Lipoprotein Profile in Immunological Non-Responders PLHIV after Antiretroviral Therapy Initiation
Abstract
:1. Introduction
2. Results
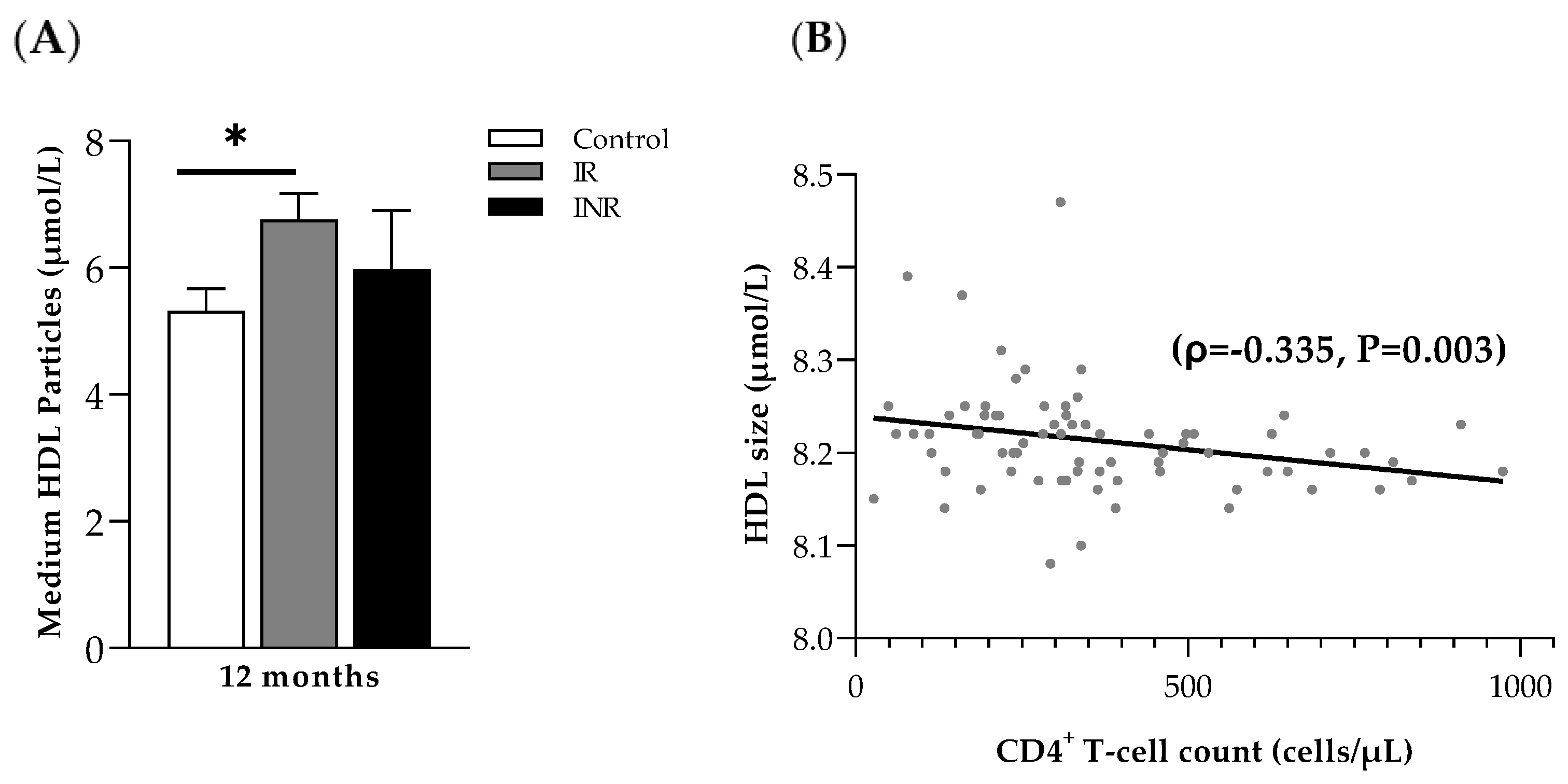
2.1. Stable ART Diminishes Differences in Lipoprotein-Based Cardiovascular Risk Markers
2.2. HDL Particle Size Correlates with CD4+ T-Cell Count at Month 12 (ART)
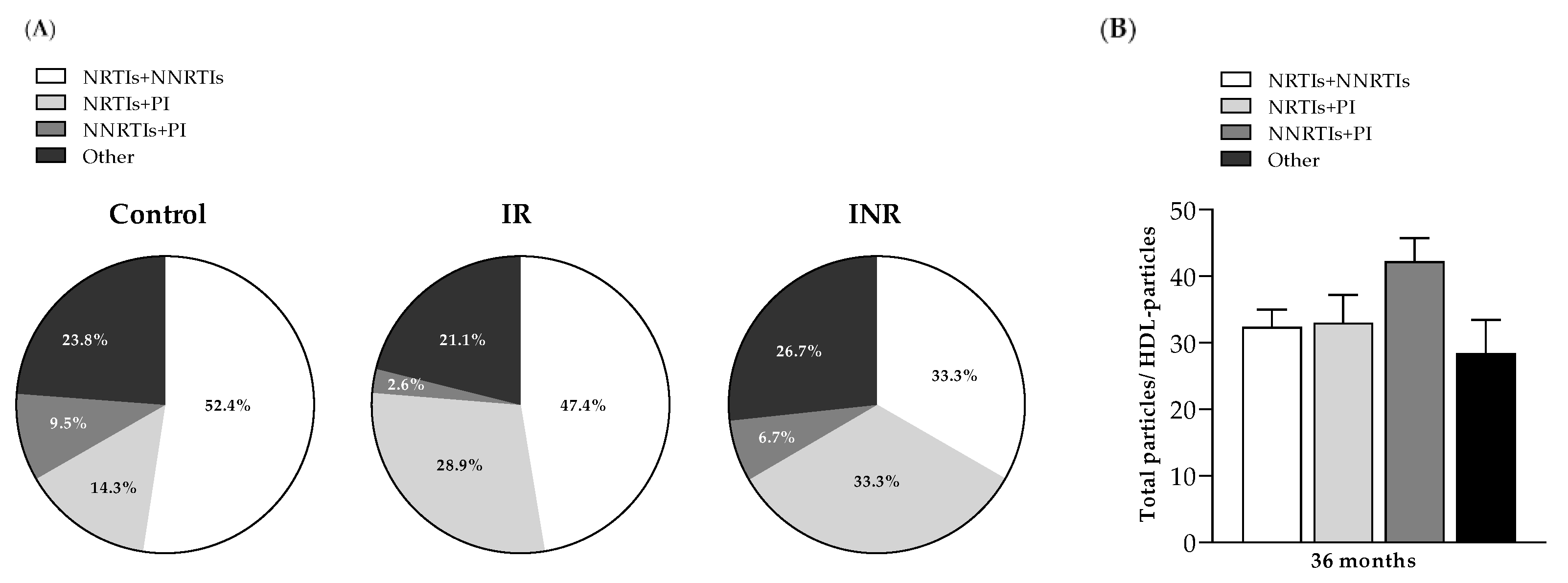
2.3. Lipoprotein Profile Evolution in PLHIV Based on the 2D 1H-NMR Liposcale Test
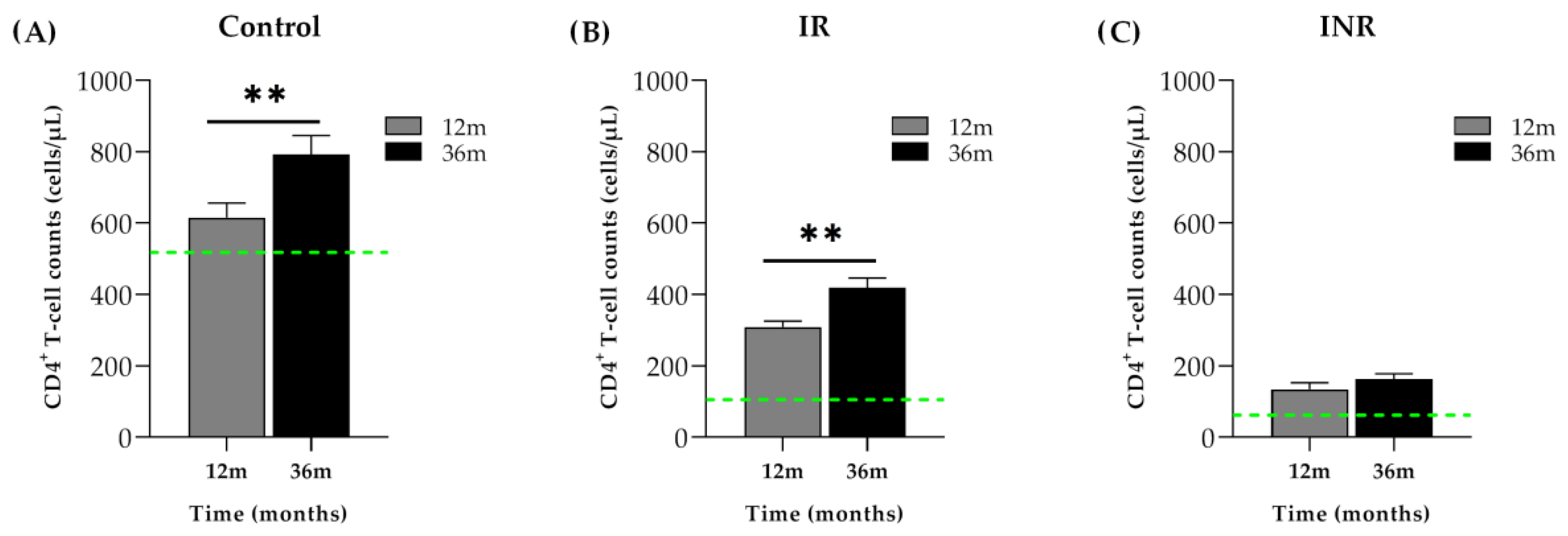
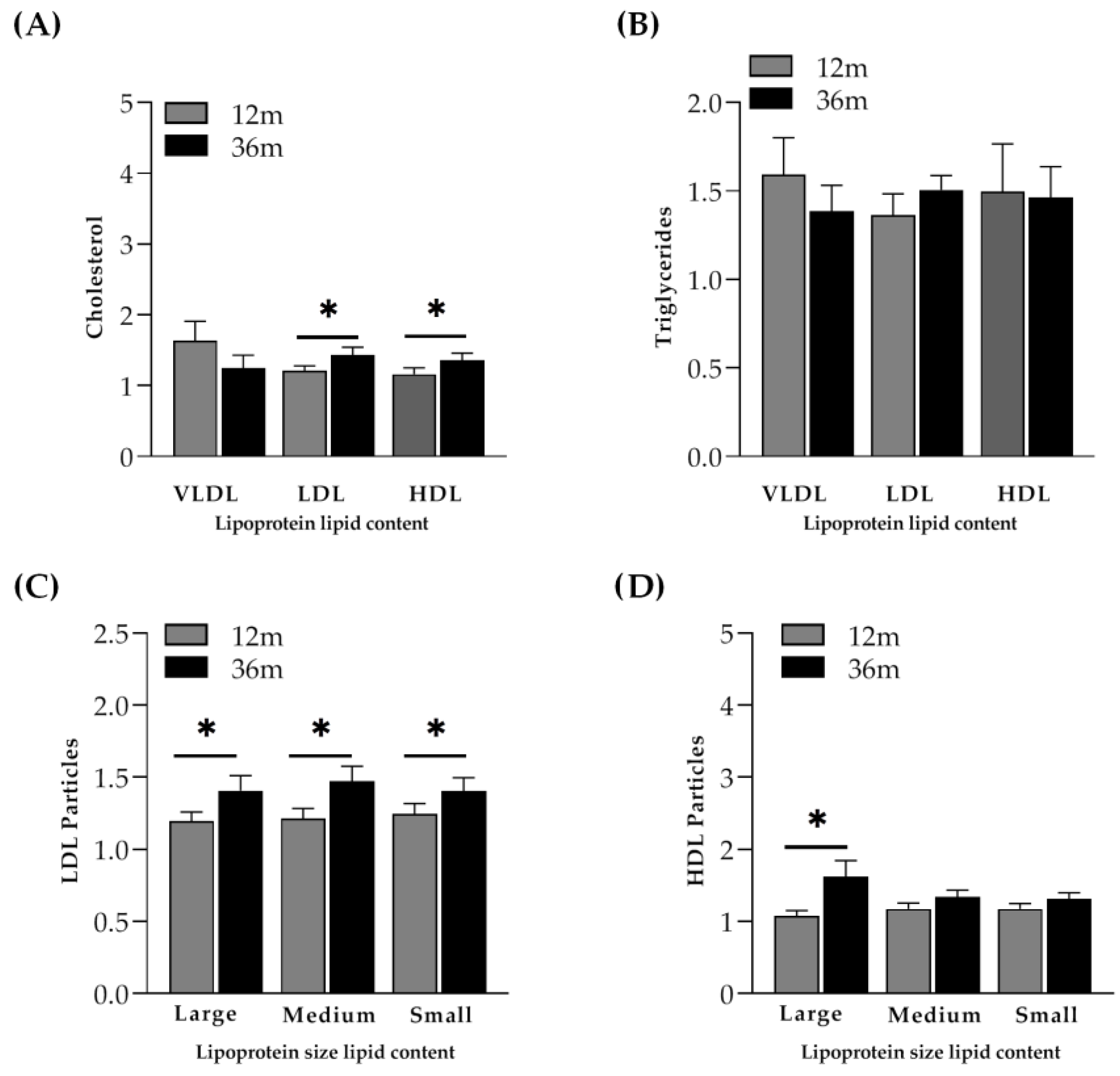
2.3.1. Lipoprotein Profile Evolution in PLHIV Starting ART with More than 350 CD4+ T-Cells/µL
2.3.2. Lipoprotein Profile Evolution in PLHIV Starting ART with Less than 200 CD4+ T-Cells/µL
Immunological Responders (IR)
Immunological Non-Responders (INR)
3. Discussion
4. Materials and Methods
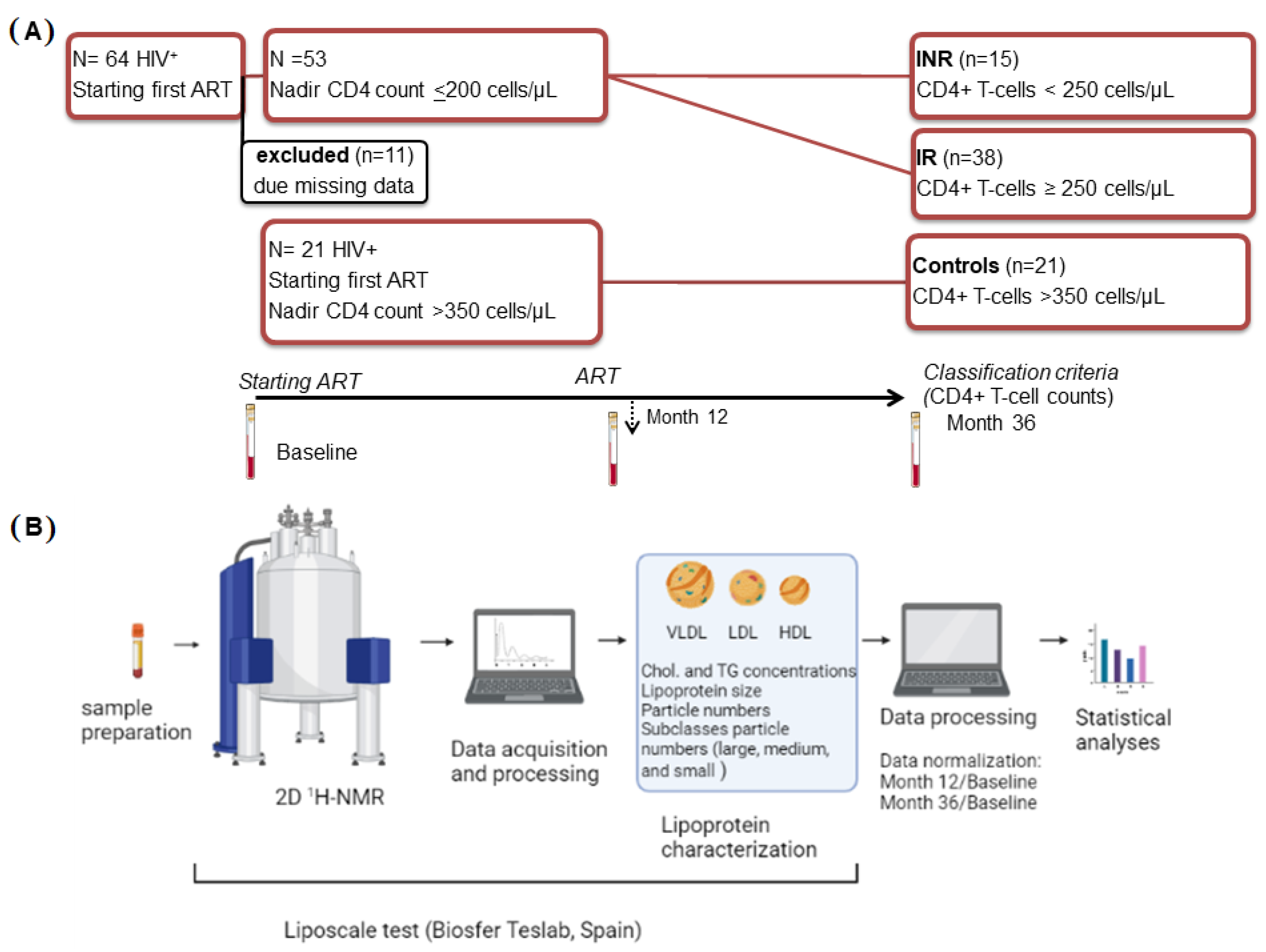
4.1. Study Design and Participants
4.2. Laboratory Measurements
4.3. D 1H-NMR Liposcale Test
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedimo, R.J.; McGinnis, K.A.; Dunlap, M.; Rodriguez-Barradas, M.C.; Justice, A.C. Incidence of Non-AIDS-Defining Malignancies in HIV-Infected versus Noninfected Patients in the HAART Era: Impact of Immunosuppression. J. Acquir. Immune Defic. Syndr. 2009, 52, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, F.; Burty, C.; Lewden, C.; Costagliola, D.; May, T.; Bouteloup, V.; Rosenthal, E.; Jougla, E.; Cacoub, P.; Salmon, D.; et al. Changes in Cancer Mortality among HIV-Infected Patients: The Mortalité 2005 Survey. Clin. Infect. Dis. 2009, 48, 633–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.S.V.; Stelzle, D.; Ken Lee, K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living with the Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis. Circulation 2018, 138, 1100. [Google Scholar] [CrossRef]
- Neuhaus, J.; Jacobs, D.R.; Baker, J.V.; Calmy, A.; Duprez, D.; Rosa, A.L.; Kuller, L.H.; Pett, S.L.; Ristola, M.; Ross, M.J.; et al. Markers of Inflammation, Coagulation, and Renal Function Are Elevated in Adults with HIV Infection. J. Infect. Dis. 2010, 201, 1788–1795. [Google Scholar] [CrossRef]
- Friis-Møller, N.; Smieja, M.; Klein, D. Antiretroviral Therapy as a Cardiovascular Disease Risk Factor: Fact or Fiction? A Review of Clinical and Surrogate Outcome Studies. Curr. Opin. HIV AIDS 2008, 3, 220–225. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The End of AIDS: HIV Infection as a Chronic Disease. Lancet 2013, 382, 1525. [Google Scholar] [CrossRef] [Green Version]
- Burdo, T.H.; Lo, J.; Abbara, S.; Wei, J.; DeLelys, M.E.; Preffer, F.; Rosenberg, E.S.; Williams, K.C.; Grinspoon, S. Soluble CD163, a Novel Marker of Activated Macrophages, Is Elevated and Associated with Noncalcified Coronary Plaque in HIV-Infected Patients. J. Infect. Dis. 2011, 204, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Kingsley, L.A.; Gange, S.J.; Benning, L.; Jacobson, L.P.; Lazar, J.; Anastos, K.; Tien, P.C.; Sharrett, A.R.; Hodis, H.N. Low CD4+ T-Cell Count as a Major Atherosclerosis Risk Factor in HIV-Infected Women and Men. AIDS 2008, 22, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Gallego, E.; Gómez, J.; Pacheco, Y.M.; Peraire, J.; Viladés, C.; Beltrán-Debón, R.; Mallol, R.; López-Dupla, M.; Veloso, S.; Alba, V.; et al. A Baseline Metabolomic Signature Is Associated with Immunological CD4+ T-Cell Recovery after 36 Months of Antiretroviral Therapy in HIV-Infected Patients. AIDS 2018, 32, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Würtz, P.; Raiko, J.R.; Magnussen, C.G.; Soininen, P.; Kangas, A.J.; Tynkkynen, T.; Thomson, R.; Laatikainen, R.; Savolainen, M.J.; Laurikka, J.; et al. High-Throughput Quantification of Circulating Metabolites Improves Prediction of Subclinical Atherosclerosis. Eur. Heart J. 2012, 33, 2307–2316. [Google Scholar] [CrossRef] [Green Version]
- Gerszten, R.E.; Wang, T.J. The Search for New Cardiovascular Biomarkers. Nature 2008, 451, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A Novel Advanced Lipoprotein Test Based on 2D Diffusion-Ordered 1H NMR Spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.H.; McBride, P.E. Should Advanced Lipoprotein Testing Be Used in Clinical Practice? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.; Kwiterovich, P.O. Update on the Detection and Treatment of Atherogenic Low-Density Lipoproteins. Curr. Opin. Endocrinol. Diabetes. Obes. 2013, 20, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.; Lichtenstein, A.H.; Chung, M.; Lau, J.; Balk, E.M. Systematic Review: Association of Low-Density Lipoprotein Subfractions with Cardiovascular Outcomes. Ann. Intern. Med. 2009, 150, 474–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKey, R.H.; Greenland, P.; Goff, D.C.; Lloyd-Jones, D.; Sibley, C.T.; Mora, S. High-Density Lipoprotein Cholesterol and Particle Concentrations, Carotid Atherosclerosis, and Coronary Events: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2012, 60, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Gowda, S.B.; Gowda, D.; Kain, V.; Chiba, H.; Hui, S.P.; Chalfant, C.E.; Parcha, V.; Arora, P.; Halade, G.V. Sphingosine-1-Phosphate Interactions in the Spleen and Heart Reflect Extent of Cardiac Repair in Mice and Failing Human Hearts. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H599–H611. [Google Scholar] [CrossRef]
- Liang, C.; Gowda, S.G.B.; Gowda, D.; Sakurai, T.; Sazaki, I.; Chiba, H.; Hui, S.P. Simple and Sensitive Method for the Quantitative Determination of Lipid Hydroperoxides by Liquid Chromatography/Mass Spectrometry. Antioxidants 2022, 11, 229. [Google Scholar] [CrossRef]
- Azzoni, L.; Foulkes, A.S.; Firnhaber, C.; Yin, X.; Crowther, N.J.; Glencross, D.; Lawrie, D.; Stevens, W.; Papasavvas, E.; Sanne, I.; et al. Metabolic and Anthropometric Parameters Contribute to ART-Mediated CD4+ T Cell Recovery in HIV-1-Infected Individuals: An Observational Study. J. Int. AIDS Soc. 2011, 14, 37. [Google Scholar] [CrossRef] [Green Version]
- Rosado-Sánchez, I.; Rodríguez-Gallego, E.; Peraire, J.; Viladés, C.; Herrero, P.; Fanjul, F.; Gutiérrez, F.; Bernal, E.; Pelazas, R.; Leal, M.; et al. Glutaminolysis and Lipoproteins Are Key Factors in Late Immune Recovery in Successfully Treated HIV-Infected Patients. Clin. Sci. 2019, 133, 997–1010. [Google Scholar] [CrossRef]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein Ratios: Physiological Significance and Clinical Usefulness in Cardiovascular Prevention. Vasc. Health Risk Manag. 2009, 5, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyslipidemia in the HIV Patient—Infectious Disease Advisor. Available online: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/infectious-diseases/dyslipidemia-in-the-hiv-patient/ (accessed on 16 May 2022).
- Pichler, G.; Amigo, N.; Tellez-Plaza, M.; Pardo-Cea, M.A.; Dominguez-Lucas, A.; Marrachelli, V.G.; Monleon, D.; Martin-Escudero, J.C.; Ascaso, J.F.; Chaves, F.J.; et al. LDL Particle Size and Composition and Incident Cardiovascular Disease in a South-European Population: The Hortega-Liposcale Follow-up Study. Int. J. Cardiol. 2018, 264, 172–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A. HDL Particle Number and Size as Predictors of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenson, R.S.; Otvos, J.D.; Freedman, D.S. Relations of Lipoprotein Subclass Levels and Low-Density Lipoprotein Size to Progression of Coronary Artery Disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) Trial. Am. J. Cardiol. 2002, 90, 89–94. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masip, J.; Jorba, R.; López-Dupla, M.; Domingo, P.; Pacheco, Y.M.; García-Pardo, G.; Martínez, E.; Viladés, C.; Veloso, S.; Alba, V.; et al. Lipoprotein Profile in Immunological Non-Responders PLHIV after Antiretroviral Therapy Initiation. Int. J. Mol. Sci. 2022, 23, 8071. https://doi.org/10.3390/ijms23158071
Masip J, Jorba R, López-Dupla M, Domingo P, Pacheco YM, García-Pardo G, Martínez E, Viladés C, Veloso S, Alba V, et al. Lipoprotein Profile in Immunological Non-Responders PLHIV after Antiretroviral Therapy Initiation. International Journal of Molecular Sciences. 2022; 23(15):8071. https://doi.org/10.3390/ijms23158071
Chicago/Turabian StyleMasip, Jenifer, Rosa Jorba, Miguel López-Dupla, Pere Domingo, Yolanda María Pacheco, Graciano García-Pardo, Esteban Martínez, Consuelo Viladés, Sergi Veloso, Verónica Alba, and et al. 2022. "Lipoprotein Profile in Immunological Non-Responders PLHIV after Antiretroviral Therapy Initiation" International Journal of Molecular Sciences 23, no. 15: 8071. https://doi.org/10.3390/ijms23158071
APA StyleMasip, J., Jorba, R., López-Dupla, M., Domingo, P., Pacheco, Y. M., García-Pardo, G., Martínez, E., Viladés, C., Veloso, S., Alba, V., Olona, M., Vidal, F., Gómez-Bertomeu, F., Peraire, J., & Rull, A. (2022). Lipoprotein Profile in Immunological Non-Responders PLHIV after Antiretroviral Therapy Initiation. International Journal of Molecular Sciences, 23(15), 8071. https://doi.org/10.3390/ijms23158071






