A Bioinformatics Approach to MicroRNA-Sequencing Analysis Based on Human Saliva Samples of Patients with Endometriosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Saliva Sample Collection
2.3. RNA Sample Extraction, Preparation and Quality Control
3. Bioinformatics
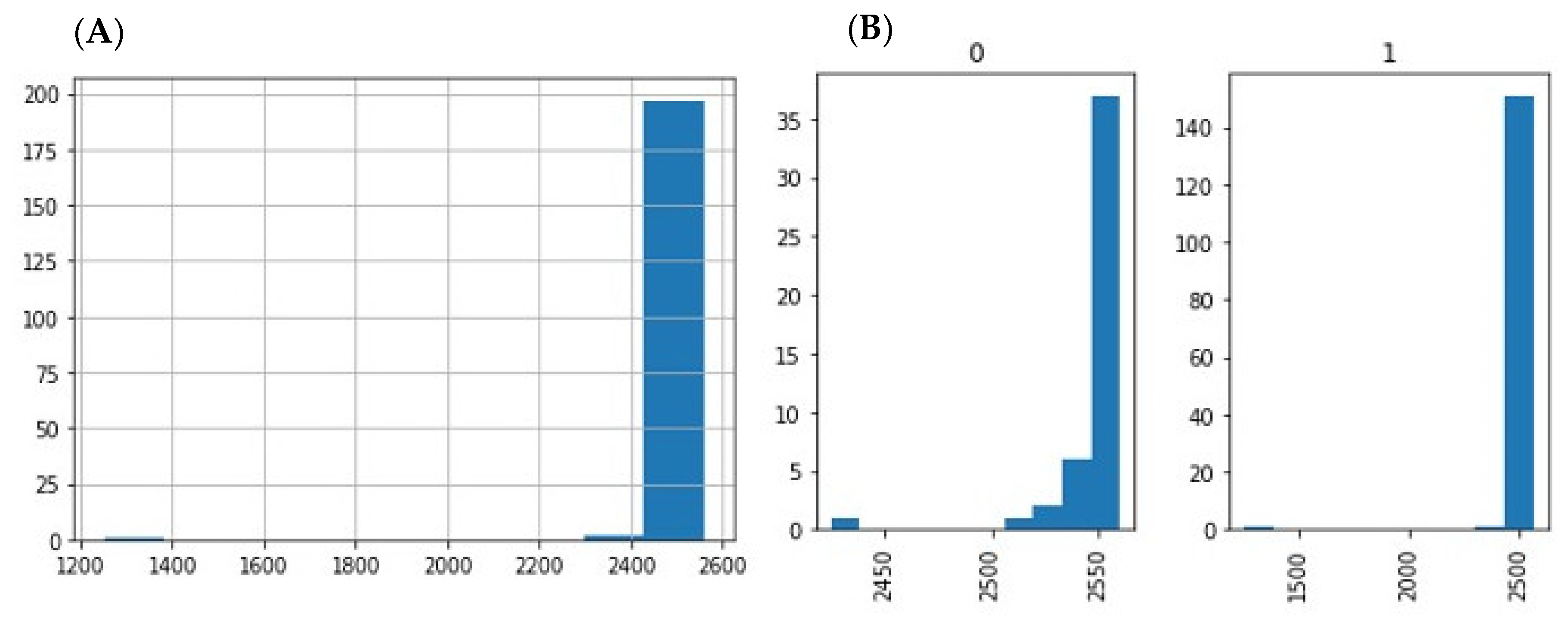
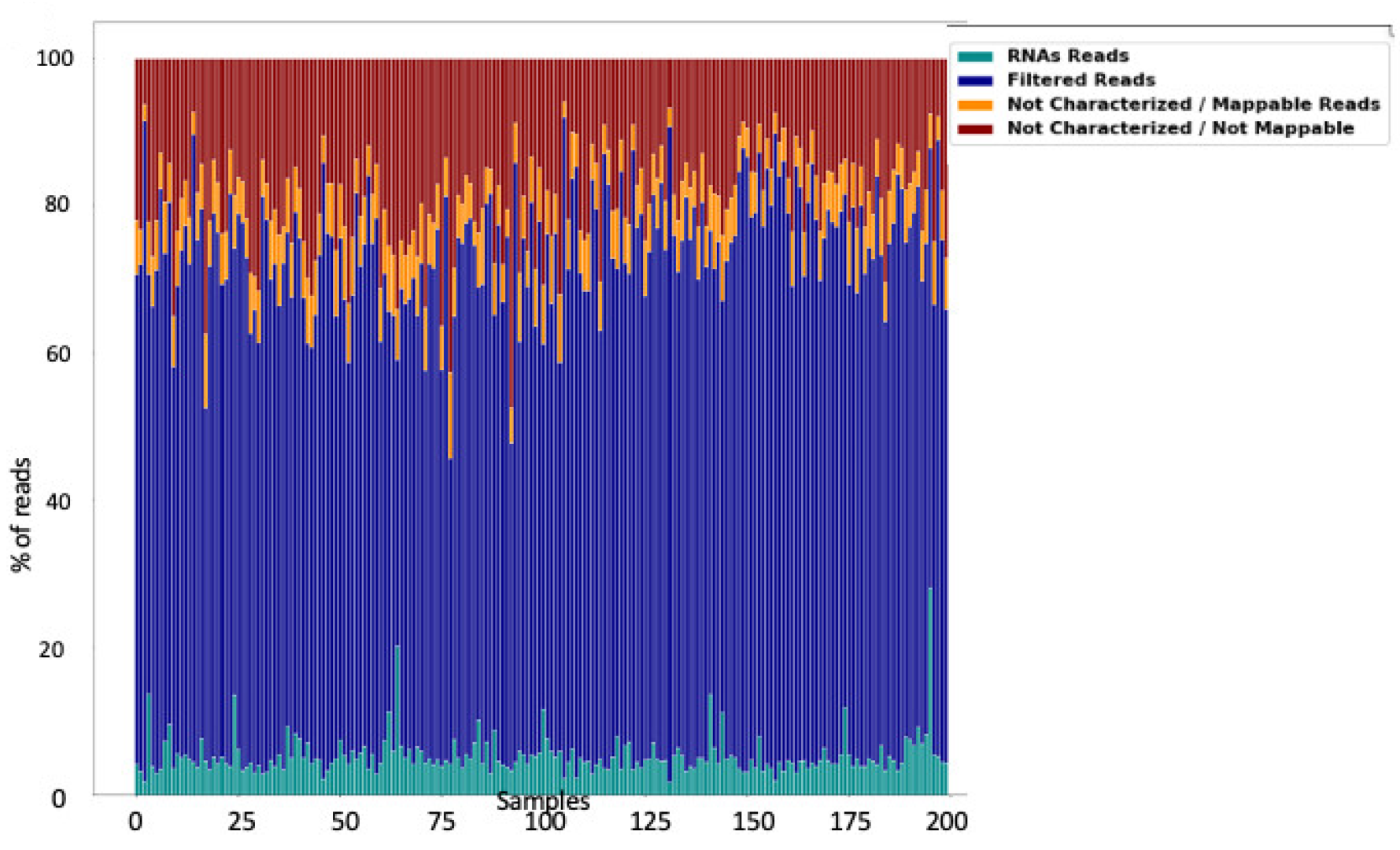
3.1. Raw Data Preprocessing (Raw, Filtered, Aligned Reads) and Quality Control
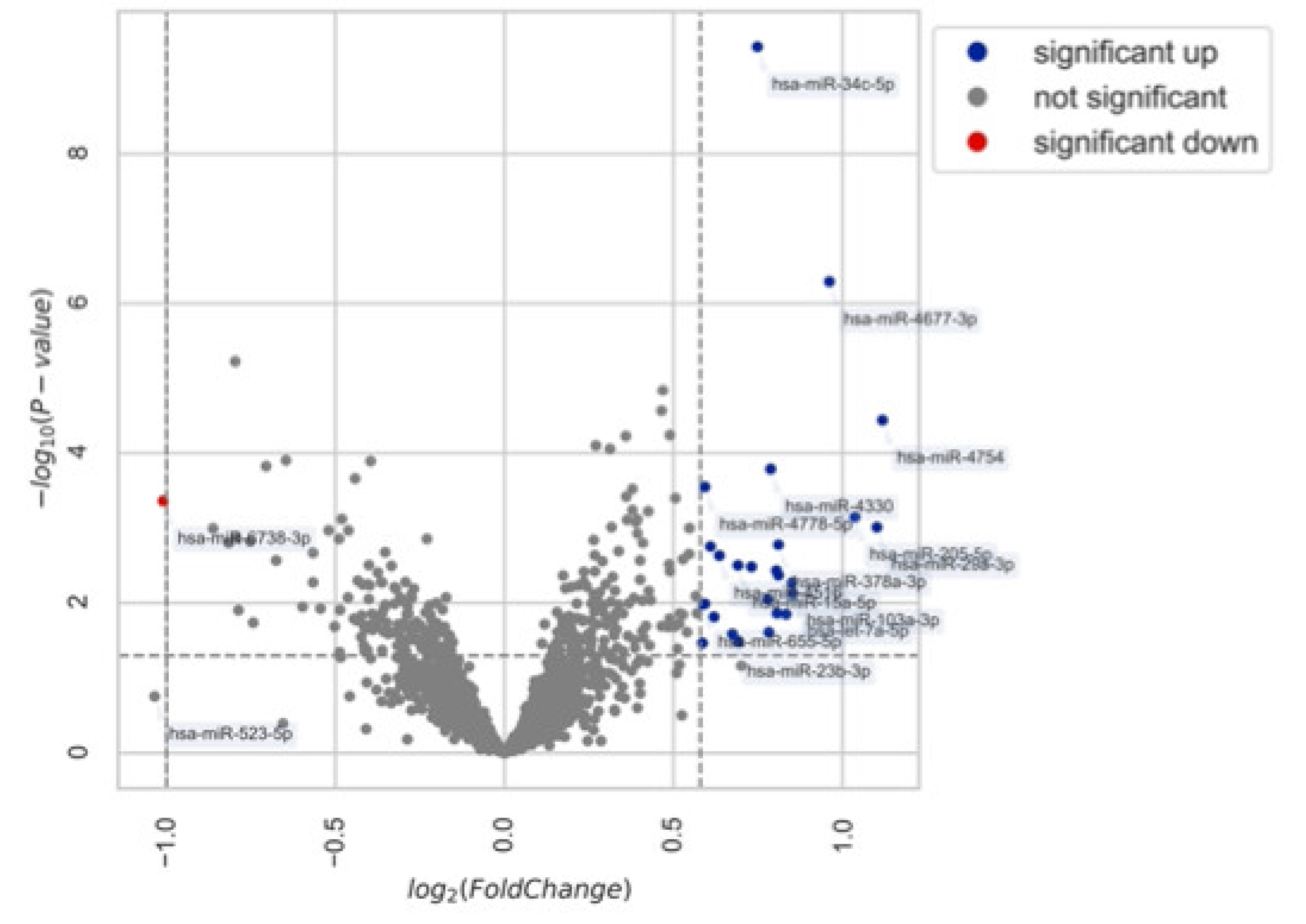
3.2. Differential Expression Analysis of miRNA
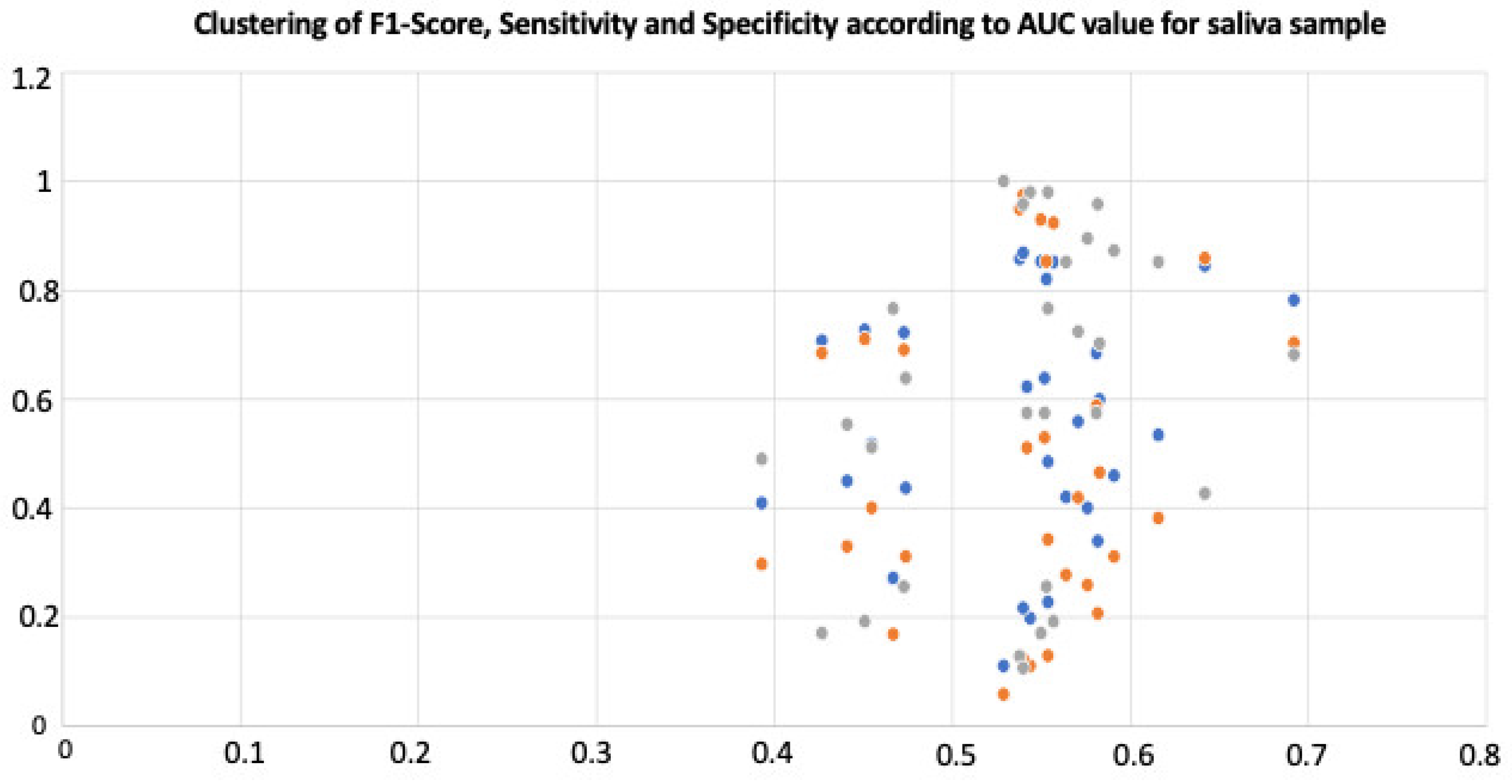
3.3. miRNA Diagnostic Accuracy
4. Results
4.1. Description of the ENDOmiARN Cohort
4.2. Global Overview of the Saliva miRNAome
4.3. miRNA Expression in Patients with and without Endometriosis
4.4. Diagnostic Accuracy of Regulated miRNAs
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.; Tapmeier, T.; Rahmioglu, N.; Kirtley, S.; Zondervan, K.; Becker, C. The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int. J. Mol. Sci. 2018, 19, 599. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Setti, G.; Pezzi, M.E.; Viani, M.V.; Pertinhez, T.A.; Cassi, D.; Magnoni, C.; Bellini, P.; Musolino, A.; Vescovi, P.; Meleti, M. Salivary MicroRNA for Diagnosis of Cancer and Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, E907. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rincon, A.; Martinez-Archundia, M.; Martinez-Ruiz, G.U.; Schoenhuth, A.; Tonda, A. Automatic discovery of 100-miRNA signature for cancer classification using ensemble feature selection. BMC Bioinform. 2019, 20, 480. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.-W.; Eun, Y.-G.; Lee, Y.-C. Diagnostic Value of Salivary miRNA in Head and Neck Squamous Cell Cancer: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 7026. [Google Scholar] [CrossRef]
- LaRocca, D.; Barns, S.; Hicks, S.D.; Brindle, A.; Williams, J.; Uhlig, R.; Johnson, P.; Neville, C.; Middleton, F.A. Comparison of serum and saliva miRNAs for identification and characterization of mTBI in adult mixed martial arts fighters. PLoS ONE 2019, 14, e0207785. [Google Scholar] [CrossRef] [Green Version]
- Rapado-González, Ó.; Majem, B.; Álvarez-Castro, A.; Díaz-Peña, R.; Abalo, A.; Suárez-Cabrera, L.; Gil-Moreno, A.; Santamaría, A.; López-López, R.; Muinelo-Romay, L.; et al. A Novel Saliva-Based miRNA Signature for Colorectal Cancer Diagnosis. J. Clin. Med. 2019, 8, E2029. [Google Scholar] [CrossRef] [Green Version]
- Monnaka, V.U.; Hernandes, C.; Heller, D.; Podgaec, S. Overview of miRNAs for the non-invasive diagnosis of endometriosis: Evidence, challenges and strategies. A systematic review. Einstein 2021, 19, eRW5704. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obs. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef] [PubMed]
- Potla, P.; Ali, S.A.; Kapoor, M. A bioinformatics approach to microRNA-sequencing analysis. Osteoarthr. Cartil. Open 2021, 3, 100131. [Google Scholar] [CrossRef]
- Gyvyte, U.; Juzenas, S.; Salteniene, V.; Kupcinskas, J.; Poskiene, L.; Kucinskas, L.; Jarmalaite, S.; Stuopelyte, K.; Steponaitiene, R.; Hemmrich-Stanisak, G.; et al. MiRNA profiling of gastrointestinal stromal tumors by next-generation sequencing. Oncotarget 2017, 8, 37225–37238. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rincon, A.; Mendoza-Maldonado, L.; Martinez-Archundia, M.; Schönhuth, A.; Kraneveld, A.D.; Garssen, J.; Tonda, A. Machine Learning-Based Ensemble Recursive Feature Selection of Circulating miRNAs for Cancer Tumor Classification. Cancers 2020, 12, E1785. [Google Scholar] [CrossRef]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod Update 2018, 24, 497–515. [Google Scholar] [CrossRef] [Green Version]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Gyvyte, U.; Kupcinskas, J.; Juzenas, S.; Inciuraite, R.; Poskiene, L.; Salteniene, V.; Link, A.; Fassan, M.; Franke, A.; Kupcinskas, L.; et al. Identification of long intergenic non-coding RNAs (lincRNAs) deregulated in gastrointestinal stromal tumors (GISTs). PLoS ONE 2018, 13, e0209342. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [Green Version]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. mirbase: Tools for microRNA genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhang, L. Construction and comprehensive analysis of a ceRNA network to reveal potential prognostic biomarkers for lung adenocarcinoma. BMC Cancer 2021, 21, 849. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Bao, Y.; Wu, Y.; You, Q. Evaluation and application of tools for the identification of known microRNAs in plants. Appl. Plant Sci. 2021, 9, e11414. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Bargaje, R.; Hariharan, M.; Scaria, V.; Pillai, B. Consensus miRNA expression profiles derived from interplatform normalization of microarray data. RNA 2010, 16, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Eijkemans, M.J.; Harrell, F.E.; Habbema, J.D. Prognostic modelling with logistic regression analysis: A comparison of selection and estimation methods in small data sets. Stat. Med. 2000, 19, 1059–1079. [Google Scholar] [CrossRef]
- Nisenblat, V.; Sharkey, D.J.; Wang, Z.; Evans, S.F.; Healey, M.; Ohlsson Teague, E.M.C.; Print, C.G.; Robertson, S.A.; Hull, M.L. Plasma miRNAs Display Limited Potential as Diagnostic Tools for Endometriosis. J. Clin. Endocrinol. Metab. 2019, 104, 1999–2022. [Google Scholar] [CrossRef]
- Courts, C.; Madea, B. Specific Micro-RNA Signatures for the Detection of Saliva and Blood in Forensic Body-fluid Identification. J. Forensic Sci. 2011, 56, 1464–1470. [Google Scholar] [CrossRef]
- El-Mogy, M.; Lam, B.; Haj-Ahmad, T.A.; McGowan, S.; Yu, D.; Nosal, L.; Rghei, N.; Roberts, P.; Haj-Ahmad, Y. Diversity and signature of small RNA in different bodily fluids using next generation sequencing. BMC Genom. 2018, 19, 408. [Google Scholar] [CrossRef]
- Ghassemi, M.; Naumann, T.; Schulam, P.; Beam, A.L.; Chen, I.Y.; Ranganath, R. A Review of Challenges and Opportunities in Machine Learning for Health. AMIA Jt Summits Transl. Sci. Proc. 2020, 2020, 191–200. [Google Scholar]
- Alexiou, A.; Zisis, D.; Kavakiotis, I.; Miliotis, M.; Koussounadis, A.; Karagkouni, D.; Hatzigeorgiou, A.G. DIANA-mAP: Analyzing miRNA from Raw NGS Data to Quantification. Genes 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, E.K.; Lubenow, H.; Ballantyne, J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal. Biochem. 2009, 387, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Jakymiw, A.; Yao, B.; Pauley, B.A.; Carcamo, W.C.; Katz, J.; Cheng, J.Q.; Chan, E.K.L. High resolution of microRNA signatures in human whole saliva. Arch. Oral Biol. 2011, 56, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Wingfield, M.; O’Herlihy, C.; Finn, M.M.; Tallon, D.F.; Fottrell, P.F. Follicular and luteal phase salivary progesterone profiles in women with endometriosis and infertility. Gynecol. Endocrinol. 1994, 8, 21–25. [Google Scholar] [CrossRef]
- Petrelluzzi, K.F.S.; Garcia, M.C.; Petta, C.A.; Grassi-Kassisse, D.M.; Spadari-Bratfisch, R.C. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress 2008, 11, 390–397. [Google Scholar] [CrossRef]
- Andreasen, D.; Fog, J.U.; Biggs, W.; Salomon, J.; Dahslveen, I.K.; Baker, A.; Mouritzen, P. Improved microRNA quantification in total RNA from clinical samples. Methods 2010, 50, S6–S9. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Yao, H.; Luo, H.; Lin, B.; Zhang, X.; Liang, X.; Sun, R.; Zhao, S.; Li, Y.; et al. miRNA Expression Profile of Saliva in Subjects of Yang Deficiency Constitution and Yin Deficiency Constitution. Cell Physiol Biochem. 2018, 49, 2088–2098. [Google Scholar] [CrossRef]
- Metselaar, P.I.; Mendoza-Maldonado, L.; Li Yim, A.Y.F.; Abarkan, I.; Henneman, P.; Te Velde, A.A.; Schönhuth, A.; Bosch, J.A.; Kraneveld, A.D.; Lopez-Rincon, A. Recursive ensemble feature selection provides a robust mRNA expression signature for myalgic encephalomyelitis/chronic fatigue syndrome. Sci. Rep. 2021, 11, 4541. [Google Scholar] [CrossRef]
- Vanhie, A.; D, O.; Peterse, D.; Beckers, A.; Cuéllar, A.; Fassbender, A.; Meuleman, C.; Mestdagh, P.; D’Hooghe, T. Plasma miRNAs as biomarkers for endometriosis. Hum. Reprod. 2019, 34, 1650–1660. [Google Scholar] [CrossRef]
- Kuokkanen, S.; Chen, B.; Ojalvo, L.; Benard, L.; Santoro, N.; Pollard, J.W. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol. Reprod. 2010, 82, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmäe, S.; Martinez-Conejero, J.A.; Esteban, F.J.; Ruiz-Alonso, M.; Stavreus-Evers, A.; Horcajadas, J.A.; Salumets, A. MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial receptivity. Reprod. Sci. 2013, 20, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Rekker, K.; Saare, M.; Roost, A.M.; Kaart, T.; Sõritsa, D.; Karro, H.; Sõritsa, A.; Simón, C.; Salumets, A.; Peters, M. Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil. Steril. 2015, 104, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Coenen-Stass, A.M.L.; Magen, I.; Brooks, T.; Ben-Dov, I.Z.; Greensmith, L.; Hornstein, E.; Fratta, P. Evaluation of methodologies for microRNA biomarker detection by next generation sequencing. RNA Biol. 2018, 15, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- ZIWIG Multicenter Validation of the Salivary miRNA Signature of Endometriosis—The ENDOmiARN Salive Test. Study. 2022. Available online: http://clinicaltrials.gov (accessed on 1 April 2022).
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]

| Controls N = 47 | Endometriosis N = 153 | ||
|---|---|---|---|
| Age years (mean ± SD) | 30.92 ± 13.79 | 31.17 ± 10.78 | 0.19 |
| BMI (body mass index) (mean ± SD) | 24.84 ± 11.10 | 24.36 ± 8.38 | 0.52 |
| rASRM classification | - | ||
| - I–II | - | 80 (52%) | |
| - III–IV | - | 73 (48%) | |
| Control diagnoses | |||
| - No abnormality | 24 (51%) | - | - |
| - Leiomyoma | 1 (2%) | ||
| - Cystadenoma | 5 (11%) | ||
| - Teratoma | 11 (23%) | ||
| - Other gynecological disorders | 6 (13%) | ||
| Dysmenorrhea | 100% | 100% | |
| Abdominal pain outside menstruation | |||
| - Yes | 21 (66%) | 89 (71%) | 0.69 |
| Patients with pain suggesting sciatica | 10 (31%) | 70 (56%) | 0.02 |
| Dyspareunia intensity at VAS (mean ± SD) | 4.95 ± 3.52 | 5.28 ± 3.95 | <0.001 |
| Patients with lower back pain outside menstruation | 20 (62%) | 101 (81%) | 0.049 |
| Intensity of pain during defecation at VAS (mean ± SD) | 2.84 ± 2.76 | 4.35 ± 3.47 | <0.001 |
| Patient with right shoulder pain during menstruation | 3 (9%) | 26 (21%) | 0.21 |
| Intensity of urinary pain during menstruation at VAS (mean ± SD) | 2.84 ± 2.76 | 4.35 ± 3.36 | <0.001 |
| Patient with blood in the stools during menstruation | 4 (12%) | 30 (24%) | 0.24 |
| Patient with blood in urine during menstruation | 8 (25%) | 21 (17%) | 0.41 |
| miRNA | Regulation | AUC | F1-Score | Sensitivity | Specificity |
|---|---|---|---|---|---|
| hsa-let-7a-5p | UP | 0.473 | 0.721 | 0.69 | 0.255 |
| hsa-let-7i-5p | UP | 0.451 | 0.726 | 0.71 | 0.191 |
| hsa-miR-101-3p | UP | 0.554 | 0.227 | 0.129 | 0.979 |
| hsa-miR-103a-3p | UP | 0.582 | 0.339 | 0.206 | 0.957 |
| hsa-miR-142-3p | UP | 0.529 | 0.11 | 0.058 | 1 |
| hsa-miR-146a-5p | UP | 0.538 | 0.857 | 0.948 | 0.128 |
| hsa-miR-15a-5p | UP | 0.571 | 0.558 | 0.419 | 0.723 |
| hsa-miR-16-5p | UP | 0.427 | 0.707 | 0.684 | 0.17 |
| hsa-miR-199a-3p | UP | 0.467 | 0.271 | 0.168 | 0.766 |
| hsa-miR-203b-5p | UP | 0.474 | 0.436 | 0.31 | 0.638 |
| hsa-miR-205-5p | UP | 0.544 | 0.197 | 0.11 | 0.979 |
| hsa-miR-223-3p | UP | 0.455 | 0.517 | 0.4 | 0.511 |
| hsa-miR-23a-3p | UP | 0.54 | 0.216 | 0.123 | 0.957 |
| hsa-miR-23b-3p | UP | 0.564 | 0.42 | 0.277 | 0.851 |
| hsa-miR-24-3p | UP | 0.542 | 0.622 | 0.51 | 0.574 |
| hsa-miR-26a-5p | UP | 0.54 | 0.868 | 0.974 | 0.106 |
| hsa-miR-29a-3p | UP | 0.552 | 0.638 | 0.529 | 0.574 |
| hsa-miR-29c-3p | UP | 0.441 | 0.449 | 0.329 | 0.553 |
| hsa-miR-3191-3p | UP | 0.55 | 0.852 | 0.929 | 0.17 |
| hsa-miR-34c-5p | UP | 0.642 | 0.844 | 0.858 | 0.426 |
| hsa-miR-378a-3p | UP | 0.554 | 0.484 | 0.342 | 0.766 |
| hsa-miR-4330 | UP | 0.393 | 0.409 | 0.297 | 0.489 |
| hsa-miR-4516 | UP | 0.581 | 0.684 | 0.587 | 0.574 |
| hsa-miR-4677-3p | UP | 0.692 | 0.781 | 0.703 | 0.681 |
| hsa-miR-4754 | UP | 0.553 | 0.82 | 0.852 | 0.255 |
| hsa-miR-4778-5p | UP | 0.583 | 0.598 | 0.465 | 0.702 |
| hsa-miR-523-5p | DOWN | 0.576 | 0.4 | 0.258 | 0.894 |
| hsa-miR-655-5p | UP | 0.616 | 0.534 | 0.381 | 0.851 |
| hsa-miR-6726-5p | UP | 0.557 | 0.851 | 0.923 | 0.191 |
| hsa-miR-6738-3p | DOWN | 0.591 | 0.459 | 0.31 | 0.872 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendifallah, S.; Dabi, Y.; Suisse, S.; Jornea, L.; Bouteiller, D.; Touboul, C.; Puchar, A.; Daraï, E. A Bioinformatics Approach to MicroRNA-Sequencing Analysis Based on Human Saliva Samples of Patients with Endometriosis. Int. J. Mol. Sci. 2022, 23, 8045. https://doi.org/10.3390/ijms23148045
Bendifallah S, Dabi Y, Suisse S, Jornea L, Bouteiller D, Touboul C, Puchar A, Daraï E. A Bioinformatics Approach to MicroRNA-Sequencing Analysis Based on Human Saliva Samples of Patients with Endometriosis. International Journal of Molecular Sciences. 2022; 23(14):8045. https://doi.org/10.3390/ijms23148045
Chicago/Turabian StyleBendifallah, Sofiane, Yohann Dabi, Stéphane Suisse, Ludmila Jornea, Delphine Bouteiller, Cyril Touboul, Anne Puchar, and Emile Daraï. 2022. "A Bioinformatics Approach to MicroRNA-Sequencing Analysis Based on Human Saliva Samples of Patients with Endometriosis" International Journal of Molecular Sciences 23, no. 14: 8045. https://doi.org/10.3390/ijms23148045
APA StyleBendifallah, S., Dabi, Y., Suisse, S., Jornea, L., Bouteiller, D., Touboul, C., Puchar, A., & Daraï, E. (2022). A Bioinformatics Approach to MicroRNA-Sequencing Analysis Based on Human Saliva Samples of Patients with Endometriosis. International Journal of Molecular Sciences, 23(14), 8045. https://doi.org/10.3390/ijms23148045






