O-Glycosylation Changes in Serum Immunoglobulin G Are Associated with Inflammation Development in Advanced Endometriosis
Abstract
:1. Introduction
2. Results
2.1. ROC Curve Analysis
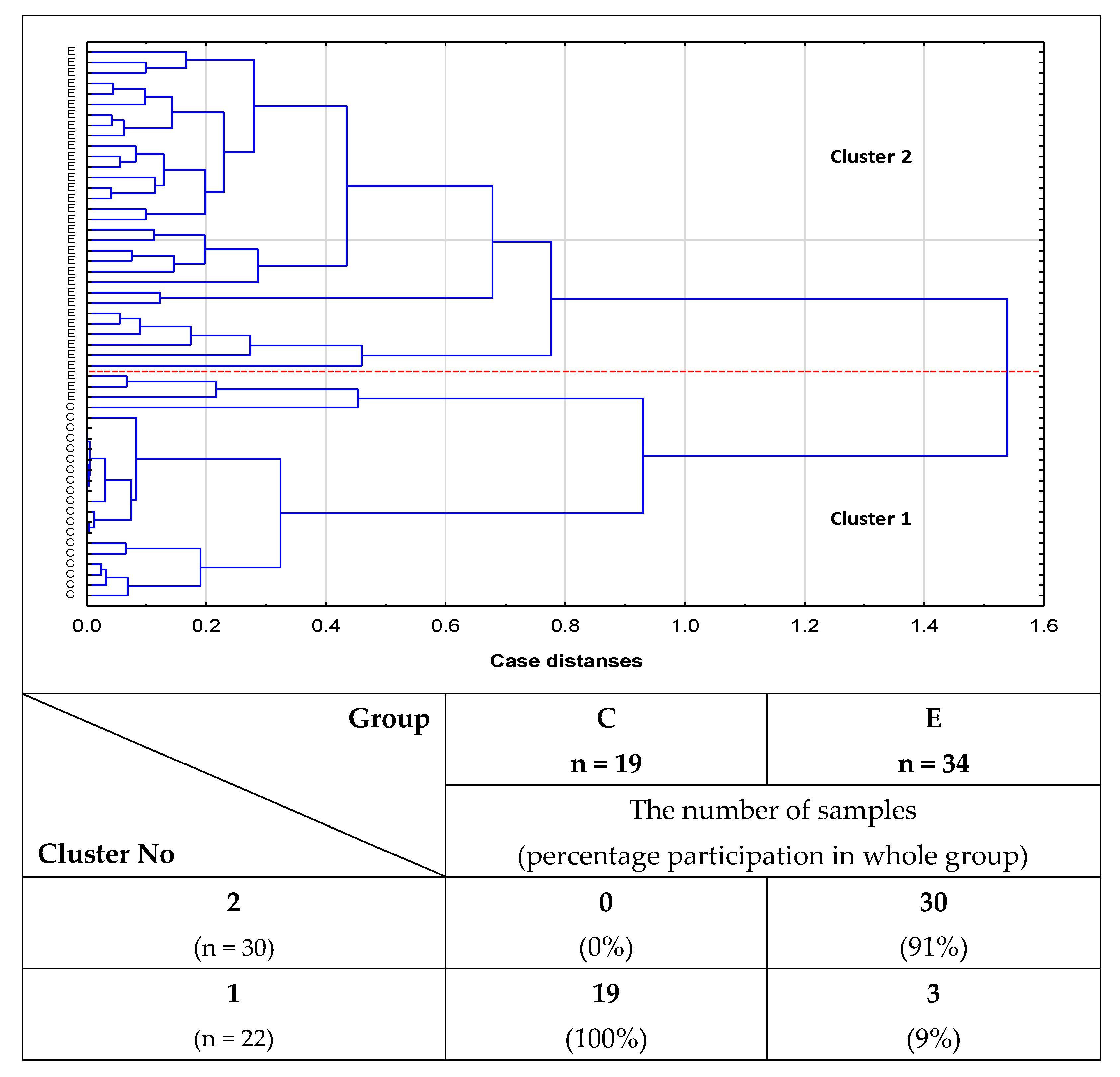
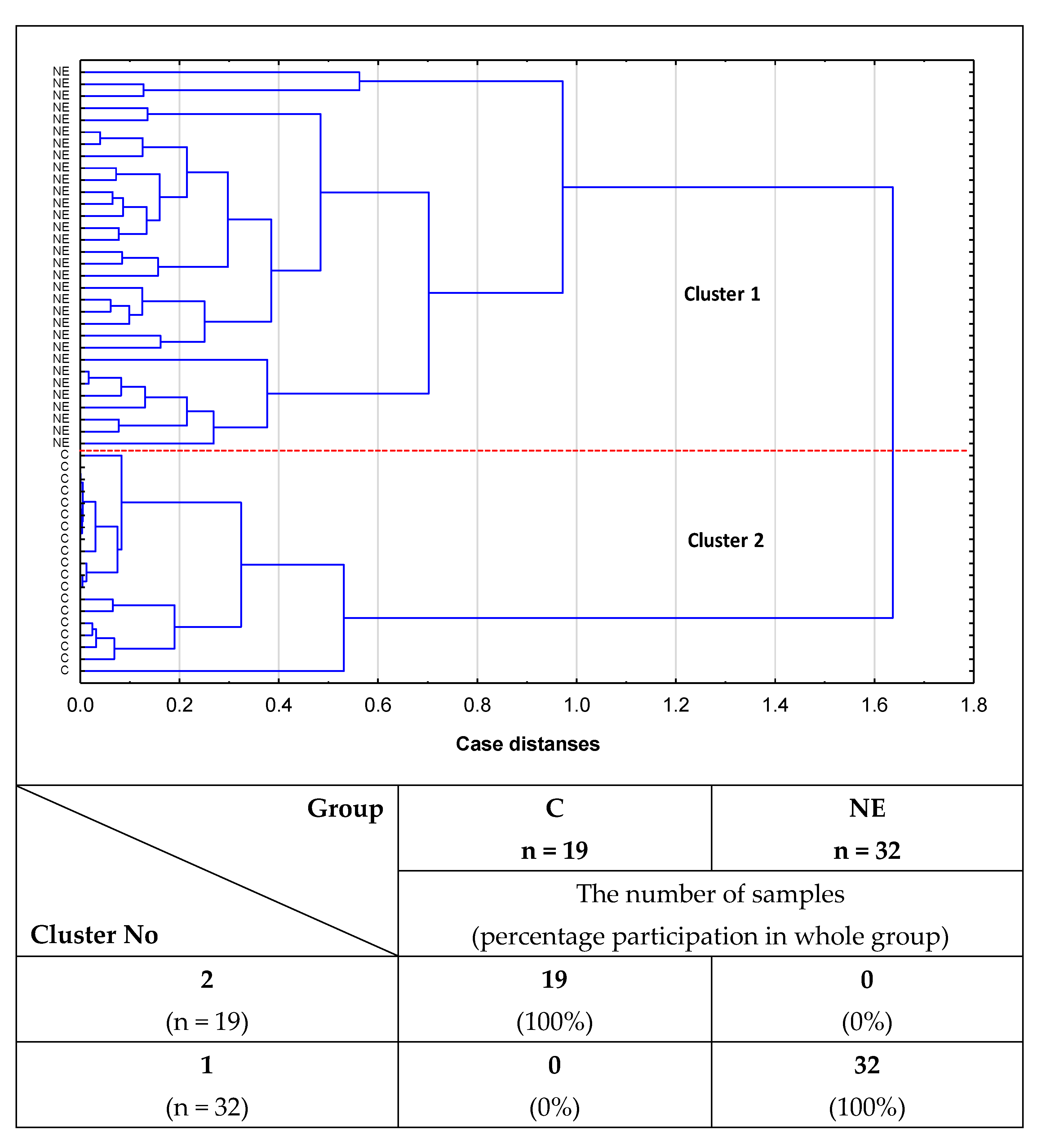
2.2. Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. IgG Isolation
4.3. Lectin-ELISA
4.4. Statistical Analysis
5. Conclusions
5.1. Strengths of the Study
- We are the first to show the presence of O-glycans in blood serum IgG in women with advanced endometriosis.
- The relative reactivities of isolated serum IgG O-glycans with specific lectins were significantly higher in women with advanced endometriosis and the group of women with gynecological diseases other than endometriosis in comparison to the group of healthy women.
- The relative reactivity of i-IgG O-glycans with Jacalin significantly differentiates the advanced endometriosis patients from women with other gynecological diseases, which was most probably caused by increased expression of core 3 type O-glycans in the case of non-endometriosis patients.
- The relative reactivities of blood serum IgG glycans with PHA-L in advanced endometriosis and non-endometriosis patients were significantly higher than in the control group of healthy women, showing increased expression of multi-branched three and tetra-antennary N-glycans in the E and NE groups.
- Cluster analysis confirmed the usefulness of the determinations of relative reactivities of i-IgG glycans with MPL, VVL, Jacalin, and PHA-L for distinguishing healthy women from women with advanced endometriosis and women without endometriosis but suffering from other gynecological diseases.
5.2. Limitations of the Study
- Significant differences in relative reactivities of IgG glycans with the lectins used were observed only for IgG isolates, which was most probably caused by better bioavailability of oligosaccharides for lectins when IgG was isolated from biological material. However, given that the process of isolation and purification of the protein is long and laborious, the differences observed between the studied groups in the relative reactivities of IgG glycans with lectins used should be treated as an additional cognitive aspect that could be difficult to apply in routine diagnostics.
- The lack of women suffering from early stages of endometriosis made it impossible to check the utility of lectin-ELISA tests used in the present study for diagnostics of the early stages of disease development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, N.; Subramanian, A. Endometriosis-Morphology, clinical presentations and molecular pathology. J. Lab. Physicians 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Herington, J.L.; Bruner-Tran, K.L.; A Lucas, J.; Osteen, K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011, 7, 611–626. [Google Scholar] [CrossRef] [Green Version]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef]
- Lefranc, M.P.; Lefranc, G. The Immunoglobulin FactsBook; Academic Press: London UK, 2001. [Google Scholar]
- Damelang, T.; Rogerson, S.J.; Kent, S.; Chung, A.W. Role of IgG3 in Infectious Diseases. Trends Immunol. 2019, 40, 197–211. [Google Scholar] [CrossRef]
- Dwek, R.A. Biological Importance of Glycosylation. In Molecular Recognition and Inclusion; Springer: Dordrecht, The Netherlands, 1998; pp. 1–6. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Dennis, J.W.; Lau, K.S.; Demetriou, M.; Nabi, I.R. Adaptive Regulation at the Cell Surface by N-Glycosylation. Traffic 2009, 10, 1569–1578. [Google Scholar] [CrossRef]
- Iwai, T.; Kudo, T.; Kawamoto, R.; Kubota, T.; Togayachi, A.; Hiruma, T.; Okada, T.; Kawamoto, T.; Morozumi, K.; Narimatsu, H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4572–4577. [Google Scholar] [CrossRef] [Green Version]
- Skropeta, D. The effect of individual N-glycans on enzyme activity. Bioorganic Med. Chem. 2009, 17, 2645–2653. [Google Scholar] [CrossRef]
- Mimura, Y.; Church, S.; Ghirlando, R.; Ashton, P.R.; Dong, S.; Goodall, M.; Lund, J.; Jefferis, R. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: Properties of a series of truncated glycoforms. Mol. Immunol. 2000, 37, 697–706. [Google Scholar] [CrossRef]
- Lee, C.-H.; Romain, G.; Yan, W.; Watanabe, M.; Charab, W.; Todorova, B.; Lee, J.; Triplett, K.; Donkor, M.; I Lungu, O.; et al. IgG Fc domains that bind C1q but not effector Fcγ receptors delineate the importance of complement-mediated effector functions. Nat. Immunol. 2017, 18, 889–898. [Google Scholar] [CrossRef]
- Niwa, R.; Natsume, A.; Uehara, A.; Wakitani, M.; Iida, S.; Uchida, K.; Satoh, M.; Shitara, K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods 2005, 306, 151–160. [Google Scholar] [CrossRef]
- Chung, A.W.; Crispin, M.; Pritchard, L.; Robinson, H.; Gorny, M.; Yu, X.; Bailey-Kellogg, C.; Ackerman, M.E.; Scanlan, C.; Zolla-Pazner, S.; et al. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS 2014, 28, 2523–2530. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-W.; Tsai, M.-H.; Li, S.-T.; Tsai, T.-I.; Chu, K.-C.; Liu, Y.-C.; Lai, M.-Y.; Wu, C.-Y.; Tseng, Y.-C.; Shivatare, S.S.; et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc. Natl. Acad. Sci. USA 2015, 112, 10611–10616. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Uh, H.-W.; Beekman, M.; Koeleman, C.A.M.; Hokke, C.H.; Westendorp, R.G.J.; Wuhrer, M.; Houwing-Duistermaat, J.J.; Slagboom, P.E.; Deelder, A.M. Decreased Levels of Bisecting GlcNAc Glycoforms of IgG Are Associated with Human Longevity. PLoS ONE 2010, 5, e12566. [Google Scholar] [CrossRef] [Green Version]
- Van De Bovenkamp, F.S.; Derksen, N.I.L.; Van Breemen, M.J.; De Taeye, S.W.; Heer, P.O.-D.; Sanders, R.W.; Rispens, T. Variable Domain N-Linked Glycans Acquired During Antigen-Specific Immune Responses Can Contribute to Immunoglobulin G Antibody Stability. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Jefferis, R. Antibody therapeutics. Expert Opin. Biol. Ther. 2007, 7, 1401–1413. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daëron, M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef]
- Brockhausen, I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006, 7, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Brockhausen, I.; Schachter, H.; Stanley, P.; Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R. O-GalNAc Glycans. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; Chapter 9. [Google Scholar]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Plomp, R.; Dekkers, G.; Rombouts, Y.; Visser, R.; Koeleman, C.A.; Kammeijer, G.S.; Jansen, B.C.; Rispens, T.; Hensbergen, P.J.; Vidarsson, G.; et al. Hinge-Region O-Glycosylation of Human Immunoglobulin G3 (IgG3). Mol. Cell. Proteom. 2015, 14, 1373–1384. [Google Scholar] [CrossRef] [Green Version]
- Julenius, K.; Mølgaard, A.; Gupta, R.; Brunak, S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 2004, 15, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Hounsell, E.F.; Davies, M.J.; Renouf, D.V. O-linked protein glycosylation structure and function. Glycoconj. J. 1996, 13, 19–26. [Google Scholar] [CrossRef]
- Van den Steen, P.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Concepts and Principles of O-Linked Glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. [Google Scholar] [CrossRef]
- Inoue, M.; Yamashina, I.; Nakada, H. Glycosylation of the Tandem Repeat Unit of the MUC2 Polypeptide Leading to the Synthesis of the Tn Antigen. Biochem. Biophys. Res. Commun. 1998, 245, 23–27. [Google Scholar] [CrossRef]
- Saldova, R.; Royle, L.; Radcliffe, C.M.; Hamid, U.M.A.; Evans, R.; Arnold, J.N.; E Banks, R.; Hutson, R.; Harvey, D.J.; Antrobus, R.; et al. Ovarian Cancer is Associated with Changes in Glycosylation in Both Acute-Phase Proteins and IgG. Glycobiology 2007, 17, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- E Van De Geijn, F.; Wuhrer, M.; Selman, M.H.; Willemsen, S.P.; A De Man, Y.; Deelder, A.M.; Hazes, J.M.; Dolhain, R.J. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: Results from a large prospective cohort study. Arthritis Res. Ther. 2009, 11, R193. [Google Scholar] [CrossRef] [Green Version]
- Bones, J.; Mittermayr, S.; O’Donoghue, N.; Guttman, A.; Rudd, P.M. Ultra Performance Liquid Chromatographic Profiling of Serum N-Glycans for Fast and Efficient Identification of Cancer Associated Alterations in Glycosylation. Anal. Chem. 2010, 82, 10208–10215. [Google Scholar] [CrossRef]
- Pučić, M.; Knežević, A.; Vidič, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Šupraha-Goreta, S.; Wormald, M.; Redžić, I.; et al. High Throughput Isolation and Glycosylation Analysis of IgG–Variability and Heritability of the IgG Glycome in Three Isolated Human Populations. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Ercan, A.; Barnes, M.G.; Hazen, M.; Tory, H.; Henderson, L.; Dedeoglu, F.; Fuhlbrigge, R.C.; Grom, A.; Holm, I.A.; Kellogg, M.; et al. Multiple juvenile idiopathic arthritis subtypes demonstrate proinflammatory IgG glycosylation. Arthritis Care Res. 2012, 64, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, P.; Wu, D.; Xu, D.; Hou, Y.; Wang, Q.; Li, M.; Li, Y.; Zeng, X.; Zhang, F.; et al. Serum IgG Subclasses in Autoimmune Diseases. Medicine 2015, 94, e387. [Google Scholar] [CrossRef]
- Chung, A.W.; Ho, T.K.; Hanson-Manful, P.; Tritscheller, S.; Raynes, J.M.; Whitcombe, A.L.; Tay, M.L.; McGregor, R.; Lorenz, N.; Oliver, J.R.; et al. Systems immunology reveals a linked IgG3-C4 response in patients with acute rheumatic fever. Immunol. Cell Biol. 2019, 98, 12–21. [Google Scholar] [CrossRef]
- Sołkiewicz, K.; Krotkiewski, H.; Jędryka, M.; Kratz, E.M. Variability of serum IgG sialylation and galactosylation degree in women with advanced endometriosis. Sci. Rep. 2021, 11, 5586. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, F.; Shan, J.; Wang, Y.; Jia, Y.; Guo, Q.; Lou, J.; Zhao, Y. Levels of serum IgG subclasses in patients with liver disease: A retrospective study. Exp. Ther. Med. 2020, 21, 1. [Google Scholar] [CrossRef]
- Sołkiewicz, K.; Krotkiewski, H.; Jędryka, M.; Czekański, A.; Kratz, E.M. The Alterations of Serum IgG Fucosylation as a Potential Additional New Diagnostic Marker in Advanced Endometriosis. J. Inflamm. Res. 2022, 15, 251–266. [Google Scholar] [CrossRef]
- Kratz, E.M.; Kałuża, A.; Zimmer, M.; Ferens-Sieczkowska, M. The Analysis of Sialylation, N-Glycan Branching, and Expression of O-Glycans in Seminal Plasma of Infertile Men. Dis. Markers 2015, 2015, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Żurawska-Płaksej, E.; Kratz, E.M.; Ferens-Sieczkowska, M.; Knapik-Kordecka, M.; Piwowar, A. Changes in glycosylation of human blood plasma chitotriosidase in patients with type 2 diabetes. Glycoconj. J. 2015, 33, 29–39. [Google Scholar] [CrossRef]
- Bossuyt, X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun. Rev. 2009, 8, 543–548. [Google Scholar] [CrossRef]
- Nothnick, W.B. Treating endometriosis as an autoimmune disease. Fertil. Steril. 2001, 76, 223–231. [Google Scholar] [CrossRef]
- Hortin, G.L.; Trimpe, B.L. Lectin affinity chromatography of proteins bearing O-linked oligosaccharides: Application of jacalin-agarose. Anal. Biochem. 1990, 188, 271–277. [Google Scholar] [CrossRef]
- Pedroso, M.M.; Pesquero, N.C.; Thomaz, S.M.; Roque-Barreira, M.C.; Faria, R.C.; Bueno, P.R. Jacalin interaction with human immunoglobulin A1 and bovine immunoglobulin G1: Affinity constant determined by piezoelectric biosensoring. Glycobiology 2011, 22, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, K.; Nakamura, S.; Wang, H.; Iwasaki, H.; Tachibana, K.; Maebara, K.; Cheng, L.; Hirabayashi, J.; Narimatsu, H. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: Quantitative analysis by frontal affinity chromatography. Glycobiology 2005, 16, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.A.; Moya, K.L.; Breen, K.C. The potential role of tau protein O-glycosylation in Alzheimer’s disease. J. Alzheimer’s Dis. 2004, 6, 489–495. [Google Scholar] [CrossRef]
- Akasaka-Manya, K.; Manya, H. The Role of APP O-Glycosylation in Alzheimer’s Disease. Biomolecules 2020, 10, 1569. [Google Scholar] [CrossRef]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Tamauchi, S.; Yoshikawa, N.; Niimi, K.; Shibata, K.; Kikkawa, F. Endometriosis and cancer. Free Radic. Biol. Med. 2018, 133, 186–192. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kokot, I.; Gilowska, I.; Faundez, R.; Kratz, E.M. The possible association of clusterin fucosylation changes with male fertility disorders. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Ey, P.; Prowse, S.; Jenkin, C. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry 1978, 15, 429–436. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Li, W.-P.; Zuber, C.; Roth, J. Use of Phaseolus vulgaris leukoagglutinating lectin in histochemical and blotting techniques: A comparison of digoxigenin- and biotin-labelled lectins. Histochem. Cell Biol. 1993, 100, 347–356. [Google Scholar] [CrossRef]
- Wu, A.M. Polyvalent GalNAcα1→Ser/Thr (Tn) and Galβ1→3GalNAcα1→Ser/Thr (T α) as the most potent recognition factors involved in Maclura pomifera agglutinin–glycan interactions. J. Biomed. Sci. 2005, 12, 135–152. [Google Scholar] [CrossRef]
- Tollefsen, S.; Kornfeld, R. [46] Vicia villosa lectins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 138, pp. 536–544. [Google Scholar] [CrossRef]
- Puri, K.D.; Gopalakrishnan, B.; Surolia, A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4). FEBS Lett. 1992, 312, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Kokot, I.; Piwowar, A.; Jędryka, M.; Sołkiewicz, K.; Kratz, E. Diagnostic Significance of Selected Serum Inflammatory Markers in Women with Advanced Endometriosis. Int. J. Mol. Sci. 2021, 22, 2295. [Google Scholar] [CrossRef]

| Relative Reactivity with Lectins (AU) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | MPL (s) | MPL (i) | VVL (s) | VVL (i) | MPL/VVL Ratio (s) | MPL/VVL Ratio (i) | Jacalin (s) | Jacalin (i) | PHA-L (s) | PHA-L (i) |
| E n = 34 | 0.118 ± 0.045 | 0.342 ± 0.160 | 0.028 ± 0.031 | 0.061 ± 0.051 | 7.361 ± 5.705 | 7.465 ± 4.439 | 0.260 ± 0.076 | 1.025 ± 0.094 | 0.197 ± 0.235 | 0.340 ± 0.148 |
| NE n = 32 | 0.169 ± 0.111 pE = 0.022764 | 0.310 ± 0.115 | 0.047 ± 0.076 | 0.080 ± 0.097 | 6.228 ± 4.742 | 5.885 ± 3.383 | 0.244 ± 0.082 | 1.103 ± 0.138 pE = 0.006401 | 0.263 ± 0.270 | 0.402 ± 0.169 |
| C n = 19 | 0.103 ± 0.042 pNE = 0.005500 | 0.020 ± 0.025 pE = 0.000000 pNE = 0.000000 | 0.029 ± 0.022 | 0.001 ± 0.002 pE = 0.000000 pNE = 0.000000 | 6.227 ± 6.382 | 2.750 ± 5.546 pE = 0.000000 pNE = 0.000317 | 0.246 ± 0.084 | 0.106 ± 0.140 pE = 0.000000 pNE = 0.000000 | 0.180 ± 0.123 | 0.002 ± 0.006 pE = 0.000000 pNE = 0.000000 |
| s-IgG | i-IgG | |
|---|---|---|
| Correlations between IgG Relative Reactivities with Lectins | Spearman Rank Coefficient (r) | Spearman Rank Coefficient (r) |
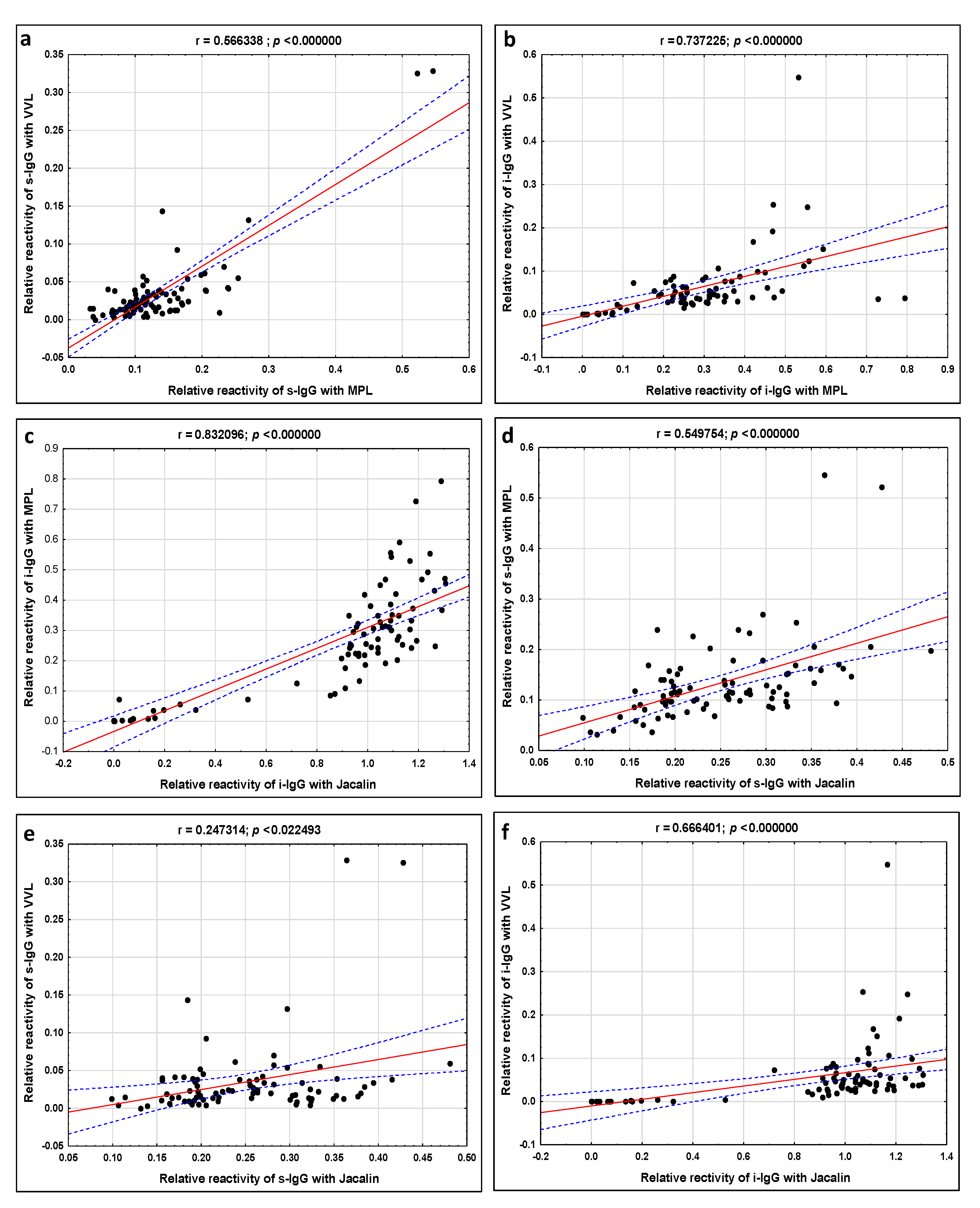
| MPL vs. VVL | 0.566 p = 0.000000 | 0.737 p = 0.000000 |
| MPL vs. Jacalin | 0.549 p = 0.000000 | 0.832 p = 0.000000 |
| VVL vs. Jacalin | 0.247 p = 0.000000 | 0.666 p = 0.000000 |
| Correlations between Relative Reactivity of Lectins with s-IgG vs. i-IgG | Spearman Rank Coefficient (r) |
|---|---|
| MPL (s) vs. MPL (i) | 0.260874 p = 0.015890 |
| VVL (s) vs. VVL (i) | |
| MPL/VVL (s) vs. MPL/VVL (i) | 0.255850 p = 0.018109 |
| Jacalin (s) vs. Jacalin (i) | |
| PHA-L (s) vs. PHA-L (i) | 0.279398 p = 0.009610 |
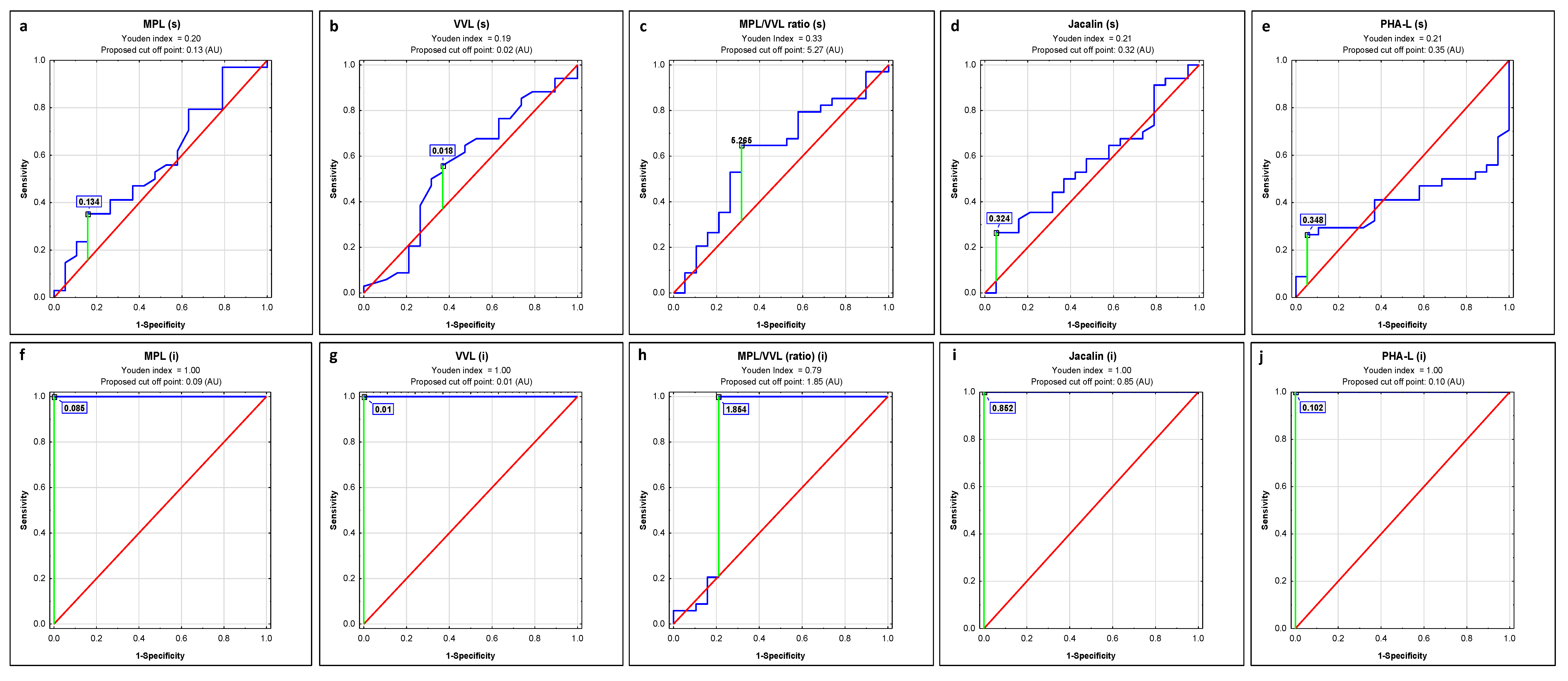
| Lectin | AUC | AUC with 95% Confidence Interval | Cut-Off Point | Sensitivity | Specificity | p |
|---|---|---|---|---|---|---|
| s-IgG | ||||||
| MPL | 0.576 | 0.414–0.738 | 0.134 | 0.353 | 0.842 | 0.3580 |
| VVL | 0.561 | 0.393–0.73 | 0.018 | 0.559 | 0.632 | 0.4771 |
| MPL/VVL | 0.611 | 0.479–0.744 | 5.265 | 0.647 | 0.684 | 0.1001 |
| Jacalin | 0.565 | 0.406–0.724 | 0.324 | 0.265 | 0.947 | 0.4242 |
| PHA-L | 0.406 | 0.254–0.557 | 0.348 | 0.265 | 0.947 | 0.2215 |
| i-IgG | ||||||
| MPL | 1 | 1–1 | 0.085 | 1.000 | 1.000 | 0.0000 |
| VVL | 1 | 1–1 | 0.01 | 1.000 | 1.000 | 0.0000 |
| MPL/VVL | 0.811 | 0.693–0.929 | 1.854 | 1.000 | 0.789 | 0.0000 |
| Jacalin | 1 | 1–1 | 0.852 | 1.000 | 1.000 | 0.0000 |
| PHA-L | 1 | 1–1 | 0.102 | 1.000 | 1.000 | 0.0000 |
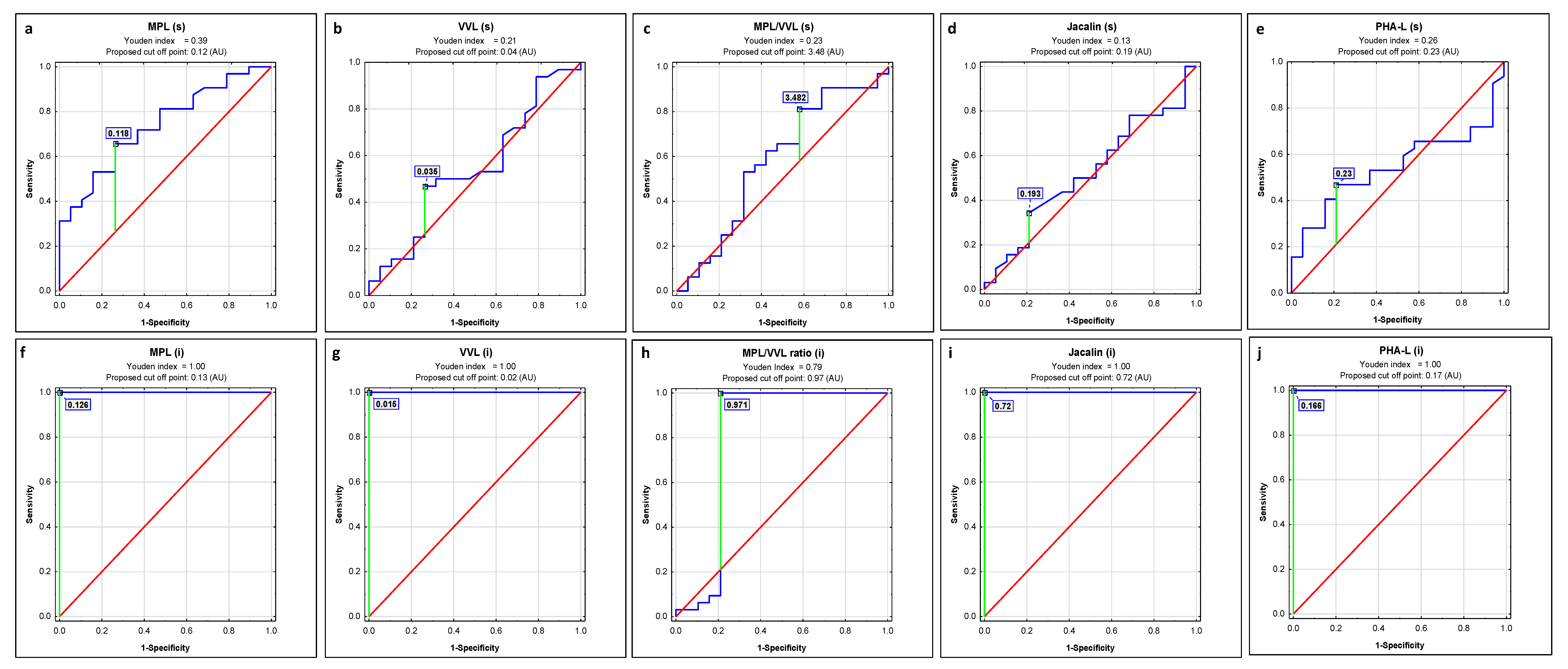
| Lectin | AUC | AUC with 95% Confidence Interval | Cut-Off Point | Sensitivity | Specificity | p |
|---|---|---|---|---|---|---|
| s-IgG | ||||||
| MPL | 0.737 | 0.601–0.873 | 0.118 | 0.656 | 0.737 | 0.0006 |
| VVL | 0.544 | 0.378–0.711 | 0.035 | 0.469 | 0.737 | 0.6011 |
| MPL/VVL | 0.582 | 0.412–0.753 | 3.48 | 0.813 | 0.421 | 0.3450 |
| Jacalin | 0.523 | 0.36–0.686 | 0.193 | 0.344 | 0.789 | 0.7823 |
| PHA-L | 0.546 | 0.388–0.704 | 0.23 | 0.469 | 0.789 | 0.5669 |
| i-IgG | ||||||
| MPL | 1 | 1–1 | 0.126 | 1.000 | 1.000 | 0.0000 |
| VVL | 1 | 1–1 | 0.015 | 1.000 | 1.000 | 0.0000 |
| MPL/VVL | 0.801 | 0.627–0.975 | 0.975 | 1.000 | 0.789 | 0.0007 |
| Jacalin | 1 | 1–1 | 0.72 | 1.000 | 1.000 | 0.0000 |
| PHA-L | 1 | 1–1 | 0.166 | 1.000 | 1.000 | 0.0000 |
| Lectin Source | Specificity for Sugar Structures |
|---|---|
| MPL (Maclura pomifera lectin) | T (Galβ1,3GalNAc) and Tn antigen (single GalNAc) [55] |
| VVL (Vicia villosa lectin) | Tn antigen (single GalNAc) [56,57] |
| Jacalin (Artocarpus integrifolia lectin) | T antigen (Galβ1,3GalNAc), Tn antigen (single GalNAc), sTn antigen (NeuAcα2,6GalNAc) [45] |
| PHA-L (Phaseolus vulgaris lectin) | tri/tetra-antennary N-glycans, binds to β1,6 branches of tri- and tetra-antennary oligosaccharides [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołkiewicz, K.; Kacperczyk, M.; Krotkiewski, H.; Jędryka, M.; Kratz, E.M. O-Glycosylation Changes in Serum Immunoglobulin G Are Associated with Inflammation Development in Advanced Endometriosis. Int. J. Mol. Sci. 2022, 23, 8087. https://doi.org/10.3390/ijms23158087
Sołkiewicz K, Kacperczyk M, Krotkiewski H, Jędryka M, Kratz EM. O-Glycosylation Changes in Serum Immunoglobulin G Are Associated with Inflammation Development in Advanced Endometriosis. International Journal of Molecular Sciences. 2022; 23(15):8087. https://doi.org/10.3390/ijms23158087
Chicago/Turabian StyleSołkiewicz, Katarzyna, Monika Kacperczyk, Hubert Krotkiewski, Marcin Jędryka, and Ewa Maria Kratz. 2022. "O-Glycosylation Changes in Serum Immunoglobulin G Are Associated with Inflammation Development in Advanced Endometriosis" International Journal of Molecular Sciences 23, no. 15: 8087. https://doi.org/10.3390/ijms23158087
APA StyleSołkiewicz, K., Kacperczyk, M., Krotkiewski, H., Jędryka, M., & Kratz, E. M. (2022). O-Glycosylation Changes in Serum Immunoglobulin G Are Associated with Inflammation Development in Advanced Endometriosis. International Journal of Molecular Sciences, 23(15), 8087. https://doi.org/10.3390/ijms23158087







