mater. 2018, 7, e1800497. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Q.; Tao, J.; Jin, Z.; Pan, Y.; Yu, C.; Yu, Z. Near infrared quantum dots in biomedical applications: Current status and future perspective. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1483. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupančič, D.; Veranič, P. Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis. Int. J. Mol. Sci. 2022, 23, 8183. https://doi.org/10.3390/ijms23158183
Zupančič D, Veranič P. Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis. International Journal of Molecular Sciences. 2022; 23(15):8183. https://doi.org/10.3390/ijms23158183
Chicago/Turabian StyleZupančič, Daša, and Peter Veranič. 2022. "Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis" International Journal of Molecular Sciences 23, no. 15: 8183. https://doi.org/10.3390/ijms23158183
APA StyleZupančič, D., & Veranič, P. (2022). Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis. International Journal of Molecular Sciences, 23(15), 8183. https://doi.org/10.3390/ijms23158183






