How Do Transposable Elements Activate Expression of Transcriptionally Silent Antibiotic Resistance Genes?
Abstract
1. Introduction
2. Silent AR Genes
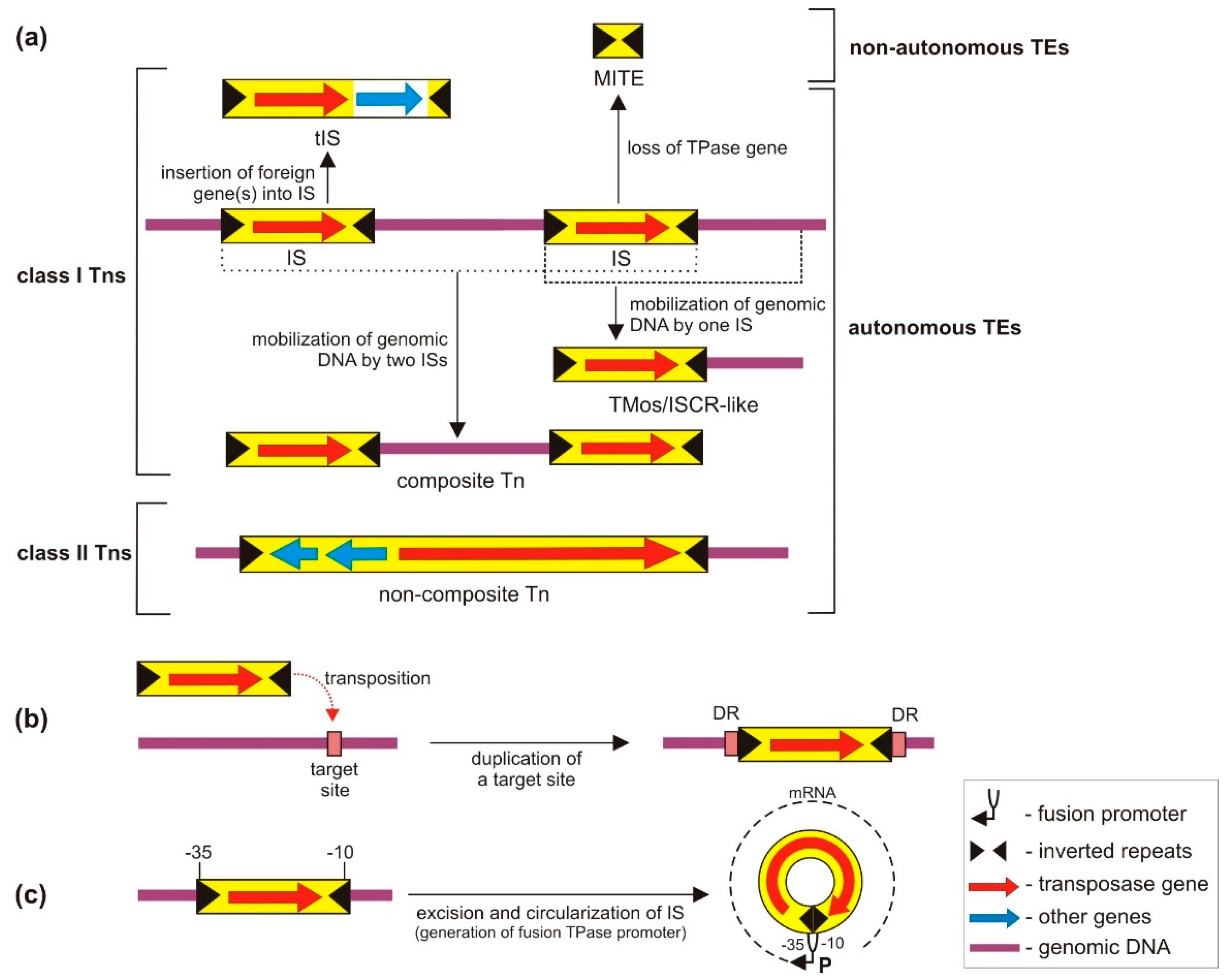
3. Classification and General Features of TEs
4. Activation of Silent AR Genes by Promoters Delivered or Generated by TEs
4.1. Transposase Gene Promoters
4.2. Fusion Promoters
| IS/Tn Family | Element | Resulting Resistance to: | Gene | Organism | Reference |
|---|---|---|---|---|---|
| IS1 | IS1 | ampicillin | blaTEM-1 | E. coli | [80] |
| fluoroquinolones (marbofloxacin, enrofloxacin, ciprofloxacin), florfenicol, erythromycin | acrEF | Salmonella enterica | [81] | ||
| IS1-like | ceftazidime, aztreonam | blaTEM-6 | E. coli | [66] | |
| IS3 | IS2 | kanamycin | neoR | E. coli | [82] |
| erythromycin, clarithromycin, azithromycin, clindamycin, linezolid | acrEF | E. coli | [83] | ||
| ampicillin | ampC | E. coli | [67] | ||
| IS6 | IS26 | β-lactams (amoxicillin, ticarcillin, piperacillin, cephalothin, cefoxitin, ceftazidime, cefotaxime, aztreonam) | blaSHV-2a | Pseudomonas aeruginosa | [84] |
| gentamicin | aacC3 | E. coli | [85] | ||
| β-lactams (penicillin, cefotaxime, aztreonam) | blaBES-1 | Serratia marcescens | [86] | ||
| neomycin, kanamycin, paromomycin, lividomycin | aphA7 | Klebsiella pneumoniae | [87] | ||
| ISOur1 | carbenicillin | blaABA-1 | Oligella urethralis | [88] | |
| IS1008 | carbapenem | blaOXA-58 | Acinetobacter baumannii | [89] | |
| IS257 | tetracycline | tetA(K) | Staphylococcus aureus | [37] | |
| trimethoprim | thyE-dfrA-orf140 | S. aureus | [90] | ||
| IS21 | ISKpn7 | imipenem | blaKPC | K. pneumoniae | [91] |
| ISBf1 (IS21-like) | ampicillin | cepA | Bacteroides fragilis | [70] | |
| IS30 | IS18 | aminoglycosides (amikacin, netilmicin, tobramycin) | aac(6′)-Ij | Acinetobacter sp. 13 strain BM2716 | [92] |
| ISAba125 | cephalosporin | blaADC | A. baumannii | [93] | |
| IS256 | IS256 | methicillin | mecA | Staphylococcus sciuri | [73] |
| llm | S. aureus | [72] | |||
| Tn3 | Tn3 | gentamicin | aacC2 | E. coli | [94] |
4.3. Chimeric TE Promoters
4.4. Complete Outward-Directed Promoters
4.5. Antisense RNA Promoters
4.6. Promoters Located within Core Regions of Composite Tns
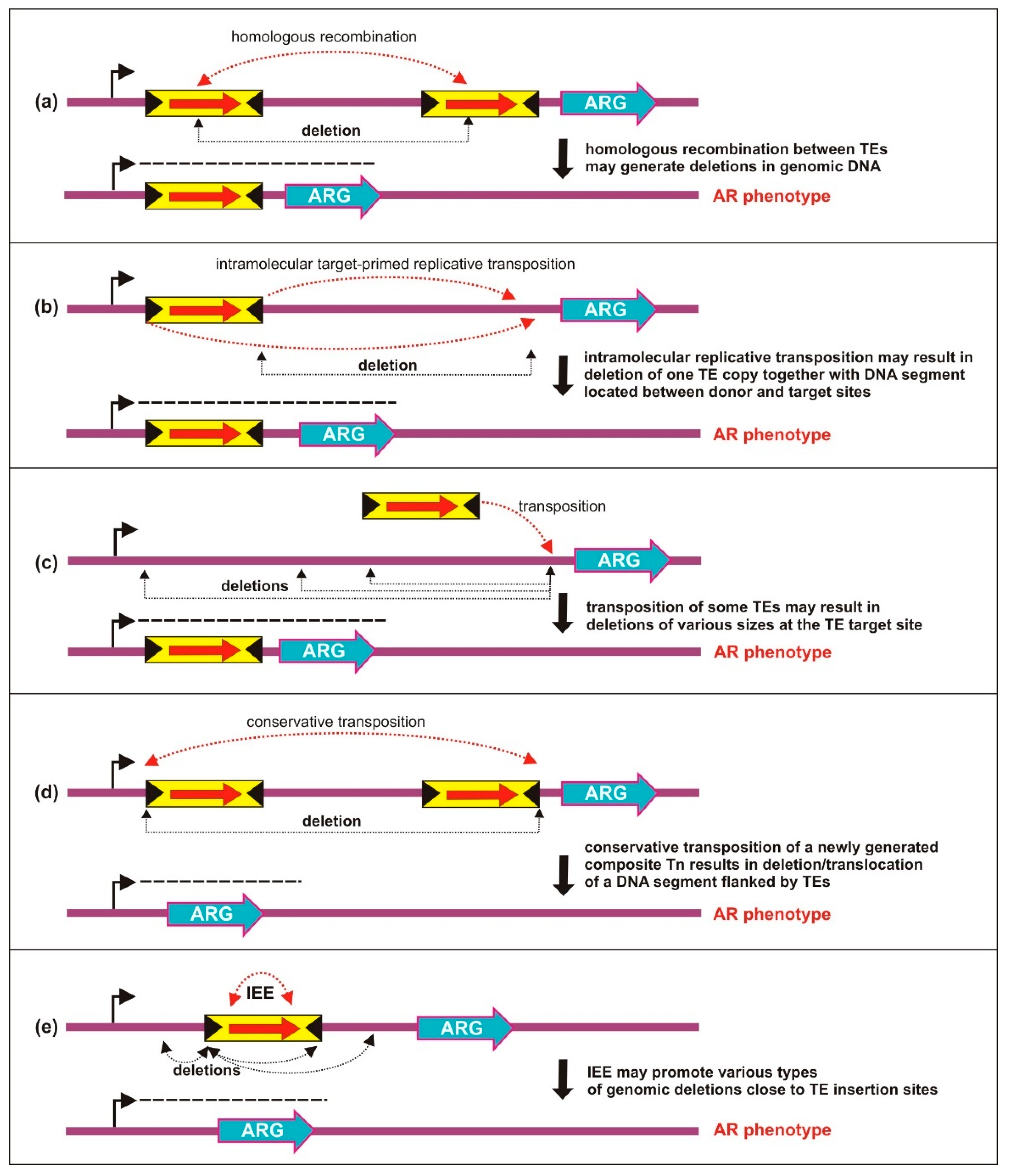
5. Activation of Silent AR Genes from Distant Promoters
5.1. Deletions Generated by Homologous Recombination
5.2. Deletions Resulting from Transposition of TEs
5.2.1. Intramolecular Target-Primed Transposition
5.2.2. Intramolecular Transposition of IS Dimers
5.2.3. Intramolecular Transposition of Composite Tns
5.2.4. Deletions Induced by IS Excision Enhancer Protein
6. Transient Trans-Replicon Activation of Silent AR Genes
7. Activation of AR Genes Carried by TEs
7.1. Composite Tns
7.2. Non-Composite Tns
7.3. TMos and ISCR-like Elements
8. Promoter Strength
9. Promoter Fixation
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- WHO. Global action plan on antimicrobial resistance. In WHO Report; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.J. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 2007, 13, 5–18. [Google Scholar] [CrossRef]
- MacLean, R.C.; San Millan, A. The evolution of antibiotic resistance. Science 2019, 365, 1082–1083. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Mahillon, J. Transposons as gene haulers. APMIS Suppl. 1998, 84, 29–36. [Google Scholar] [CrossRef]
- Aziz, R.K.; Breitbart, M.; Edwards, R.A. Transposases are the most abundant, most ubiquitous genes in nature. Nucleic Acids Res. 2010, 38, 4207–4217. [Google Scholar] [CrossRef]
- Touchon, M.; Rocha, E.P. Causes of insertion sequences abundance in prokaryotic genomes. Mol. Biol. Evol. 2007, 24, 969–981. [Google Scholar] [CrossRef]
- Genilloud, O.; Blázquez, J.; Mazodier, P.; Moreno, F. A clinical isolate of transposon Tn5 expressing streptomycin resistance in Escherichia coli. J. Bacteriol. 1988, 170, 1275–1278. [Google Scholar] [CrossRef]
- Teo, J.W.; Tan, T.M.; Poh, C.L. Genetic determinants of tetracycline resistance in Vibrio harveyi. Antimicrob. Agents Chemother. 2002, 46, 1038–1045. [Google Scholar] [CrossRef]
- Partridge, S.R. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol. Rev. 2011, 35, 820–855. [Google Scholar] [CrossRef]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Van Houdt, R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef]
- Stasiak, M.; Maćkiw, E.; Kowalska, J.; Kucharek, K.; Postupolski, J. Silent genes: Antimicrobial resistance and antibiotic production. Pol. J. Microbiol. 2021, 70, 421–429. [Google Scholar] [CrossRef]
- Navarre, W.W.; Porwollik, S.; Wang, Y.; McClelland, M.; Rosen, H.; Libby, S.J.; Fang, F.C. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 2006, 313, 236–238. [Google Scholar] [CrossRef]
- Qin, L.; Erkelens, A.M.; Ben Bdira, F.; Dame, R.T. The architects of bacterial DNA bridges: A structurally and functionally conserved family of proteins. Open Biol. 2019, 9, 190223. [Google Scholar] [CrossRef]
- Lanz, R.; Kuhnert, P.; Boerlin, P. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 2003, 91, 73–84. [Google Scholar] [CrossRef]
- Walsh, T.R. The emergence and implications of metallo-beta-lactamases in Gram-negative bacteria. Clin. Microbiol. Infect. 2005, 11 (Suppl. S6), 2–9. [Google Scholar] [CrossRef]
- Deekshit, V.K.; Kumar, B.K.; Rai, P.; Srikumar, S.; Karunasagar, I.; Karunasagar, I. Detection of class 1 integrons in Salmonella Weltevreden and silent antibiotic resistance genes in some seafood-associated nontyphoidal isolates of Salmonella in south-west coast of India. J. Appl. Microbiol. 2012, 112, 1113–1122. [Google Scholar] [CrossRef]
- Jiang, Y.; Yao, L.; Li, F.; Tan, Z.; Zhai, Y.; Wang, L. Characterization of antimicrobial resistance of Vibrio parahaemolyticus from cultured sea cucumbers (Apostichopus japonicas). Lett. Appl. Microbiol. 2014, 59, 147–154. [Google Scholar] [CrossRef]
- Yan, J.J.; Ko, W.C.; Tsai, S.H.; Wu, H.M.; Wu, J.J. Outbreak of infection with multidrug-resistant Klebsiella pneumoniae carrying bla(IMP-8) in a university medical center in Taiwan. J. Clin. Microbiol. 2001, 39, 4433–4439. [Google Scholar] [CrossRef]
- Mazel, D. Integrons: Agents of bacterial evolution. Nat. Rev. Microbiol. 2006, 4, 608–620. [Google Scholar] [CrossRef]
- Nagy, Z.; Chandler, M. Regulation of transposition in bacteria. Res. Microbiol. 2004, 155, 387–398. [Google Scholar] [CrossRef]
- Chandler, M.; Mahillon, J. Insertion sequences revisited. In Mobile DNA II; Craig, N.L., Cragie, R., Gellert, M., Lambowitz, A.M., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 303–366. [Google Scholar]
- Roberts, A.P.; Chandler, M.; Courvalin, P.; Guédon, G.; Mullany, P.; Pembroke, T.; Rood, J.I.; Smith, C.J.; Summers, A.O.; Tsuda, M.; et al. Revised nomenclature for transposable genetic elements. Plasmid 2008, 60, 167–173. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Varani, A.; Ton-Hoang, B.; Chandler, M. Everyman’s guide to bacterial insertion sequences. In Mobile DNA III; Craig, N.L., Chandler, M., Gellert, M., Lambowitz, A.M., Rice, P.A., Sandmeyer, S.B., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 555–590. [Google Scholar]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Known knowns, known unknowns and unknown unknowns in prokaryotic transposition. Curr. Opin. Microbiol. 2017, 38, 171–180. [Google Scholar] [CrossRef]
- Bartosik, D.; Putyrski, M.; Dziewit, L.; Malewska, E.; Szymanik, M.; Jagiello, E.; Lukasik, J.; Baj, J. Transposable modules generated by a single copy of insertion sequence ISPme1 and their influence on structure and evolution of natural plasmids of Paracoccus methylutens DM12. J. Bacteriol. 2008, 190, 3306–3313. [Google Scholar] [CrossRef]
- Szuplewska, M.; Bartosik, D. Identification of a mosaic transposable element of Paracoccus marcusii composed of insertion sequence ISPmar4 (ISAs1 family) and an IS1247a-driven transposable module (TMo). FEMS Microbiol. Lett. 2009, 292, 216–221. [Google Scholar] [CrossRef]
- Toleman, M.A.; Bennett, P.M.; Walsh, T.R. ISCR elements: Novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 2006, 70, 296–316. [Google Scholar] [CrossRef]
- Nicolas, E.; Lambin, M.; Dandoy, D.; Galloy, C.; Nguyen, N.; Oger, C.A.; Hallet, B. The Tn3-family of replicative transposons. In Mobile DNA III; Craig, N.L., Chandler, M., Gellert, M., Lambowitz, A.M., Rice, P.A., Sandmeyer, S.B., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 693–726. [Google Scholar]
- Delihas, N. Impact of small repeat sequences on bacterial genome evolution. Genome Biol. Evol. 2011, 3, 959–973. [Google Scholar] [CrossRef]
- Hickman, A.B.; Dyda, F. Mechanisms of DNA transposition. In Mobile DNA III; Craig, N.L., Chandler, M., Gellert, M., Lambowitz, A.M., Rice, P.A., Sandmeyer, S.B., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 529–553. [Google Scholar]
- Vandecraen, J.; Monsieurs, P.; Mergeay, M.; Leys, N.; Aertsen, A.; Van Houdt, R. Zinc-induced transposition of insertion sequence elements contributes to increased adaptability of Cupriavidus metallidurans. Front. Microbiol. 2016, 7, 359. [Google Scholar] [CrossRef]
- Podglajen, I.; Breuil, J.; Collatz, E. Insertion of a novel DNA sequence, 1S1186, upstream of the silent carbapenemase gene cfiA, promotes expression of carbapenem resistance in clinical isolates of Bacteroides fragilis. Mol. Microbiol. 1994, 12, 105–114. [Google Scholar] [CrossRef]
- Simpson, A.E.; Skurray, R.A.; Firth, N. An IS257-derived hybrid promoter directs transcription of a tetA(K) tetracycline resistance gene in the Staphylococcus aureus chromosomal mec region. J. Bacteriol. 2000, 182, 3345–3352. [Google Scholar] [CrossRef][Green Version]
- Lartigue, M.F.; Poirel, L.; Nordmann, P. Diversity of genetic environment of bla(CTX-M) genes. FEMS Microbiol. Lett. 2004, 234, 201–207. [Google Scholar] [CrossRef]
- Sóki, J.; Gal, M.; Brazier, J.S.; Rotimi, V.O.; Urbán, E.; Nagy, E.; Duerden, B.I. Molecular investigation of genetic elements contributing to metronidazole resistance in Bacteroides strains. J. Antimicrob. Chemother. 2006, 57, 212–220. [Google Scholar] [CrossRef]
- Héritier, C.; Poirel, L.; Nordmann, P. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 2006, 12, 123–130. [Google Scholar] [CrossRef]
- Aubert, D.; Naas, T.; Héritier, C.; Poirel, L.; Nordmann, P. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of beta-lactam resistance genes. J. Bacteriol. 2006, 188, 6506–6514. [Google Scholar] [CrossRef]
- Podglajen, I.; Breuil, J.; Rohaut, A.; Monsempes, C.; Collatz, E. Multiple mobile promoter regions for the rare carbapenem resistance gene of Bacteroides fragilis. J. Bacteriol. 2001, 183, 3531–3535. [Google Scholar] [CrossRef][Green Version]
- Kato, N.; Yamazoe, K.; Han, C.G.; Ohtsubo, E. New insertion sequence elements in the upstream region of cfiA in imipenem-resistant Bacteroides fragilis strains. Antimicrob. Agents Chemother. 2003, 47, 979–985. [Google Scholar] [CrossRef]
- Segal, H.; Nelson, E.C.; Elisha, B.G. Genetic environment and transcription of ampC in an Acinetobacter baumannii clinical isolate. Antimicrob. Agents Chemother. 2004, 48, 612–614. [Google Scholar] [CrossRef]
- Corvec, S.; Caroff, N.; Espaze, E.; Giraudeau, C.; Drugeon, H.; Reynaud, A. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J. Antimicrob. Chemother. 2003, 52, 629–635. [Google Scholar] [CrossRef]
- Wachino, J.; Yamane, K.; Kimura, K.; Shibata, N.; Suzuki, S.; Ike, Y.; Arakawa, Y. Mode of transposition and expression of 16S rRNA methyltransferase gene rmtC accompanied by ISEcp1. Antimicrob. Agents Chemother. 2006, 50, 3212–3215. [Google Scholar] [CrossRef] [PubMed]
- Dziewit, L.; Baj, J.; Szuplewska, M.; Maj, A.; Tabin, M.; Czyzkowska, A.; Skrzypczyk, G.; Adamczuk, M.; Sitarek, T.; Stawinski, P.; et al. Insights into the transposable mobilome of Paracoccus spp. (Alphaproteobacteria). PLoS ONE 2012, 7, e32277. [Google Scholar] [CrossRef] [PubMed]
- Zerbib, D.; Polard, P.; Escoubas, J.M.; Galas, D.; Chandler, M. The regulatory role of the IS1-encoded InsA protein in transposition. Mol. Microbiol. 1990, 4, 471–477. [Google Scholar] [CrossRef]
- Escoubas, J.M.; Prère, M.F.; Fayet, O.; Salvignol, I.; Galas, D.; Zerbib, D.; Chandler, M. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 1991, 10, 705–712. [Google Scholar] [CrossRef]
- Vigil-Stenman, T.; Ininbergs, K.; Bergman, B.; Ekman, M. High abundance and expression of transposases in bacteria from the Baltic Sea. ISME J. 2017, 11, 2611–2623. [Google Scholar] [CrossRef]
- Luque, I.; Andújar, A.; Jia, L.; Zabulon, G.; de Marsac, N.T.; Flores, E.; Houmard, J. Regulated expression of glutamyl-tRNA synthetase is directed by a mobile genetic element in the cyanobacterium Tolypothrix sp. PCC 7601. Mol. Microbiol. 2006, 60, 1276–1288. [Google Scholar] [CrossRef]
- Carlson, P.E., Jr.; Horzempa, J.; O’Dee, D.M.; Robinson, C.M.; Neophytou, P.; Labrinidis, A.; Nau, G.J. Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J. Bacteriol. 2009, 191, 6855–6864. [Google Scholar] [CrossRef]
- Bartosik, D.; Sochacka, M.; Baj, J. Identification and characterization of transposable elements of Paracoccus pantotrophus. J. Bacteriol. 2003, 185, 3753–3763. [Google Scholar] [CrossRef][Green Version]
- Ludvigsen, J.; Amdam, G.V.; Rudi, K.; L’Abée-Lund, T.M. Detection and characterization of streptomycin resistance (strA-strB) in a honeybee gut symbiont (Snodgrassella alvi) and the associated risk of antibiotic resistance transfer. Microb. Ecol. 2018, 76, 588–591. [Google Scholar] [CrossRef]
- L’Abée-Lund, T.M.; Sørum, H. Functional Tn5393-like transposon in the R plasmid pRAS2 from the fish pathogen Aeromonas salmonicida subspecies salmonicida isolated in Norway. Appl. Environ. Microbiol. 2000, 66, 5533–5535. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Q.; Jin, L.; Guo, Y.; Yin, Y.; Wang, R.; Bi, L.; Zhang, R.; Han, Y.; Wang, H. Identification of multiple transfer units and novel subtypes of tmexCD-toprJ gene clusters in clinical carbapenem-resistant Enterobacter cloacae and Klebsiella oxytoca. J. Antimicrob. Chemother. 2022, 77, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.; Fayet, O.; Rousseau, P.; Hoang, B.T.; Duval-Valentin, G. Copy-out—Paste-in transposition of IS911: A major transposition pathway. In Mobile DNA III; Craig, N.L., Chandler, M., Gellert, M., Lambowitz, A.M., Rice, P.A., Sandmeyer, S.B., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 591–607. [Google Scholar]
- Ton-Hoang, B.; Bétermier, M.; Polard, P.; Chandler, M. Assembly of a strong promoter following IS911 circularization and the role of circles in transposition. EMBO J. 1997, 16, 3357–3371. [Google Scholar] [CrossRef] [PubMed]
- Duval-Valentin, G.; Normand, C.; Khemici, V.; Marty, B.; Chandler, M. Transient promoter formation: A new feedback mechanism for regulation of IS911 transposition. EMBO J. 2001, 20, 5802–5811. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.A.; Gadura, N.; Greene, M.; Saby, R.; Grindley, N.D. The basis of asymmetry in IS2 transposition. Mol. Microbiol. 2001, 42, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.C.; Chen, H.W.; Tsai, Y.K.; Kuo, Y.C.; Lin, C.F.; Kuo, S.Y.; Lin, T.H. Formation of an inverted repeat junction in the transposition of insertion sequence ISLC3 isolated from Lactobacillus casei. Microbiology 2008, 154, 1047–1058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reimmann, C.; Moore, R.; Little, S.; Savioz, A.; Willetts, N.S.; Haas, D. Genetic structure, function and regulation of the transposable element IS21. Mol. Gen. Genet. 1989, 215, 416–424. [Google Scholar] [CrossRef]
- Kiss, J.; Olasz, F. Formation and transposition of the covalently closed IS30 circle: The relation between tandem dimers and monomeric circles. Mol. Microbiol. 1999, 34, 37–52. [Google Scholar] [CrossRef]
- Prudhomme, M.; Turlan, C.; Claverys, J.P.; Chandler, M. Diversity of Tn4001 transposition products: The flanking IS256 elements can form tandem dimers and IS circles. J. Bacteriol. 2002, 184, 433–443. [Google Scholar] [CrossRef]
- Partridge, S.R.; Hall, R.M. The IS1111 family members IS4321 and IS5075 have subterminal inverted repeats and target the terminal inverted repeats of Tn21 family transposons. J. Bacteriol. 2003, 185, 6371–6384. [Google Scholar] [CrossRef]
- Goussard, S.; Sougakoff, W.; Mabilat, C.; Bauernfeind, A.; Courvalin, P. An IS1-like element is responsible for high-level synthesis of extended-spectrum beta-lactamase TEM-6 in Enterobacteriaceae. J. Gen. Microbiol. 1991, 137, 2681–2687. [Google Scholar] [CrossRef]
- Jaurin, B.; Normark, S. Insertion of IS2 creates a novel ampC promoter in Escherichia coli. Cell 1983, 32, 809–816. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Stork, M.; Tolmasky, M.E.; Actis, L.A.; Farrell, D.; Welch, T.J.; Crosa, L.M.; Wertheimer, A.M.; Chen, Q.; Salinas, P.; et al. Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J. Bacteriol. 2003, 185, 5822–5830. [Google Scholar] [CrossRef]
- Jones, L.A.; McIver, C.J.; Kim, M.J.; Rawlinson, W.D.; White, P.A. The aadB gene cassette is associated with blaSHV genes in Klebsiella species producing extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 2005, 49, 794–797. [Google Scholar] [CrossRef]
- Rogers, M.B.; Bennett, T.K.; Payne, C.M.; Smith, C.J. Insertional activation of cepA leads to high-level beta-lactamase expression in Bacteroides fragilis clinical isolates. J. Bacteriol. 1994, 176, 4376–4384. [Google Scholar] [CrossRef][Green Version]
- Dalrymple, B. Novel rearrangements of IS30 carrying plasmids leading to the reactivation of gene expression. Mol. Gen. Genet. 1987, 207, 413–420. [Google Scholar] [CrossRef]
- Maki, H.; Murakami, K. Formation of potent hybrid promoters of the mutant llm gene by IS256 transposition in methicillin-resistant Staphylococcus aureus. J. Bacteriol. 1997, 179, 6944–6948. [Google Scholar] [CrossRef][Green Version]
- Couto, I.; Wu, S.W.; Tomasz, A.; de Lencastre, H. Development of methicillin resistance in clinical isolates of Staphylococcus sciuri by transcriptional activation of the mecA homologue native to species. J. Bacteriol. 2003, 185, 645–653. [Google Scholar] [CrossRef]
- López de Felipe, F.; Magni, C.; de Mendoza, D.; López, P. Transcriptional activation of the citrate permease P gene of Lactococcus lactis biovar diacetylactis by an insertion sequence-like element present in plasmid pCIT264. Mol. Gen. Genet. 1996, 250, 428–436. [Google Scholar] [CrossRef]
- Zerbib, D.; Gamas, P.; Chandler, M.; Prentki, P.; Bass, S.; Galas, D. Specificity of insertion of IS1. J. Mol. Biol. 1985, 185, 517–524. [Google Scholar] [CrossRef]
- Kivistik, P.A.; Kivisaar, M.; Hõrak, R. Target site selection of Pseudomonas putida transposon Tn4652. J. Bacteriol. 2007, 189, 3918–3921. [Google Scholar] [CrossRef]
- Lozinski, T.; Markiewicz, W.T.; Wyrzykiewicz, T.K.; Wierzchowski, K.L. Effect of the sequence-dependent structure of the 17 bp AT spacer on the strength of consensuslike E. coli promoters in vivo. Nucleic Acids Res. 1989, 17, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tolstorukov, M.; Zhurkin, V.; Garges, S.; Adhya, S. A mutant spacer sequence between -35 and -10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proc. Natl. Acad. Sci. USA 2004, 101, 6911–6916. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Saunders, N.J.; Jeffries, A.; Rest, R.F. Genome analysis and strain comparison of correia repeats and correia repeat-enclosed elements in pathogenic Neisseria. J. Bacteriol. 2002, 184, 6163–6173. [Google Scholar] [CrossRef] [PubMed]
- Prentki, P.; Teter, B.; Chandler, M.; Galas, D.J. Functional promoters created by the insertion of transposable element IS1. J. Mol. Biol. 1986, 191, 383–393. [Google Scholar] [CrossRef]
- Olliver, A.; Vallé, M.; Chaslus-Dancla, E.; Cloeckaert, A. Overexpression of the multidrug efflux operon acrEF by insertional activation with IS1 or IS10 elements in Salmonella enterica serovar Typhimurium DT204 acrB mutants selected with fluoroquinolones. Antimicrob. Agents Chemother. 2005, 49, 289–301. [Google Scholar] [CrossRef]
- Oliveira, P.H.; Prazeres, D.M.; Monteiro, G.A. Deletion formation mutations in plasmid expression vectors are unfavored by runaway amplification conditions and differentially selected under kanamycin stress. J. Biotechnol. 2009, 143, 231–238. [Google Scholar] [CrossRef]
- Jellen-Ritter, A.S.; Kern, W.V. Enhanced expression of the multidrug efflux pumps AcrAB and AcrEF associated with insertion element transposition in Escherichia coli mutants selected with a fluoroquinolone. Antimicrob. Agents Chemother. 2001, 45, 1467–1472. [Google Scholar] [CrossRef]
- Naas, T.; Philippon, L.; Poirel, L.; Ronco, E.; Nordmann, P. An SHV-derived extended-spectrum beta-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1999, 43, 1281–1284. [Google Scholar] [CrossRef][Green Version]
- Allmansberger, R.; Bräu, B.; Piepersberg, W. Genes for gentamicin-(3)-N-acetyl-transferases III and IV. II. Nucleotide sequences of three AAC(3)-III genes and evolutionary aspects. Mol. Gen. Genet. 1985, 198, 514–520. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Poirel, L.; Sampaio, J.L.; Nordmann, P. Complete sequence of broad-host-range plasmid pRIO-5 harboring the extended-spectrum-β-lactamase gene blaBES−1. Antimicrob. Agents Chemother. 2012, 56, 1116–1119. [Google Scholar] [CrossRef]
- Lee, K.Y.; Hopkins, J.D.; Syvanen, M. Direct involvement of IS26 in an antibiotic resistance operon. J. Bacteriol. 1990, 172, 3229–3236. [Google Scholar] [CrossRef]
- Mammeri, H.; Poirel, L.; Mangeney, N.; Nordmann, P. Chromosomal integration of a cephalosporinase gene from Acinetobacter baumannii into Oligella urethralis as a source of acquired resistance to beta-lactams. Antimicrob. Agents Chemother. 2003, 47, 1536–1542. [Google Scholar] [CrossRef]
- Chen, T.L.; Wu, R.C.; Shaio, M.F.; Fung, C.P.; Cho, W.L. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 2573–2580. [Google Scholar] [CrossRef]
- Leelaporn, A.; Firth, N.; Byrne, M.E.; Roper, E.; Skurray, R.A. Possible role of insertion sequence IS257 in dissemination and expression of high- and low-level trimethoprim resistance in staphylococci. Antimicrob. Agents Chemother. 1994, 38, 2238–2244. [Google Scholar] [CrossRef][Green Version]
- Naas, T.; Cuzon, G.; Truong, H.V.; Nordmann, P. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob. Agents Chemother. 2012, 56, 4753–4759. [Google Scholar] [CrossRef]
- Rudant, E.; Courvalin, P.; Lambert, T. Characterization of IS18, an element capable of activating the silent aac(6′)-Ij gene of Acinetobacter sp. 13 strain BM2716 by transposition. Antimicrob. Agents Chemother. 1998, 42, 2759–2761. [Google Scholar] [CrossRef][Green Version]
- Lopes, B.S.; Amyes, S.G.B. Role of ISAba1 and ISAba125 in governing the expression of blaADC in clinically relevant Acinetobacter baumannii strains resistant to cephalosporins. J. Med. Microbiol. 2012, 61, 1103–1108. [Google Scholar] [CrossRef]
- Jung, J.S.; Lee, H.Y.; Chung, J.H. Increased expression of the gentamicin resistance gene by a Tn3 sequence located at the upstream region. Mol. Cells 1998, 8, 201–204. [Google Scholar]
- Bayley, D.P.; Rocha, E.R.; Smith, C.J. Analysis of cepA and other Bacteroides fragilis genes reveals a unique promoter structure. FEMS Microbiol. Lett. 2000, 193, 149–154. [Google Scholar] [CrossRef]
- Evans, B.A.; Hamouda, A.; Amyes, S.G. The rise of carbapenem-resistant Acinetobacter baumannii. Curr. Pharm. Des. 2013, 19, 223–238. [Google Scholar] [CrossRef]
- Strateva, T.; Sirakov, I.; Stoeva, T.; Stratev, A.; Dimov, S.; Savov, E.; Mitov, I. Carbapenem-resistant Acinetobacter baumannii: Current status of the problem in four Bulgarian university hospitals (2014–2016). J. Glob. Antimicrob. Resist. 2019, 16, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Pham, S.C.; Ly, A.K.; Nguyen, C.V.V.; Vu, T.T.; Ha, T.M. Overexpression of blaOXA-58 gene driven by ISAba3 is associated with imipenem resistance in a clinical Acinetobacter baumannii isolate from Vietnam. Biomed. Res. Int. 2020, 2020, 7213429. [Google Scholar] [CrossRef] [PubMed]
- Ravasi, P.; Limansky, A.S.; Rodriguez, R.E.; Viale, A.M.; Mussi, M.A. ISAba825, a functional insertion sequence modulating genomic plasticity and bla(OXA-58) expression in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Chang, W.C.; Kuo, S.C.; Lee, Y.T.; Chen, C.P.; Siu, L.K.; Cho, W.L.; Fung, C.P. Contribution of a plasmid-borne blaOXA-58 gene with its hybrid promoter provided by IS1006 and an ISAba3-like element to beta-lactam resistance in acinetobacter genomic species 13TU. Antimicrob. Agents Chemother. 2010, 54, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, S.; Poirel, L.; Papa, A.; Koulourida, V.; Nordmann, P. Overexpression of the naturally occurring blaOXA-51 gene in Acinetobacter baumannii mediated by novel insertion sequence ISAba9. Antimicrob. Agents Chemother. 2009, 53, 4045–4047. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, C.K.; Lee, H.; Jeong, S.H.; Yong, D.; Lee, K. A novel insertion sequence, ISAba10, inserted into ISAba1 adjacent to the bla(OXA-23) gene and disrupting the outer membrane protein gene carO in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2006, 50, 1442–1448. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, J.; Zhou, H.; Jiang, Y.; Fu, Y.; Yu, Y.; Zhou, J. Characterization of a novel plasmid type and various genetic contexts of bla OXA-58 in Acinetobacter spp. from multiple cities in China. PLoS ONE 2014, 9, e84680. [Google Scholar] [CrossRef]
- Coleman, N.V.; Richardson-Harris, J.; Wilson, N.L.; Holmes, A.J. Insertion sequence ISPst4 activates pUC plasmid replication in Pseudomonas stutzeri. FEMS Microbiol. Lett. 2014, 356, 242–249. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Patterson, J.D.; Shoma, S.; Ginn, A.N.; Partridge, S.R.; Iredell, J.R. Relative strengths of promoters provided by common mobile genetic elements associated with resistance gene expression in gram-negative bacteria. Antimicrob. Agents Chemother. 2015, 59, 5088–5091. [Google Scholar] [CrossRef]
- Kallastu, A.; Hõrak, R.; Kivisaar, M. Identification and characterization of IS1411, a new insertion sequence which causes transcriptional activation of the phenol degradation genes in Pseudomonas putida. J. Bacteriol. 1998, 180, 5306–5312. [Google Scholar] [CrossRef]
- Zong, Z.; Partridge, S.R.; Iredell, J.R. ISEcp1-mediated transposition and homologous recombination can explain the context of bla(CTX-M-62) linked to qnrB2. Antimicrob. Agents Chemother. 2010, 54, 3039–3042. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, T.Y.; Xie, M.H.; Zhang, Y. Characterization of the variants, flanking genes, and promoter activity of the Leifsonia xyli subsp. cynodontis insertion sequence IS1237. J. Bacteriol. 2007, 189, 3217–3227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corvec, S.; Poirel, L.; Naas, T.; Drugeon, H.; Nordmann, P. Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-23 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Cabanne, L.; Vahaboglu, H.; Nordmann, P. Genetic environment and expression of the extended-spectrum beta-lactamase blaPER-1 gene in gram-negative bacteria. Antimicrob. Agents Chemother. 2005, 49, 1708–1713. [Google Scholar] [CrossRef]
- Aubert, D.; Naas, T.; Nordmann, P. IS1999 increases expression of the extended-spectrum beta-lactamase VEB-1 in Pseudomonas aeruginosa. J. Bacteriol. 2003, 185, 5314–5319. [Google Scholar] [CrossRef][Green Version]
- Haggoud, A.; Reysset, G.; Azeddoug, H.; Sebald, M. Nucleotide sequence analysis of two 5-nitroimidazole resistance determinants from Bacteroides strains and of a new insertion sequence upstream of the two genes. Antimicrob. Agents Chemother. 1994, 38, 1047–1051. [Google Scholar] [CrossRef]
- Trinh, S.; Haggoud, A.; Reysset, G.; Sebald, M. Plasmids pIP419 and pIP421 from Bacteroides: 5-nitroimidazole resistance genes and their upstream insertion sequence elements. Microbiology 1995, 141 Pt 4, 927–935. [Google Scholar] [CrossRef]
- Al-Hassan, L.; Opazo, A.; Lopes, B.S.; Mahallawy, H.E.; Amyes, S.G. Variations in IS6 promoters alter the expression of carbapenem resistance in related strains of Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2015, 3, 5–8. [Google Scholar] [CrossRef]
- Rasmussen, J.L.; Odelson, D.A.; Macrina, F.L. Complete nucleotide sequence of insertion element IS4351 from Bacteroides fragilis. J. Bacteriol. 1987, 169, 3573–3580. [Google Scholar] [CrossRef]
- Lopes, B.S.; Al-Hassan, L.; Amyes, S.G. ISAba825 controls the expression of the chromosomal bla(OXA-51-like) and the plasmid borne bla(OXA-58) gene in clinical isolates of Acinetobacter baumannii isolated from the USA. Clin. Microbiol. Infect. 2012, 18, E446–E451. [Google Scholar] [CrossRef]
- Karim, A.; Poirel, L.; Nagarajan, S.; Nordmann, P. Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 2001, 201, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Cao, V.; Lambert, T.; Courvalin, P. ColE1-like plasmid pIP843 of Klebsiella pneumoniae encoding extended-spectrum beta-lactamase CTX-M-17. Antimicrob. Agents Chemother. 2002, 46, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Decousser, J.W.; Nordmann, P. Insertion sequence ISEcp1B is involved in expression and mobilization of a bla(CTX-M) beta-lactamase gene. Antimicrob. Agents Chemother. 2003, 47, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, M.F.; Poirel, L.; Aubert, D.; Nordmann, P. In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring beta-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob. Agents Chemother. 2006, 50, 1282–1286. [Google Scholar] [CrossRef]
- Nakano, R.; Okamoto, R.; Nagano, N.; Inoue, M. Resistance to gram-negative organisms due to high-level expression of plasmid-encoded ampC beta-lactamase blaCMY-4 promoted by insertion sequence ISEcp1. J. Infect. Chemother. 2007, 13, 18–23. [Google Scholar] [CrossRef]
- Walsh, T.R.; Onken, A.; Haldorsen, B.; Toleman, M.A.; Sundsfjord, A. Characterization of a carbapenemase-producing clinical isolate of Bacteroides fragilis in Scandinavia: Genetic analysis of a unique insertion sequence. Scand. J. Infect. Dis. 2005, 37, 676–679. [Google Scholar] [CrossRef]
- Sóki, J.; Fodor, E.; Hecht, D.W.; Edwards, R.; Rotimi, V.O.; Kerekes, I.; Urbán, E.; Nagy, E. Molecular characterization of imipenem-resistant, cfiA-positive Bacteroides fragilis isolates from the USA, Hungary and Kuwait. J. Med. Microbiol. 2004, 53, 413–419. [Google Scholar] [CrossRef]
- Simons, R.W.; Hoopes, B.C.; McClure, W.R.; Kleckner, N. Three promoters near the termini of IS10: pIN, pOUT, and pIII. Cell 1983, 34, 673–682. [Google Scholar] [CrossRef]
- Arini, A.; Keller, M.P.; Arber, W. An antisense RNA in IS30 regulates the translational expression of the transposase. Biol. Chem. 1997, 378, 1421–1431. [Google Scholar] [CrossRef]
- Ellis, M.J.; Trussler, R.S.; Haniford, D.B. A cis-encoded sRNA, Hfq and mRNA secondary structure act independently to suppress IS200 transposition. Nucleic Acids Res. 2015, 43, 6511–6527. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hwang, J.; Yi, H.; Ulrich, R.L.; Yu, Y.; Nierman, W.C.; Kim, H.S. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathog. 2010, 6, e1000922. [Google Scholar] [CrossRef] [PubMed]
- Sommer, H.; Schumacher, B.; Saedler, H. A new type of IS1-mediated deletion. Mol. Gen. Genet. 1981, 184, 300–307. [Google Scholar] [CrossRef]
- Grindley, N.D.; Whiteson, K.L.; Rice, P.A. Mechanisms of site-specific recombination. Annu. Rev. Biochem. 2006, 75, 567–605. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.T. Generation of deletions and duplications using transposons as portable regions of homology with emphasis on mud and Tn10 transposons. Methods Enzymol. 2007, 421, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Pilla, G.; McVicker, G.; Tang, C.M. Genetic plasticity of the Shigella virulence plasmid is mediated by intra- and inter-molecular events between insertion sequences. PLoS Genet. 2017, 13, e1007014. [Google Scholar] [CrossRef]
- Sampson, S.L.; Warren, R.M.; Richardson, M.; Victor, T.C.; Jordaan, A.M.; van der Spuy, G.D.; van Helden, P.D. IS6110-mediated deletion polymorphism in the direct repeat region of clinical isolates of Mycobacterium tuberculosis. J. Bacteriol. 2003, 185, 2856–2866. [Google Scholar] [CrossRef]
- Shitikov, E.; Guliaev, A.; Bespyatykh, J.; Malakhova, M.; Kolchenko, S.; Smirnov, G.; Merker, M.; Niemann, S.; Mokrousov, I.; Ilina, E.; et al. The role of IS6110 in micro- and macroevolution of Mycobacterium tuberculosis lineage 2. Mol. Phylogenet. Evol. 2019, 139, 106559. [Google Scholar] [CrossRef]
- Turlan, C.; Chandler, M. IS1-mediated intramolecular rearrangements: Formation of excised transposon circles and replicative deletions. EMBO J. 1995, 14, 5410–5421. [Google Scholar] [CrossRef]
- He, S.; Hickman, A.B.; Varani, A.M.; Siguier, P.; Chandler, M.; Dekker, J.P.; Dyda, F. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 2015, 6, e00762. [Google Scholar] [CrossRef]
- Starlinger, P. IS elements and transposons. Plasmid 1980, 3, 241–259. [Google Scholar] [CrossRef]
- Grindley, N.D.F. The movement of Tn3-like elements: Transposition and cointegrate resolution. In Mobile DNA II; Craig, N.L., Cragie, R., Gellert, M., Lambowitz, A.M., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 272–302. [Google Scholar]
- Ahmed, A. Evidence for replicative transposition of Tn5 and Tn9. J. Mol. Biol. 1986, 191, 75–84. [Google Scholar] [CrossRef]
- Blackwell, G.A.; Nigro, S.J.; Hall, R.M. Evolution of AbGRI2-0, the progenitor of the AbGRI2 resistance island in global clone 2 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 60, 1421–1429. [Google Scholar] [CrossRef]
- He, S.; Chandler, M.; Varani, A.M.; Hickman, A.B.; Dekker, J.P.; Dyda, F. Mechanisms of evolution in high-consequence drug resistance plasmids. mBio 2016, 7, e01987-16. [Google Scholar] [CrossRef]
- Bernardi, F.; Bernardi, A. Intramolecular transposition of IS102. Gene 1986, 42, 11–19. [Google Scholar] [CrossRef]
- Bishop, R.; Sherratt, D. Transposon Tn1 intra-molecular transposition. Mol. Gen. Genet. 1984, 196, 117–122. [Google Scholar] [CrossRef]
- Berger, B.; Haas, D. Transposase and cointegrase: Specialized transposition proteins of the bacterial insertion sequence IS21 and related elements. Cell. Mol. Life Sci. 2001, 58, 403–419. [Google Scholar] [CrossRef]
- Olasz, F.; Stalder, R.; Arber, W. Formation of the tandem repeat (IS30)2 and its role in IS30-mediated transpositional DNA rearrangements. Mol. Gen. Genet. 1993, 239, 177–187. [Google Scholar] [CrossRef]
- Watanabe, S.; Ito, T.; Morimoto, Y.; Takeuchi, F.; Hiramatsu, K. Precise excision and self-integration of a composite transposon as a model for spontaneous large-scale chromosome inversion/deletion of the Staphylococcus haemolyticus clinical strain JCSC1435. J. Bacteriol. 2007, 189, 2921–2925. [Google Scholar] [CrossRef]
- Kleckner, N.; Chalmers, R.M.; Kwon, D.; Sakai, J.; Bolland, S. Tn10 and IS10 transposition and chromosome rearrangements: Mechanism and regulation in vivo and in vitro. Curr. Top. Microbiol. Immunol. 1996, 204, 49–82. [Google Scholar] [CrossRef]
- Kusumoto, M.; Ooka, T.; Nishiya, Y.; Ogura, Y.; Saito, T.; Sekine, Y.; Iwata, T.; Akiba, M.; Hayashi, T. Insertion sequence-excision enhancer removes transposable elements from bacterial genomes and induces various genomic deletions. Nat. Commun. 2011, 2, 152. [Google Scholar] [CrossRef]
- Wagner, A. Cooperation is fleeting in the world of transposable elements. PLoS Comput. Biol. 2006, 2, e162. [Google Scholar] [CrossRef]
- Rothstein, S.J.; Reznikoff, W.S. The functional differences in the inverted repeats of Tn5 are caused by a single base pair nonhomology. Cell 1981, 23, 191–199. [Google Scholar] [CrossRef]
- Yoon, E.J.; Goussard, S.; Touchon, M.; Krizova, L.; Cerqueira, G.; Murphy, C.; Lambert, T.; Grillot-Courvalin, C.; Nemec, A.; Courvalin, P. Origin in Acinetobacter guillouiae and dissemination of the aminoglycoside-modifying enzyme Aph(3′)-VI. mBio 2014, 5, e01972-14. [Google Scholar] [CrossRef]
- Lima-Mendez, G.; Oliveira Alvarenga, D.; Ross, K.; Hallet, B.; Van Melderen, L.; Varani, A.M.; Chandler, M. Toxin-antitoxin gene pairs found in Tn3 family transposons appear to be an integral part of the transposition module. mBio 2020, 11, e00452-20. [Google Scholar] [CrossRef]
- Sundin, G.W.; Bender, C.L. Expression of the strA-strB streptomycin resistance genes in Pseudomonas syringae and Xanthomonas campestris and characterization of IS6100 in X. campestris. Appl. Environ. Microbiol. 1995, 61, 2891–2897. [Google Scholar] [CrossRef]
- Smith, B.; Dyson, P. Inducible transposition in Streptomyces lividans of insertion sequence IS6100 from Mycobacterium fortuitum. Mol. Microbiol. 1995, 18, 933–941. [Google Scholar] [CrossRef]
- Liebert, C.A.; Hall, R.M.; Summers, A.O. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 1999, 63, 507–522. [Google Scholar] [CrossRef]
- Huang, J.; Hu, X.; Zhao, Y.; Shi, Y.; Ding, H.; Xv, J.; Ren, J.; Wu, R.; Zhao, Z. Genetic factors associated with enhanced bla(KPC) expression in Tn3/Tn4401 chimeras. Antimicrob. Agents Chemother. 2020, 64, e01836-19. [Google Scholar] [CrossRef]
- Petrova, M.; Gorlenko, Z.; Mindlin, S. Molecular structure and translocation of a multiple antibiotic resistance region of a Psychrobacter psychrophilus permafrost strain. FEMS Microbiol. Lett. 2009, 296, 190–197. [Google Scholar] [CrossRef]
- Dhanji, H.; Doumith, M.; Hope, R.; Livermore, D.M.; Woodford, N. ISEcp1-mediated transposition of linked blaCTX-M-3 and blaTEM-1b from the IncI1 plasmid pEK204 found in clinical isolates of Escherichia coli from Belfast, UK. J. Antimicrob. Chemother. 2011, 66, 2263–2265. [Google Scholar] [CrossRef]
- Poirel, L.; Lartigue, M.F.; Decousser, J.W.; Nordmann, P. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob. Agents Chemother. 2005, 49, 447–450. [Google Scholar] [CrossRef]
- Fang, L.X.; Li, X.P.; Li, L.; Chen, M.Y.; Wu, C.Y.; Li, L.L.; Liao, X.P.; Liu, Y.H.; Sun, J. ISEcp1-mediated transposition of chromosome-borne bla(CMY-2) into an endogenous ColE1-like plasmid in Escherichia coli. Infect. Drug Resist. 2018, 11, 995–1005. [Google Scholar] [CrossRef]
- Mendiola, M.V.; de la Cruz, F. IS91 transposase is related to the rolling-circle-type replication proteins of the pUB110 family of plasmids. Nucleic Acids Res. 1992, 20, 3521. [Google Scholar] [CrossRef][Green Version]
- Tavakoli, N.; Comanducci, A.; Dodd, H.M.; Lett, M.C.; Albiger, B.; Bennett, P. IS1294, a DNA element that transposes by RC transposition. Plasmid 2000, 44, 66–84. [Google Scholar] [CrossRef]
- Mendiola, M.V.; Bernales, I.; de la Cruz, F. Differential roles of the transposon termini in IS91 transposition. Proc. Natl. Acad. Sci. USA 1994, 91, 1922–1926. [Google Scholar] [CrossRef]
- Bardaji, L.; Añorga, M.; Echeverría, M.; Ramos, C.; Murillo, J. The toxic guardians—Multiple toxin-antitoxin systems provide stability, avoid deletions and maintain virulence genes of Pseudomonas syringae virulence plasmids. Mob. DNA 2019, 10, 7. [Google Scholar] [CrossRef]
- Toleman, M.A.; Walsh, T.R. Combinatorial events of insertion sequences and ICE in Gram-negative bacteria. FEMS Microbiol. Rev. 2011, 35, 912–935. [Google Scholar] [CrossRef]
- Shimada, T.; Yamazaki, Y.; Tanaka, K.; Ishihama, A. The whole set of constitutive promoters recognized by RNA polymerase RpoD holoenzyme of Escherichia coli. PLoS ONE 2014, 9, e90447. [Google Scholar] [CrossRef]
- Ma, L.; Siu, L.K.; Lu, P.L. Effect of spacer sequences between bla(CTX-M) and ISEcp1 on bla(CTX-M) expression. J. Med. Microbiol. 2011, 60, 1787–1792. [Google Scholar] [CrossRef]
- Typas, A.; Hengge, R. Role of the spacer between the -35 and -10 regions in sigmas promoter selectivity in Escherichia coli. Mol. Microbiol. 2006, 59, 1037–1051. [Google Scholar] [CrossRef]
- Potron, A.; Rondinaud, E.; Poirel, L.; Belmonte, O.; Boyer, S.; Camiade, S.; Nordmann, P. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int. J. Antimicrob. Agents 2013, 41, 325–329. [Google Scholar] [CrossRef]
- Naas, T.; Aubert, D.; Lambert, T.; Nordmann, P. Complex genetic structures with repeated elements, a sul-type class 1 integron, and the blaVEB extended-spectrum beta-lactamase gene. Antimicrob. Agents Chemother. 2006, 50, 1745–1752. [Google Scholar] [CrossRef]
- Razavi, M.; Kristiansson, E.; Flach, C.F.; Larsson, D.G.J. The association between insertion sequences and antibiotic resistance genes. mSphere 2020, 5, e00418-20. [Google Scholar] [CrossRef]
- Schreiber, F.; Szekat, C.; Josten, M.; Sahl, H.G.; Bierbaum, G. Antibiotic-induced autoactivation of IS256 in Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 6381–6384. [Google Scholar] [CrossRef][Green Version]



| IS/Tn Family | Element | Resulting Resistance to: | Gene | Organism | Reference |
|---|---|---|---|---|---|
| IS4 | ISAba1 | ceftazidime | ampC | A. baumannii | [40,45] |
| β-lactams (ticarcillin, piperacillin, aztreonam) | |||||
| cephalosporins (cefuroxime, cefoxitin, cefotaxime, ceftazidime, cephalothin) | ampC | A. baumannii | [44] | ||
| carbapenem | blaOXA-23 | A. baumannii | [110] | ||
| cephalosporins (ceftazidime, cefepime), gatifloxacin) | blaADC | A. baumannii | [93] | ||
| ISPa12 | β-lactams (amoxicillin, ticarcillin, piperacillin, cefuroxime, ceftazidime, cefotaxime, cefepime, aztreonam) | blaPER-1 | S. enterica | [111] | |
| (amoxicillin, ticarcillin, cefuroxime, ceftazidime, cefotaxime, cefepime, aztreonam) | P. aeruginosa | [111] | |||
| IS10 | fluoroquinolones (marbofloxacin, enrofloxacin, ciprofloxacin), florfenicol, erythromycin | acrEF | S. enterica | [81] | |
| IS1999 | ceftazidime | blaOXA-48 | K. pneumoniae | [41] | |
| blaVEB-1 | P. aeruginosa | [112] | |||
| IS5 | IS1168 | 5-nitroimidazole | nimA, nimB | Bacteroides | [39,113] |
| IS1169 | 5-nitroimidazole | nimD | B. fragilis | [114] | |
| IS1186 | carbapenem | cfiA | B. fragilis | [36] | |
| IS6 | IS257 | tetracycline | tetA(K) | S. aureus | [37] |
| IS1006 | imipenem, meropenem | blaOXA-58 | A. baumannii | [115] | |
| IS1008 | imipenem, meropenem | blaOXA-58 | A. baumannii | [115] | |
| IS30 | IS4351 | tetracycline, chloramphenicol | B. fragilis | [116] | |
| IS982 | IS1187 | carbapenem | cfiA | B. fragilis | [42] |
| ISAba4 | carbapenem | blaOXA-23 | A. baumannii | [110] | |
| ISAba825 | carbapenem | blaOXA-58-like, blaOXA-65 | A. baumannii | [117] | |
| IS1380 | ISEcp1 | β-lactams (amoxicillin, ticarcillin, piperacillin, cephalothin, cefoxitin, ceftazidime, cefotaxime, cefpirome, aztreonam) | blaCTX-M-15 | Enterobacteriaceae (E. coli, K. pneumoniae, Enterobacter aerogenes) | [118] |
| β-lactams (amoxicillin, piperacillin, cephalothin, cefuroxime, cefotaxime, aztreonam) | blaCTX-M-17 | K. pneumoniae | [119] | ||
| β-lactams | blaCTX-M-19 | K. pneumoniae | [120] | ||
| cefotaxime | blaCTX-M-2 | Kluyvera ascorbata | [121] | ||
| extended-spectrum cephalosporin (cephalothin, cefpodoxime, cefotaxime, ceftazidime, cefmetazole), ampicillin, aztreonam | blaCMY-4 | K. pneumoniae | [122] | ||
| aminoglycosides (gentamicin, streptomycin) | rmtC | E. coli | [46] | ||
| IS612 | imipenem | cfiA | B. fragilis | [43] | |
| IS613 | imipenem | cfiA | B. fragilis | [43] | |
| IS613-like | imipenem | cfiA | B. fragilis | [123] | |
| IS614 | imipenem | cfiA | B. fragilis | [43] | |
| IS615 | imipenem | cfiA | B. fragilis | [43] | |
| IS616 | imipenem | cfiA | B. fragilis | [43] | |
| IS942 | imipenem | cfiA | B. fragilis | [42] | |
| IS943 | imipenem | cfiA | B. fragilis | [124] | |
| IS1188 | carbapenem | cfiA | B. fragilis | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipszyc, A.; Szuplewska, M.; Bartosik, D. How Do Transposable Elements Activate Expression of Transcriptionally Silent Antibiotic Resistance Genes? Int. J. Mol. Sci. 2022, 23, 8063. https://doi.org/10.3390/ijms23158063
Lipszyc A, Szuplewska M, Bartosik D. How Do Transposable Elements Activate Expression of Transcriptionally Silent Antibiotic Resistance Genes? International Journal of Molecular Sciences. 2022; 23(15):8063. https://doi.org/10.3390/ijms23158063
Chicago/Turabian StyleLipszyc, Aleksander, Magdalena Szuplewska, and Dariusz Bartosik. 2022. "How Do Transposable Elements Activate Expression of Transcriptionally Silent Antibiotic Resistance Genes?" International Journal of Molecular Sciences 23, no. 15: 8063. https://doi.org/10.3390/ijms23158063
APA StyleLipszyc, A., Szuplewska, M., & Bartosik, D. (2022). How Do Transposable Elements Activate Expression of Transcriptionally Silent Antibiotic Resistance Genes? International Journal of Molecular Sciences, 23(15), 8063. https://doi.org/10.3390/ijms23158063






