Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice
Abstract
1. Introduction
2. Results
2.1. Chronic Intraperitoneally Injected GnIH Increases Male Mice Obesity and Photophase Food Intake and Alters Meal Microstructure
2.2. Chronic Intraperitoneally Injected GnIH Alters Organ Indexes and Serum Biochemical Indexes in Male Mice
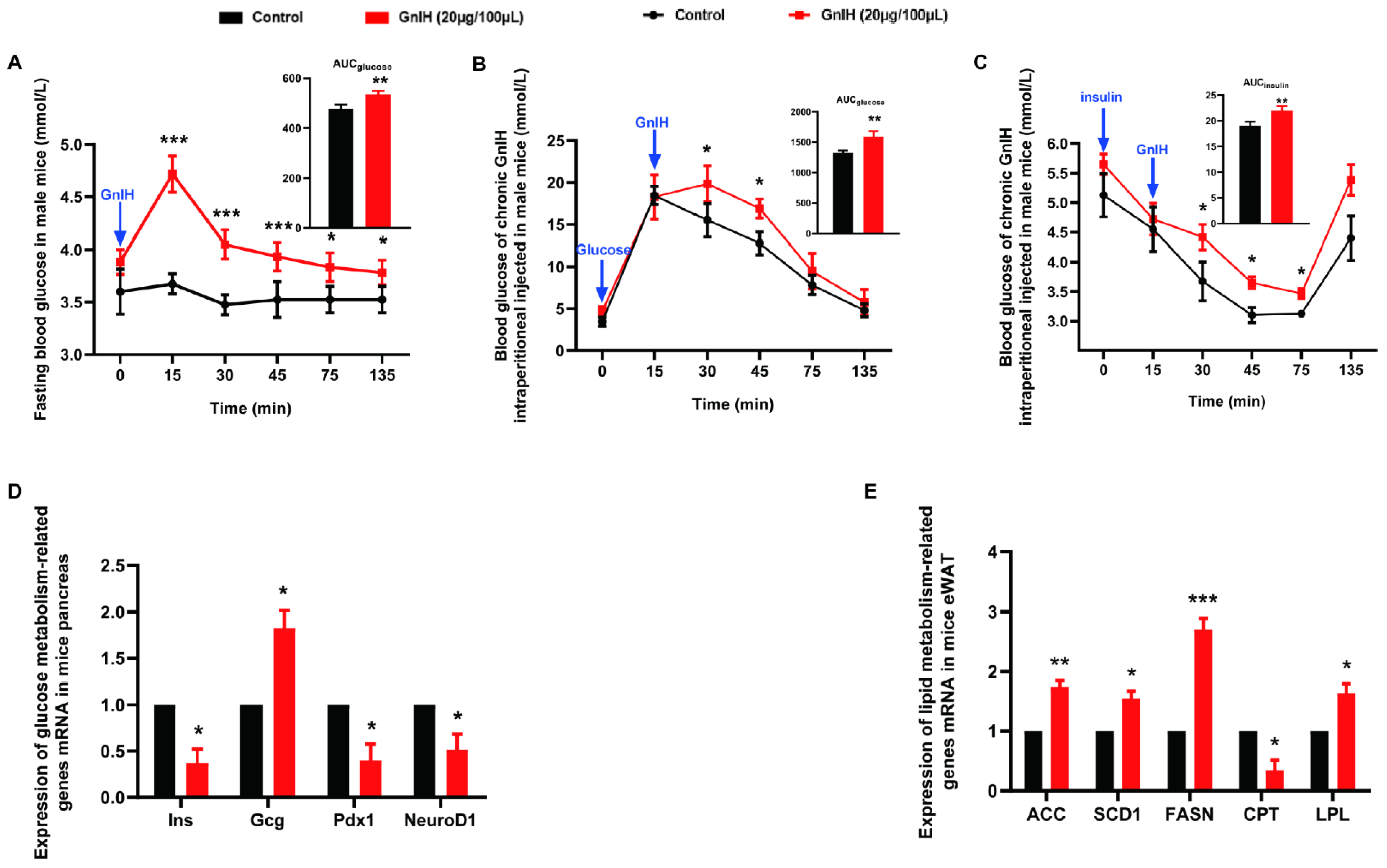
2.3. Chronic Intraperitoneal Injection of GnIH Impaired Glucose Homeostasis in Male Mice
2.4. Chronic Intraperitoneal Injection of GnIH Alters the Expression of Genes Related to Glucose and Lipid Metabolism in Pancreas and eWAT
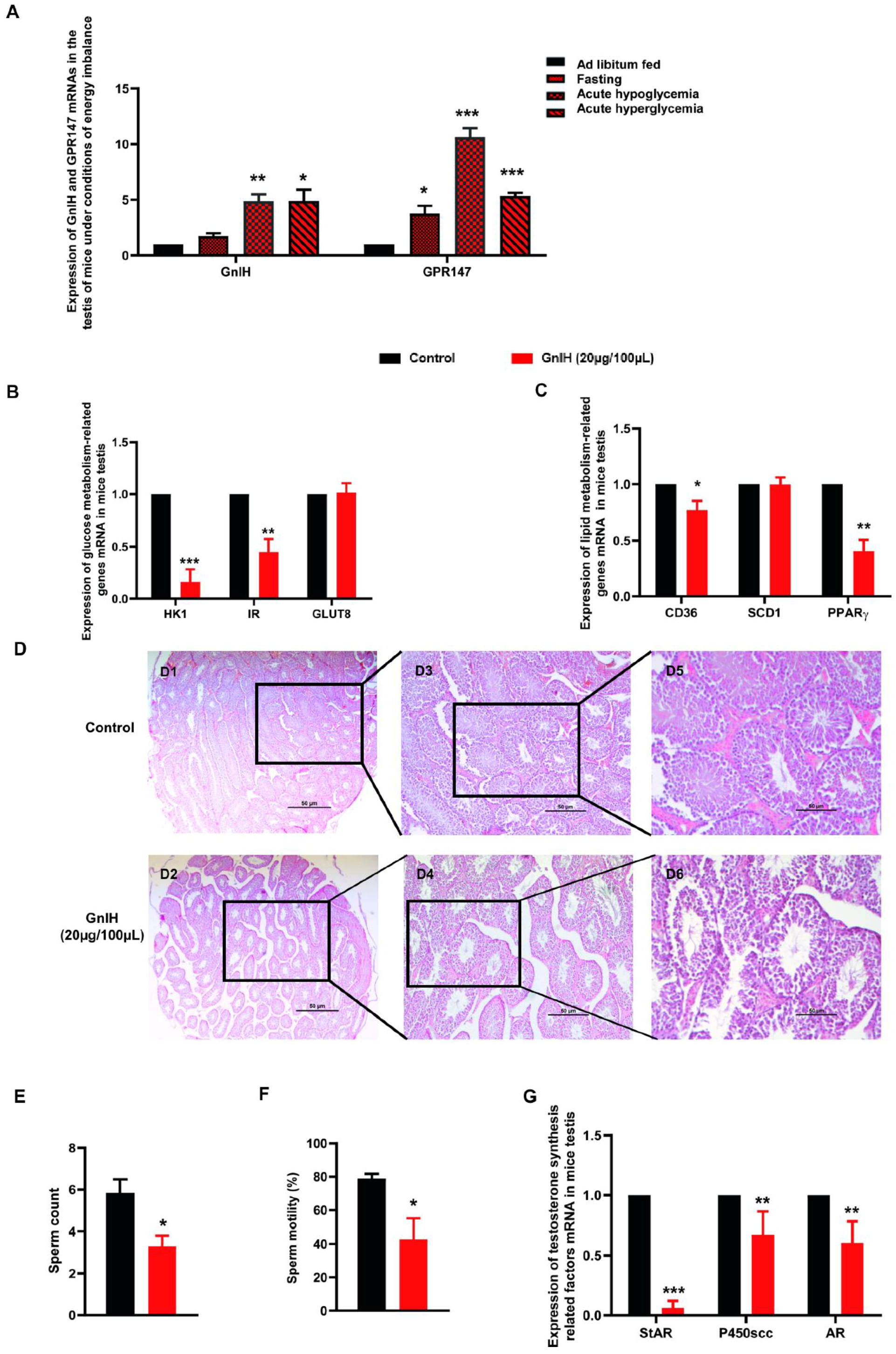
2.5. GnIH and Its Receptor GPR147 Are Involved in Glucolipid Metabolic Disorder-Induced Testicular Dysfunction
3. Discussion
4. Materials and Methods
4.1. Animals and GnIH
4.2. Food Intake, Meal Microstructure, Weight, and Serum Biochemical Index Measurements
4.3. Blood Glucose Measurements
4.4. Glucose Tolerance Test
4.5. Insulin Tolerance Test
4.6. Gene Expression
4.7. Sperm Parameter Analysis of Epididymis
4.8. Histological Examination of Testicular Tissue
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castellano, J.M.; Bentsen, A.H.; Mikkelsen, J.D.; Tena-Sempere, M. Kisspeptins: Bridging energy homeostasis and reproduction. Brain Res. 2010, 1364, 129–138. [Google Scholar] [CrossRef]
- Crown, A.; Clifton, D.K.; Steiner, R.A. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 2007, 86, 175–182. [Google Scholar] [CrossRef]
- Hansen, M.; Flatt, T.; Aguilaniu, H. Reproduction, fat metabolism, and life span: What is the connection? Cell Metab. 2013, 17, 10–19. [Google Scholar] [CrossRef]
- Goulis, D.G.; Tarlatzis, B.C. Metabolic syndrome and reproduction: I. testicular function. Gynecol. Endocrinol. 2008, 24, 33–39. [Google Scholar] [CrossRef]
- Hart, R.J.; Doherty, D.A.; Mori, T.A.; Adams, L.A.; Huang, R.C.; Minaee, N.; Handelsman, D.J.; McLachlan, R.; Norman, R.J.; Dickinson, J.E.; et al. Features of the metabolic syndrome in late adolescence are associated with impaired testicular function at 20 years of age. Hum. Reprod. 2019, 34, 389–402. [Google Scholar] [CrossRef]
- Wahab, F.; Atika, B.; Shahab, M. Kisspeptin as a link between metabolism and reproduction: Evidences from rodent and primate studies. Metabolism 2013, 62, 898–910. [Google Scholar] [CrossRef]
- Cunningham, M.J. Galanin-like peptide as a link between metabolism and reproduction. J. Neuroendocrinol. 2004, 16, 717–723. [Google Scholar] [CrossRef]
- Xu, Y.; Faulkner, L.D.; Hill, J.W. Cross-Talk between Metabolism and Reproduction: The Role of POMC and SF1 Neurons. Front. Endocrinol. 2011, 2, 98. [Google Scholar] [CrossRef]
- Tena-Sempere, M. Interaction between energy homeostasis and reproduction: Central effects of leptin and ghrelin on the reproductive axis. Horm. Metab. Res. 2013, 45, 919–927. [Google Scholar] [CrossRef]
- Tsutsui, K.; Saigoh, E.; Ukena, K.; Teranishi, H.; Fujisawa, Y.; Kikuchi, M.; Ishii, S.; Sharp, P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000, 275, 661–667. [Google Scholar] [CrossRef]
- Smith, J.T.; Clarke, I.J. Gonadotropin inhibitory hormone function in mammals. Trends Endocrinol. Metab. 2010, 21, 255–260. [Google Scholar] [CrossRef]
- Ubuka, T.; McGuire, N.L.; Calisi, R.M.; Perfito, N.; Bentley, G.E. The control of reproductive physiology and behavior by gonadotropin-inhibitory hormone. Integr. Comp. Biol. 2008, 48, 560–569. [Google Scholar] [CrossRef]
- Ubuka, T.; Tsutsui, K. Evolution of gonadotropin-inhibitory hormone receptor and its ligand. Gen. Comp. Endocrinol. 2014, 209, 148–161. [Google Scholar] [CrossRef]
- Tachibana, T.; Sato, M.; Takahashi, H.; Ukena, K.; Tsutsui, K.; Furuse, M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005, 1050, 94–100. [Google Scholar] [CrossRef]
- Johnson, M.A.; Tsutsui, K.; Fraley, G.S. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav. 2007, 51, 171–180. [Google Scholar] [CrossRef]
- Huo, K.; Li, X.; Hu, W.; Song, X.; Zhang, D.; Zhang, X.; Chen, X.; Yuan, J.; Zuo, J.; Wang, X. RFRP-3, the Mammalian Ortholog of GnIH, Is a Novel Modulator Involved in Food Intake and Glucose Homeostasis. Front. Endocrinol. 2020, 11, 194. [Google Scholar] [CrossRef]
- Anjum, S.; Krishna, A.; Tsutsui, K. Possible Role of GnIH as a Mediator between Adiposity and Impaired Testicular Function. Front. Endocrinol. 2016, 7, 6. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ubuka, T. Gonadotropin-inhibitory hormone (GnIH): A new key neurohormone controlling reproductive physiology and behavior. Front. Neuroendocrinol. 2021, 61, 100900. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ubuka, T.; Ukena, K. Advancing reproductive neuroendocrinology through research on the regulation of GnIH and on its diverse actions on reproductive physiology and behavior. Front. Neuroendocrinol. 2021, 64, 100955. [Google Scholar] [CrossRef]
- Ubuka, T.; Tsutsui, K. Reproductive neuroendocrinology of mammalian gonadotropin-inhibitory hormone. Reprod. Med. Biol. 2019, 18, 225–233. [Google Scholar] [CrossRef]
- Anjum, S.; Khattak, M.N.K.; Tsutsui, K.; Krishna, A. RF-amide related peptide-3 (RFRP-3): A novel neuroendocrine regulator of energy homeostasis, metabolism, and reproduction. Mol. Biol. Rep. 2021, 48, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- Wilsterman, K.; Alonge, M.M.; Bao, X.; Conner, K.A.; Bentley, G.E. Food access modifies GnIH, but not CRH, cell number in the hypothalamus in a female songbird. Gen. Comp. Endocrinol. 2020, 292, 113438. [Google Scholar] [CrossRef] [PubMed]
- McConn, B.R.; Yi, J.; Gilbert, E.R.; Siegel, P.B.; Chowdhury, V.S.; Furuse, M.; Cline, M.A. Stimulation of food intake after central administration of gonadotropin-inhibitory hormone is similar in genetically selected low and high body weight lines of chickens. Gen. Comp. Endocrinol. 2016, 232, 96–100. [Google Scholar] [CrossRef]
- Clarke, I.J.; Smith, J.T.; Henry, B.A.; Oldfield, B.J.; Stefanidis, A.; Millar, R.P.; Sari, I.P.; Chng, K.; Fabre-Nys, C.; Caraty, A.; et al. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 2012, 95, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Inoue, K.; Fukuda, Y.; Mizuno, T.; Ukena, K.; Kriegsfeld, L.J.; Tsutsui, K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 2012, 153, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Birnie, M.; Lincoln, G.A.; Hazlerigg, D.G. RFamide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. J. Neuroendocrinol. 2008, 20, 1252–1259. [Google Scholar] [CrossRef]
- Smith, J.T.; Coolen, L.M.; Kriegsfeld, L.J.; Sari, I.P.; Jaafarzadehshirazi, M.R.; Maltby, M.; Bateman, K.; Goodman, R.L.; Tilbrook, A.J.; Ubuka, T.; et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: A novel medium for seasonal breeding in the sheep. Endocrinology 2008, 149, 5770–5782. [Google Scholar] [CrossRef]
- Ubuka, T.; Bentley, G.E.; Ukena, K.; Wingfield, J.C.; Tsutsui, K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl. Acad. Sci. USA 2005, 102, 3052–3057. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ubuka, T.; Bentley, G.E.; Kriegsfeld, L.J. Gonadotropin-inhibitory hormone (GnIH): Discovery, progress and prospect. Gen. Comp. Endocrinol. 2012, 177, 305–314. [Google Scholar] [CrossRef]
- Guyenet, S.J.; Schwartz, M.W. Clinical review: Regulation of food intake, energy balance, and body fat mass: Implications for the pathogenesis and treatment of obesity. J. Clin. Endocrinol. Metab. 2012, 97, 745–755. [Google Scholar] [CrossRef]
- Rosenheck, R. Fast food consumption and increased caloric intake: A systematic review of a trajectory towards weight gain and obesity risk. Obes. Rev. 2008, 9, 535–547. [Google Scholar] [CrossRef]
- Elfhag, K.; Barkeling, B.; Carlsson, A.M.; Rossner, S. Microstructure of eating behavior associated with Rorschach characteristics in obesity. J. Pers. Assess. 2003, 81, 40–50. [Google Scholar] [CrossRef]
- Moriwaki, S.; Narimatsu, Y.; Fukumura, K.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ukena, K. Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice. Int. J. Mol. Sci. 2020, 21, 8606. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Yu, X.; Jia, A.; Ming, J.; Ji, Q. RFamide-related peptide-3 promotes alpha TC1 clone 6 cell survival likely via GPR147. Peptides 2018, 107, 39–44. [Google Scholar] [CrossRef]
- Carpentier, A.C. 100(th) anniversary of the discovery of insulin perspective: Insulin and adipose tissue fatty acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E653–E670. [Google Scholar] [CrossRef]
- Habegger, K.M.; Heppner, K.M.; Geary, N.; Bartness, T.J.; DiMarchi, R.; Tschop, M.H. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 2010, 6, 689–697. [Google Scholar] [CrossRef]
- Mileti, E.; Kwok, K.H.M.; Andersson, D.P.; Mathelier, A.; Raman, A.; Backdahl, J.; Jalkanen, J.; Massier, L.; Thorell, A.; Gao, H.; et al. Human White Adipose Tissue Displays Selective Insulin Resistance in the Obese State. Diabetes 2021, 70, 1486–1497. [Google Scholar] [CrossRef]
- Ubuka, T.; Ukena, K.; Sharp, P.J.; Bentley, G.E.; Tsutsui, K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology 2006, 147, 1187–1194. [Google Scholar] [CrossRef]
- McGuire, N.L.; Kangas, K.; Bentley, G.E. Effects of melatonin on peripheral reproductive function: Regulation of testicular GnIH and testosterone. Endocrinology 2011, 152, 3461–3470. [Google Scholar] [CrossRef]
- Batool, A.; Naz, R.; Wazir, M.; Azam, A.; Ullah, R.; Wahab, F.; Shahab, M. Acute fasting-induced repression of the hypothalamic-pituitary-gonadal axis is reversed by RF-9 administration in the adult male macaque. Horm. Metab. Res. 2014, 46, 832–927. [Google Scholar] [CrossRef]
- Ubuka, T.; Son, Y.L.; Tobari, Y.; Narihiro, M.; Bentley, G.E.; Kriegsfeld, L.J.; Tsutsui, K. Central and direct regulation of testicular activity by gonadotropin-inhibitory hormone and its receptor. Front. Endocrinol. 2014, 5, 8. [Google Scholar] [CrossRef]
- Zheng, L.; Su, J.; Fang, R.; Jin, M.; Lei, Z.; Hou, Y.; Ma, Z.; Guo, T. Developmental changes in the role of gonadotropin-inhibitory hormone (GnIH) and its receptors in the reproductive axis of male Xiaomeishan pigs. Anim. Reprod. Sci. 2015, 154, 113–120. [Google Scholar] [CrossRef]
- Anjum, S.; Krishna, A.; Tsutsui, K. Inhibitory roles of the mammalian GnIH ortholog RFRP3 in testicular activities in adult mice. J. Endocrinol. 2014, 223, 79–91. [Google Scholar] [CrossRef]
- Nakamura, N.; Komiyama, M.; Fujioka, M.; Mori, C. Sorting specificity of spermatogenic cell specific region of mouse hexokinase-s (mHk1-s). Mol. Reprod. Dev. 2003, 64, 113–119. [Google Scholar] [CrossRef]
- Kokk, K.; Verajankorva, E.; Wu, X.K.; Tapfer, H.; Poldoja, E.; Simovart, H.E.; Pollanen, P. Expression of insulin signaling transmitters and glucose transporters at the protein level in the rat testis. Ann. N. Y. Acad. Sci. 2007, 1095, 262–273. [Google Scholar] [CrossRef]
- Gillot, I.; Jehl-Pietri, C.; Gounon, P.; Luquet, S.; Rassoulzadegan, M.; Grimaldi, P.; Vidal, F. Germ cells and fatty acids induce translocation of CD36 scavenger receptor to the plasma membrane of Sertoli cells. J. Cell Sci. 2005, 118 Pt 14, 3027–3035. [Google Scholar] [CrossRef][Green Version]
- Olia Bagheri, F.; Alizadeh, A.; Sadighi Gilani, M.A.; Shahhoseini, M. Role of peroxisome proliferator-activated receptor gamma (PPARgamma) in the regulation of fatty acid metabolism related gene expressions in testis of men with impaired spermatogenesis. Reprod. Biol. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Couret, D.; Bourane, S.; Catan, A.; Nativel, B.; Planesse, C.; Dorsemans, A.C.; Ait-Arsa, I.; Cournot, M.; Rondeau, P.; Patche, J.; et al. A hemorrhagic transformation model of mechanical stroke therapy with acute hyperglycemia in mice. J. Comp. Neurol. 2018, 526, 1006–1016. [Google Scholar] [CrossRef]
- Park, M.J.; Yoo, S.W.; Choe, B.S.; Dantzer, R.; Freund, G.G. Acute hypoglycemia causes depressive-like behaviors in mice. Metabolism 2012, 61, 229–236. [Google Scholar] [CrossRef]
- Goebel, M.; Stengel, A.; Wang, L.; Tache, Y. Central nesfatin-1 reduces the nocturnal food intake in mice by reducing meal size and increasing inter-meal intervals. Peptides 2011, 32, 36–43. [Google Scholar] [CrossRef]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef]
- Li, X.; Su, J.; Lei, Z.; Zhao, Y.; Jin, M.; Fang, R.; Zheng, L.; Jiao, Y. Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig: cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle. Peptides 2012, 36, 176–185. [Google Scholar] [CrossRef]
- Chinoy, N.J.; Sequeira, E. Effects of fluoride on the histoarchitecture of reproductive organs of the male mouse. Reprod. Toxicol. 1989, 3, 261–267. [Google Scholar] [CrossRef]
- Leblond, C.P.; Clermont, Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad Sci. 1952, 55, 548–573. [Google Scholar] [CrossRef]


| HK1 | IR | GLUT8 | |
| StAR | r = 0.85 ** p < 0.001 | NS | NS |
| P450scc | r = 0.08 p > 0.05 | NS | NS |
| AR | r = −0.48 ** p < 0.001 | NS | NS |
| Testis index | r = 0.25 p > 0.05 | r = −0.07 p > 0.05 | r = −0.18 p > 0.05 |
| Sperm motility | NS | r = −0.06 p > 0.05 | r = 0.44 * p < 0.05 |
| CD36 | SCD1 | PPARγ | |
| StAR | r = 0.38 * p < 0.05 | r = 0.35 p > 0.05 | r = 0.35 p > 0.05 |
| P450scc | r = −0.30 p > 0.05 | r = −0.68 ** p < 0.001 | r = −0.13 p > 0.05 |
| AR | r = 0.32 p > 0.05 | r = 0.27 p > 0.05 | r = 0.28 p > 0.05 |
| Testis index | r = −0.12 p > 0.05 | r = −0.17 p > 0.05 | r = 0.09 p > 0.05 |
| Sperm motility | NS | NS | r = −0.09 p > 0.05 |
| Genes | Primer Sequence (5′–3′) | Annealing (°C) | Gene Accession Number |
|---|---|---|---|
| StAR | F: AAGGAAAGCCAGCAGGAGAAC | 60 | XM_021169998.1 |
| R: TCCATGCGGTCCACAAGTT | |||
| P450scc | F: CAGATGCAGAGTTTCCAA | 60 | NM_019779.4 |
| R: TGAGAAGAGTATCGACGCATCCT | |||
| AR | F: CTGGGAAGGGTCTACCCAC | 60 | XM_021188305.2 |
| R: GGTGCTATGTTAGCGGCCTC | |||
| HK1 | F: TGCCATGCGGCTCTCTGATG | 60 | XM_006513247.2 |
| R: CTTGACGGAGGCCGTTGGGTT | |||
| GLUT8 | F: TTCATGGCCTTTCTAGTGACC | 60 | XM_036162406.1 |
| R: GAGTCCTGCCTTTAGTCTCAG | |||
| IR | F: GCAGTTTGTGGAACGGTGCT | 55 | NM_001330056.1 |
| R: CCAGGCACTCTTTGTGGCAG | |||
| CD36 | F: CAGATGACGTGGCAAAGAAC | 55 | NM_001159558.1 |
| R: TGGCTCCATTGGGCTGTA | |||
| PPARγ | F: TCACAAGAGCTGACCCAATGGT | 55 | NM_001308354 |
| R: ATAATAAGGTGGAGATGCAGGTTCTAC | |||
| SCD1 | F: AGGCCTGTACGGGATCATACT | 60 | NM_009127.4 |
| R: AGAGGCTGGTCATGTAGTAG | |||
| Gcg | F: CCACTCACAGGGCACATTC | 55 | XM_006516200.5 |
| R: CGGTTCCTCTTGGTGTCA | |||
| Pdx1 | F: CCCCAGTTTACAAGCTCGCT | 55 | NM_008814.4 |
| R: CTCGGTTCCATTCGGGAAAGG | |||
| NeuroD1 | F: CTTGGCCAAGAACTACATCTGG | 55 | XM_021193023.2 |
| R: GGAGTAGGGATGCACCGGGAA | |||
| Ins | F: GCTTCTTCTACACACCCATGTC | 55 | XM_021204833.1 |
| R: AGCACTGATCTACAATGCCAC | |||
| GnIH | F: GAGGAATCCCAAAAGGGGTAAAGG | 60 | XM_021165817.2 |
| R: GTGATGCGTCTGGCTGTTGTTCT | |||
| GPR147 | F: AGCCTCACCTTCTCCTCCTACTACC | 60 | XM_021205580.2 |
| R: AGTGATAAGGTTGTCCACAAGGGTT | |||
| R: TCCACCACCCTGTTGCTGTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, R.; Chen, L.; Song, X.; Zhang, X.; Xu, W.; Han, D.; Zuo, J.; Hu, W.; Shi, Y.; Cao, Y.; et al. Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice. Int. J. Mol. Sci. 2022, 23, 8066. https://doi.org/10.3390/ijms23158066
Luo R, Chen L, Song X, Zhang X, Xu W, Han D, Zuo J, Hu W, Shi Y, Cao Y, et al. Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice. International Journal of Molecular Sciences. 2022; 23(15):8066. https://doi.org/10.3390/ijms23158066
Chicago/Turabian StyleLuo, Rongrong, Lei Chen, Xingxing Song, Xin Zhang, Wenhao Xu, Dongyang Han, Jianyu Zuo, Wen Hu, Yan Shi, Yajie Cao, and et al. 2022. "Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice" International Journal of Molecular Sciences 23, no. 15: 8066. https://doi.org/10.3390/ijms23158066
APA StyleLuo, R., Chen, L., Song, X., Zhang, X., Xu, W., Han, D., Zuo, J., Hu, W., Shi, Y., Cao, Y., Ma, R., Liu, C., Xu, C., Li, Z., & Li, X. (2022). Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice. International Journal of Molecular Sciences, 23(15), 8066. https://doi.org/10.3390/ijms23158066






