Mechanical Compression by Simulating Orthodontic Tooth Movement in an In Vitro Model Modulates Phosphorylation of AKT and MAPKs via TLR4 in Human Periodontal Ligament Cells
Abstract
:1. Introduction
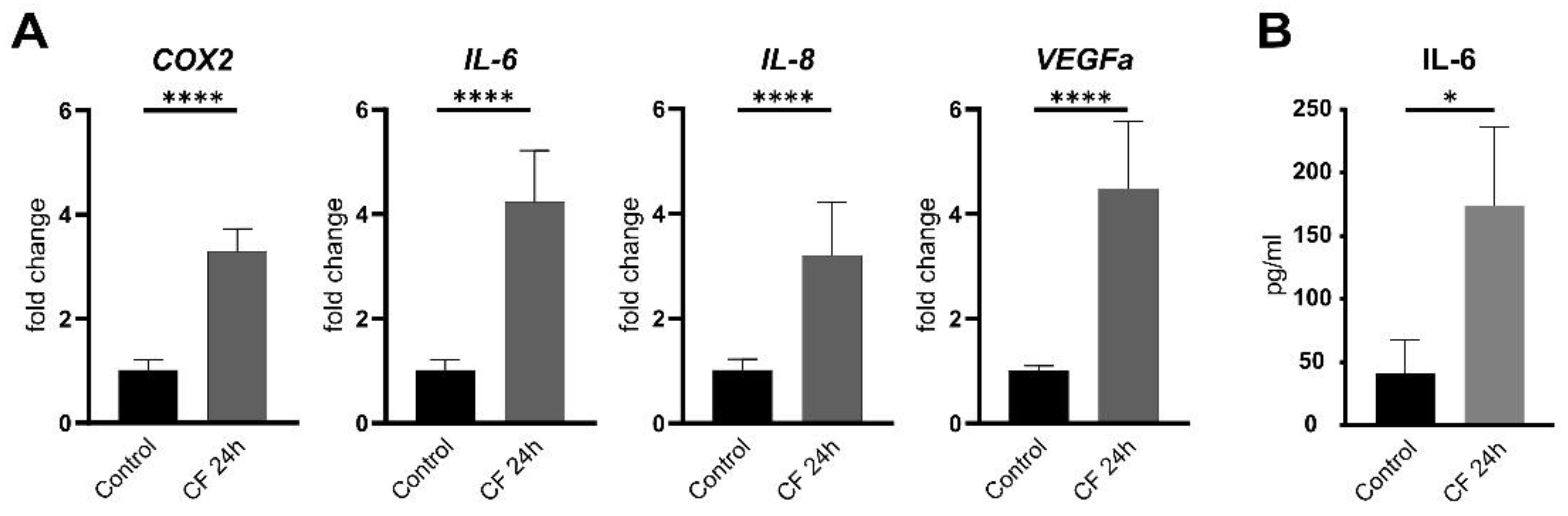
2. Results
2.1. Characterization of Primary Human PDL Cells
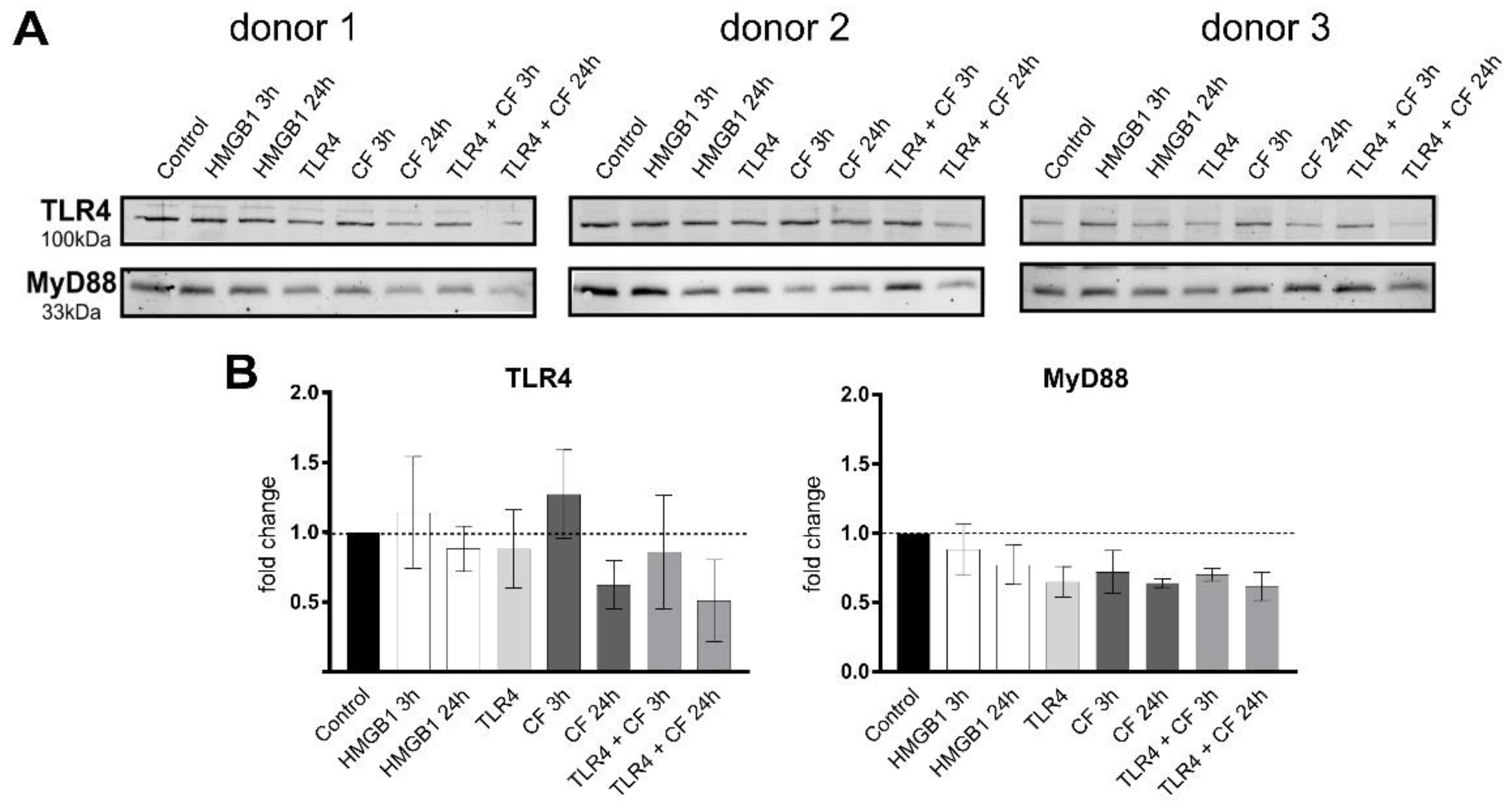
2.2. Changes in the TLR4 Production under Compressive Forces
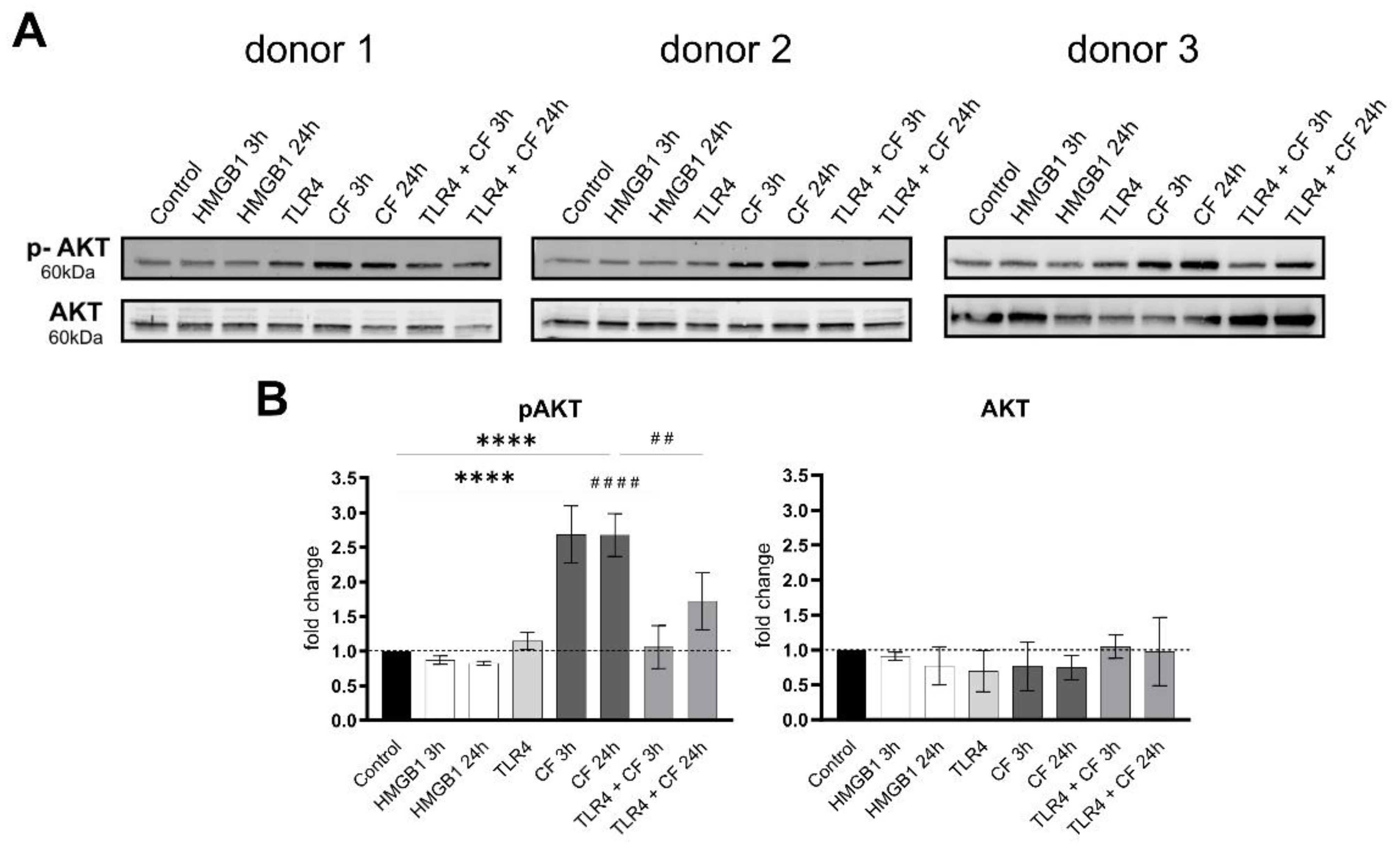
2.3. Phospho-AKT Was Upregulated by Compressive Force
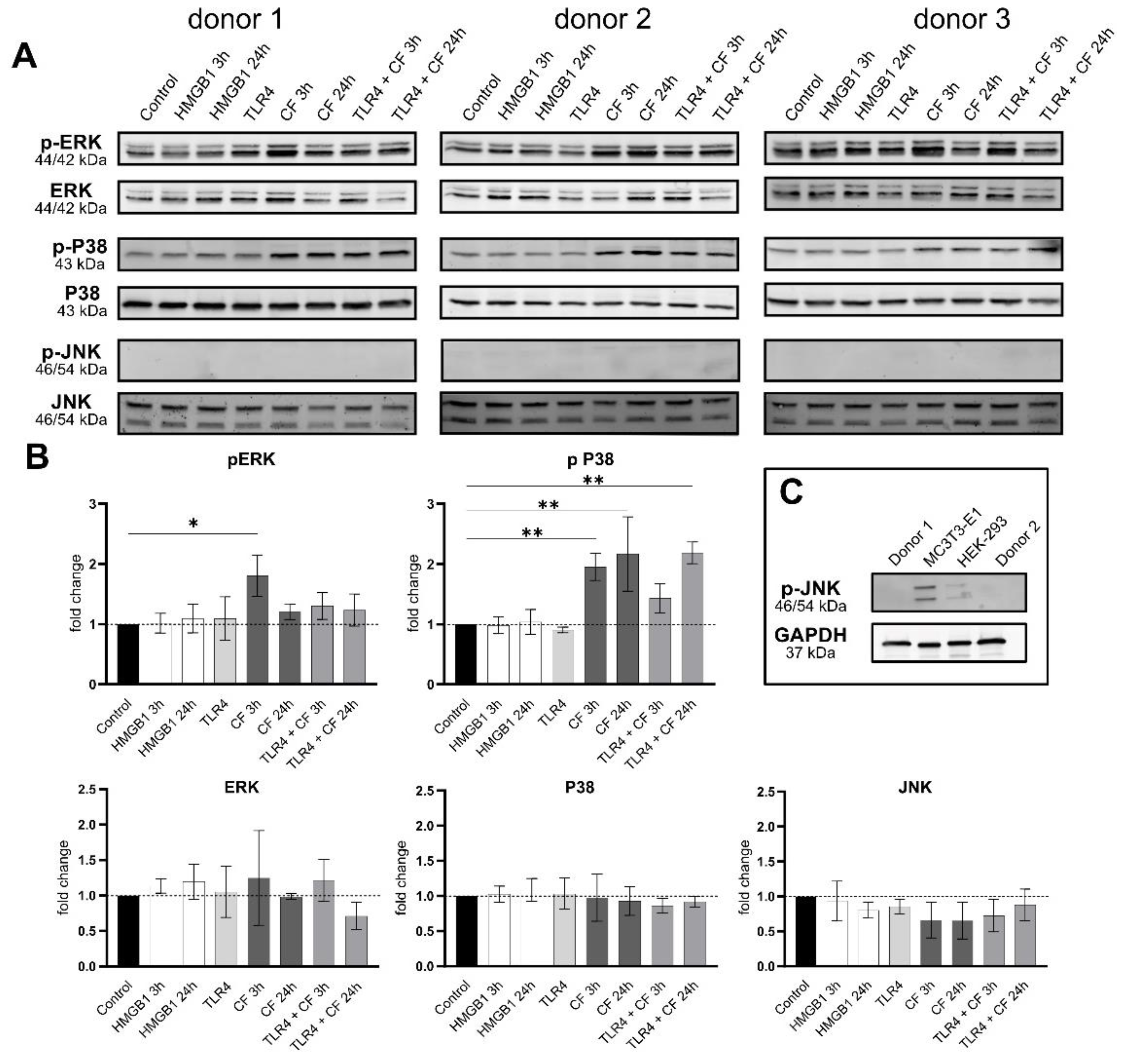
2.4. Phospho-ERK and Phospho-p38 Were Significantly Upregulated under Compressive Force
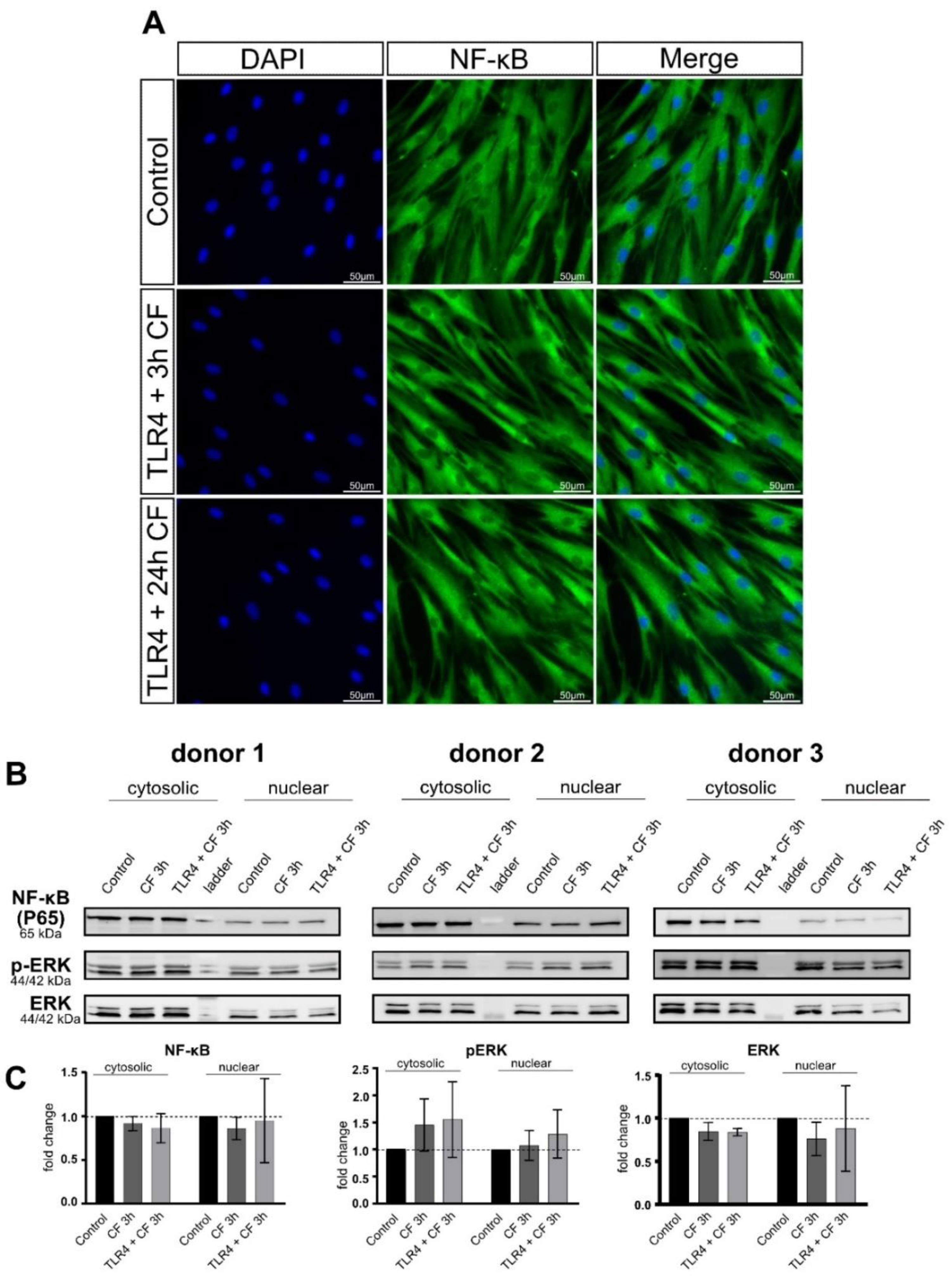
2.5. Moderate Compressive Forces on PDL Cells Is Not Able to Translocate NF-kB and ERK to the Nucleus
3. Discussion
4. Materials and Methods
4.1. Reagents and Methods
4.2. Primary Human Periodontal Ligament (hPDL) Cell Isolation
4.3. Characterization of hPDL Cells Flow Cytometry Analysis of Cell Surface Markers
4.4. PDL Cell Differentiation
4.5. Isolation and Purification of RNA
4.6. Quantitative Realtime-RT-PCR Analysis (RT-qPCR)
4.7. ELISA
4.8. In Vitro Compressive Stimulation Model
4.9. Isolation of Total Protein Respective Cytoplasmic and Nuclear Fractions
4.10. Immunoblotting Analysis
4.11. NF-kB/DAPI Staining
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richmond, S.; Shaw, W.C.; O’brien, K.D.; Buchanan, I.B.; Jones, R.; Stephens, C.D.; Roberts, C.T.; Andrews, M. The Development of the PAR Index (Peer Assessment Rating): Reliability and Validity; European Orthodontic Society: London, UK, 1992; Volume 14. [Google Scholar]
- Meikle, M.C. The Tissue, Cellular, and Molecular Regulation of Orthodontic Tooth Movement: 100 Years after Carl Sandstedt. Eur. J. Orthod. 2006, 28, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Beertsen, W.; Mcculloch, C.A.G.; Sodek, J. The Periodontal Ligament: A Unique, Multifunctional Connective Tissue. Periodontol. 2000 1997, 13, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Bresin, A.; Kiliaridis, S.; Strid, K.G. Effect of Masticatory Function on the Internal Bone Structure in the Mandible of the Growing Rat. Eur. J. Oral Sci. 1999, 107, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.J.; Merzel, J. Alveolar Bone Sharpey Fibers of the Rat Incisor in Normal and Altered Functional Conditions Examined by Scanning Electron Microscopy. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 279, 792–797. [Google Scholar] [CrossRef]
- Pagella, P.; de Vargas Roditi, L.; Stadlinger, B.; Moor, A.E.; Mitsiadis, T.A. A Single-Cell Atlas of Human Teeth. iScience 2021, 24, 102405. [Google Scholar] [CrossRef]
- Hlaing, E.E.H.; Ishihara, Y.; Wang, Z.; Odagaki, N.; Kamioka, H. Role of Intracellular Ca2+–Based Mechanotransduction of Human Periodontal Ligament Fibroblasts. FASEB J. 2019, 33, 10409–10424. [Google Scholar] [CrossRef] [Green Version]
- Marciniak, J.; Lossdörfer, S.; Knaup, I.; Bastian, A.; Craveiro, R.B.; Jäger, A.; Wolf, M. Orthodontic Cell Stress Modifies Proinflammatory Cytokine Expression in Human PDL Cells and Induces Immunomodulatory Effects via TLR-4 Signaling in Vitro. Clin. Oral Investig. 2020, 24, 1411–1419. [Google Scholar] [CrossRef]
- Brockhaus, J.; Craveiro, R.B.; Azraq, I.; Niederau, C.; Schröder, S.K.; Weiskirchen, R.; Jankowski, J.; Wolf, M. In Vitro Compression Model for Orthodontic Tooth Movement Modulates Human Periodontal Ligament Fibroblast Proliferation, Apoptosis and Cell Cycle. Biomolecules 2021, 11, 932. [Google Scholar] [CrossRef]
- Schröder, A.; Käppler, P.; Nazet, U.; Jantsch, J.; Proff, P.; Cieplik, F.; Deschner, J.; Kirschneck, C. Effects of Compressive and Tensile Strain on Macrophages during Simulated Orthodontic Tooth Movement. Mediat. Inflamm. 2020, 2020, 2814015. [Google Scholar] [CrossRef]
- Weider, M.; Schröder, A.; Docheva, D.; Rodrian, G.; Enderle, I.; Seidel, C.L.; Andreev, D.; Wegner, M.; Bozec, A.; Deschner, J.; et al. A Human Periodontal Ligament Fibroblast Cell Line as a New Model to Study Periodontal Stress. Int. J. Mol. Sci. 2020, 21, 7961. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorsa, T.; Tja¨derhane, L.T.; Salo, T. Matrix Metalloproteinases (MMPs) in Oral Diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Moscalu, M.; Goriuc, A.; Nucci, L.; Tatarciuc, M.; Martu, I.; Covasa, M. Using Salivary MMP-9 to Successfully Quantify Periodontal Inflammation during Orthodontic Treatment. J. Clin. Med. 2021, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P. Cytokine Expression Pattern in Compression and Tension Sides of the Periodontal Ligament during Orthodontic Tooth Movement in Humans. Eur. J. Oral Sci. 2007, 115, 355–362. [Google Scholar] [CrossRef]
- Konermann, A.; Beyer, M.; Deschner, J.; Allam, J.P.; Novak, N.; Winter, J.; Jepsen, S.; Jäger, A. Human Periodontal Ligament Cells Facilitate Leukocyte Recruitment and Are Influenced in Their Immunomodulatory Function by Th17 Cytokine Release. Cell. Immunol. 2012, 272, 137–143. [Google Scholar] [CrossRef]
- Konermann, A.; Stabenow, D.; Knolle, P.A.; Held, S.A.E.; Deschner, J.; Jäger, A. Regulatory Role of Periodontal Ligament Fibroblasts for Innate Immune Cell Function and Differentiation. Innate Immun. 2012, 18, 745–752. [Google Scholar] [CrossRef]
- Bartold, P.M.; McCulloch, C.A.G.; Narayanan, A.S.; Pitaru, S. Tissue Engineering: A New Paradigm for Periodontal Regeneration Based on Molecular and Cell Biology. Periodontol. 2000 2000, 24, 253–269. [Google Scholar] [CrossRef]
- Shimono, M.; Ishikawa, T.; Ishikawa, H.; Matsuzaki, H.; Hashimoto, S.; Muramatsu, T.; Shima, K.; Matsuzaka, K.I.; Inoue, T. Regulatory Mechanisms of Periodontal Regeneration. Microsc. Res. Tech. 2003, 60, 491–502. [Google Scholar] [CrossRef]
- Hayashi, S.I.; Tsuneto, M.; Yamada, T.; Nose, M.; Yoshino, M.; Shultz, L.D.; Yamazaki, H. Lipopolysaccharide-Induced Osteoclastogenesis in Src Homology 2-Domain Phosphatase-1-Deficient Viable Motheaten Mice. Endocrinology 2004, 145, 2721–2729. [Google Scholar] [CrossRef] [Green Version]
- AlQranei, M.S.; Senbanjo, L.T.; Aljohani, H.; Hamza, T.; Chellaiah, M.A. Lipopolysaccharide- TLR-4 Axis Regulates Osteoclastogenesis Independent of RANKL/RANK Signaling. BMC Immunol. 2021, 22, 23. [Google Scholar] [CrossRef]
- Yim, M. The Role of Toll-like Receptors in Osteoclastogenesis. J. Bone Metab. 2020, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, B.S.; Kim, J.I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, M.; Lossdörfer, S.; Römer, P.; Kirschneck, C.; Küpper, K.; Deschner, J.; Jäger, A. Short-Term Heat Pre-Treatment Modulates the Release of HMGB1 and pro-Inflammatory Cytokines in HPDL Cells Following Mechanical Loading and Affects Monocyte Behavior. Clin. Oral Investig. 2016, 20, 923–931. [Google Scholar] [CrossRef]
- Kanzaki, H.; Nakamura, Y. Orthodontic Tooth Movement and HMGB1. J. Oral Biosci. 2018, 60, 49–53. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of Chromatin Protein HMGB1 by Necrotic Cells Triggers Inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Chiba, M.; Shimizu, Y.; Mitani, H. Periodontal Ligament Cells under Mechanical Stress Induce Osteoclastogenesis by Receptor Activator of Nuclear Factor ΚB Ligand Up-Regulation via Prostaglandin E2 Synthesis. J. Bone Miner. Res. 2002, 17, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Brasier, A.R. The Nuclear Factor-B-Interleukin-6 Signalling Pathway Mediating Vascular Inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Kircheis, R.; Haasbach, E.; Lueftenegger, D.; Heyken, W.T.; Ocker, M.; Planz, O. NF-ΚB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front. Immunol. 2020, 11, 598444. [Google Scholar] [CrossRef]
- Janjic, M.; Docheva, D.; Trickovic Janjic, O.; Wichelhaus, A.; Baumert, U. In Vitro Weight-Loaded Cell Models for Understanding Mechanodependent Molecular Pathways Involved in Orthodontic Tooth Movement: A Systematic Review. Stem Cells Int. 2018, 2018, 3208285. [Google Scholar] [CrossRef] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–184. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Lele, T.P. Periodontal Cell Mechanotransduction. Open Biol. 2018, 8, 180053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Li, M. Functions of Periostin in Dental Tissues and Its Role in Periodontal Tissues’ Regeneration. Cell. Mol. Life Sci. 2017, 74, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Belgardt, E.; Steinberg, T.; Husari, A.; Dieterle, M.P.; Hülter-Hassler, D.; Jung, B.; Tomakidi, P. Force-Responsive Zyxin Modulation in Periodontal Ligament Cells Is Regulated by YAP Rather than TAZ. Cell Signal 2020, 72, 109662. [Google Scholar] [CrossRef] [PubMed]
- Premaraj, S.; Souza, I.; Premaraj, T. Mechanical Loading Activates β-Catenin Signaling in Periodontal Ligament Cells. Angle Orthod. 2011, 81, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, N.; Alonso, A.; Steinberg, T.; Woodnutt, D.; Kohl, A.; Müssig, E.; Schulz, S.; Tomakidi, P. Mechano-Transduction in Periodontal Ligament Cells Identifies Activated States of MAP-Kinases P42/44 and P38-Stress Kinase as a Mechanism for MMP-13 Expression. BMC Cell Biol. 2010, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, J.; Tamai, R.; Sugiyama, A.; Akashi, S.; Sugawara, S.; Takada, H. Contrasting Responses of Human Gingival and Periodontal Ligament Fibroblasts to Bacterial Cell-Surface Components through the CD14/Toll-like Receptor System. Oral Microbiol. Immunol. 2003, 18, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhou, X.; Wang, Q.; Zhang, L.; Wang, Y.; Li, X.; Huang, D. Expression of TRAF6 and Pro-Inflammatory Cytokines through Activation of TLR2, TLR4, NOD1, and NOD2 in Human Periodontal Ligament Fibroblasts. Arch Oral Biol. 2011, 56, 1064–1072. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal Ligament Stem Cells: Current Status, Concerns, and Future Prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef] [Green Version]
- Andersson, U.; Tracey, K.J. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.; Lossdörfer, S.; Küpper, K.; Jäger, A. Regulation of High Mobility Group Box Protein 1 Expression Following Mechanical Loading by Orthodontic Forces in Vitro and in Vivo. Eur. J. Orthod. 2014, 36, 624–631. [Google Scholar] [CrossRef]
- Wolf, M.; Lossdörfer, S.; Römer, P.; Bastos Craveiro, R.; Deschner, J.; Jäger, A. Anabolic Properties of High Mobility Group Box Protein-1 in Human Periodontal Ligament Cells in Vitro. Mediat. Inflamm. 2014, 2014, 347585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, Y.; Zmijewski, J.; Xu, Z.; Abraham, E. HMGB1 Develops Enhanced Proinflammatory Activity by Binding to Cytokines. J. Immunol. 2008, 180, 2531–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, M.E. HMGB1 Loves Company. J. Leukoc. Biol. 2009, 86, 573–576. [Google Scholar] [CrossRef]
- Rouhiainen, A.; Tumova, S.; Valmu, L.; Kalkkinen, N.; Rauvala, H. Analysis of Proinflammatory Activity of Highly Purified Eukaryotic Recombinant HMGB1 (Amphoterin). J. Leukoc. Biol. 2007, 81, 49–58. [Google Scholar] [CrossRef]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT Pathways Are Crucial for IL-6 Trans-Signaling-Mediated pro-Inflammatory Response in Human Vascular Endothelial Cells. Cell Commun. Signal. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Hülter-Hassler, D.; Wein, M.; Schulz, S.D.; Proksch, S.; Steinberg, T.; Jung, B.A.; Tomakidi, P. Biomechanical Strain-Induced Modulation of Proliferation Coincides with an ERK1/2-Independent Nuclear YAP Localization. Exp. Cell Res. 2017, 361, 93–100. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, J.; Sun, P.P.; Guan, S.; Xu, J. Wedelolactone Inhibits LPS-Induced pro-Inflammation via NF-KappaB Pathway in RAW 264.7 Cells. J. Biomed. Sci. 2013, 20, 84. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Yang, R.; Zhou, Y.-H. Mechanobiology of Periodontal Ligament Stem Cells in Orthodontic Tooth Movement. Stem Cells Int. 2018, 2018, 6531216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.I.; Park, K.H.; Kim, S.J.; Kang, Y.G.; Lee, Y.M.; Kim, E.C. Mechanical Stress-Activated Immune Response Genes via Sirtuin 1 Expression in Human Periodontal Ligament Cells. Clin. Exp. Immunol. 2012, 168, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diomede, F.; Zingariello, M.; Cavalcanti, M.F.X.B.; Merciaro, I.; Pizzicannella, J.; de Isla, N.; Caputi, S.; Ballerini, P.; Trubiani, O. MyD88/ERK/NFkB Pathways and pro-Inflammatory Cytokines Release in Periodontal Ligament Stem Cells Stimulated by Porphyromonas Gingivalis. Eur. J. Histochem. 2017, 61, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Aveic, S.; Craveiro, R.B.; Wolf, M.; Fischer, H. Current Trends in In Vitro Modeling to Mimic Cellular Crosstalk in Periodontal Tissue. Adv. Healthc. Mater. 2021, 10, 2001269. [Google Scholar] [CrossRef]

| Gene Symbol | Gene Name (Mus Musculus) | Gene Function | Accession Number (NCBI Gene Bank) | Chromosoma Location (Length) | 5′-Forward Primer-3′ (Length/Tm/GC) | 5′reverse Primer-3′ (Length/Tm/GC) | Primer Location | Amplicon Length | Amplicon Location (bp of Start/Stop) | Intron-Flanking (Length) | Variants Targeted (Transcript/ Splice) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RPL22 | ribosomal protein L22 | translation of mRNA in protein | NM_000983 | 1; 1p36.31 (2061 bp) | tgattgcacccaccctgtag (20 bp/59.67 °C/55%GC) | ggttcccagcttttccgttc (20 bp/59.4 °C/55%GC) | Exon 2/ Exon 3 | 98 | 91/188 | yes | yes |
| IL-6 | Interleukin 6 | important role in bone metabolism; osteoclastogenesis | NM_000600 | 7; 7p15.3 (1127 bp) | catcctcgacggcatctcag (20 bp/60.32 °C/60%GC) | tcaccaggcaagtctcctca (20 bp/60.47 °C/55%GC) | Exon 2/ Exon 4 | 164 | 240/403 | yes | yes |
| IL-8 | Interleukin 8 | important role in bone metabolism; osteoclastogenesis | NM_000584 | 4; 4q13.3 (1642 bp) | catactccaaacctttccacc (21 bp/57.9 °C/47,6%GC) | cttcaaaaacttctccacaacc (22 bp/56.9 °C/40.9%GC) | Exon 2/ Exon 3 | 167 | 206/372 | yes | yes |
| VEGF A | vascular endothelial growth factor A | induces proliferation and migration of vascular endothelial cells | NM 001171623 | 6p21.1 (3660 bp) |
GGAGGGCAGAATCATCACGAA (21 bp/60.1 °C/52.3%GC) |
GGTACTCCTGGAAGATGTCCAC (22 bp/59.8 °C/54.5%GC) | Exon 2/ Exon 3 | 100 | 1153/1211 | yes | yes |
| PTGS2 COX2 | prostaglandin-endoperoxide synthase 2 | involved in prostaglandin synthesis | NM_000963 | 1q31.1 (4510 pb) | GATGATTGCCCGACTCCCTT (20 bp/59.8 °C/55%GC) | GGCCCTCGCTTATGATCTGT (20 pb/59.6 °C/55%GC) | Exon 4/ Exon 5 | 185 | 560/725 | yes | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, C.E.; Craveiro, R.B.; Niederau, C.; Malyaran, H.; Neuss, S.; Jankowski, J.; Wolf, M. Mechanical Compression by Simulating Orthodontic Tooth Movement in an In Vitro Model Modulates Phosphorylation of AKT and MAPKs via TLR4 in Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2022, 23, 8062. https://doi.org/10.3390/ijms23158062
Roth CE, Craveiro RB, Niederau C, Malyaran H, Neuss S, Jankowski J, Wolf M. Mechanical Compression by Simulating Orthodontic Tooth Movement in an In Vitro Model Modulates Phosphorylation of AKT and MAPKs via TLR4 in Human Periodontal Ligament Cells. International Journal of Molecular Sciences. 2022; 23(15):8062. https://doi.org/10.3390/ijms23158062
Chicago/Turabian StyleRoth, Charlotte E., Rogerio B. Craveiro, Christian Niederau, Hanna Malyaran, Sabine Neuss, Joachim Jankowski, and Michael Wolf. 2022. "Mechanical Compression by Simulating Orthodontic Tooth Movement in an In Vitro Model Modulates Phosphorylation of AKT and MAPKs via TLR4 in Human Periodontal Ligament Cells" International Journal of Molecular Sciences 23, no. 15: 8062. https://doi.org/10.3390/ijms23158062
APA StyleRoth, C. E., Craveiro, R. B., Niederau, C., Malyaran, H., Neuss, S., Jankowski, J., & Wolf, M. (2022). Mechanical Compression by Simulating Orthodontic Tooth Movement in an In Vitro Model Modulates Phosphorylation of AKT and MAPKs via TLR4 in Human Periodontal Ligament Cells. International Journal of Molecular Sciences, 23(15), 8062. https://doi.org/10.3390/ijms23158062







