Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine
Abstract
1. Introduction
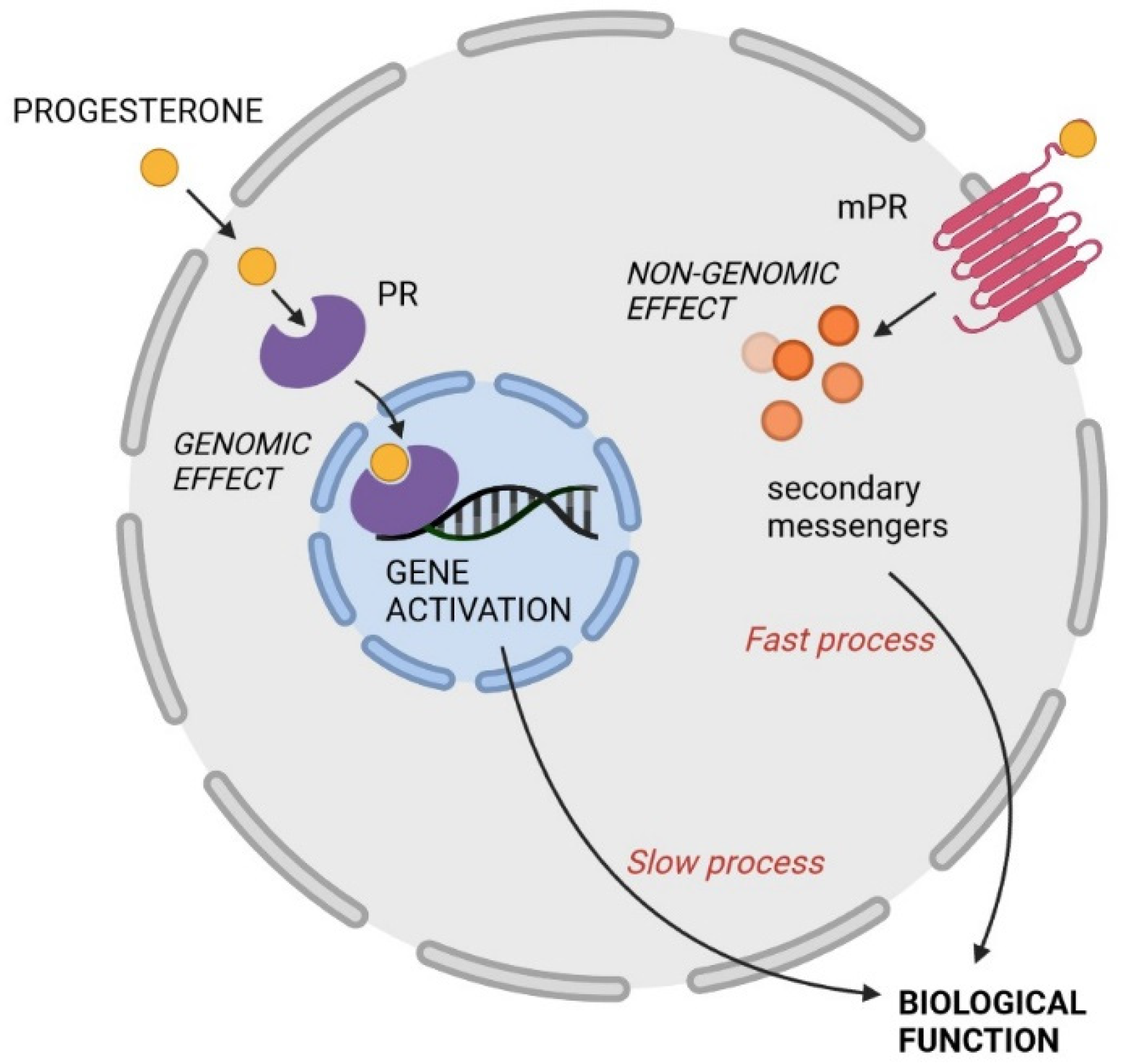
2. Mechanisms of Action
3. Physiological Actions of Progesterone in Females
4. Progesterone and Its Other Effects
4.1. Analgetic Effects of Progesterone
4.2. Progesterone as a Neuroactive Steroid
4.2.1. Synthesis of Neuroactive Steroids—Progesterone Metabolites
4.2.2. Mechanism of Action of Pregnane Steroids—Modulation of GABAA and NMDA Receptors
4.2.3. Modulation of Other Neurotransmitter Systems
4.3. Immunomodulatory Effects of Progesterone
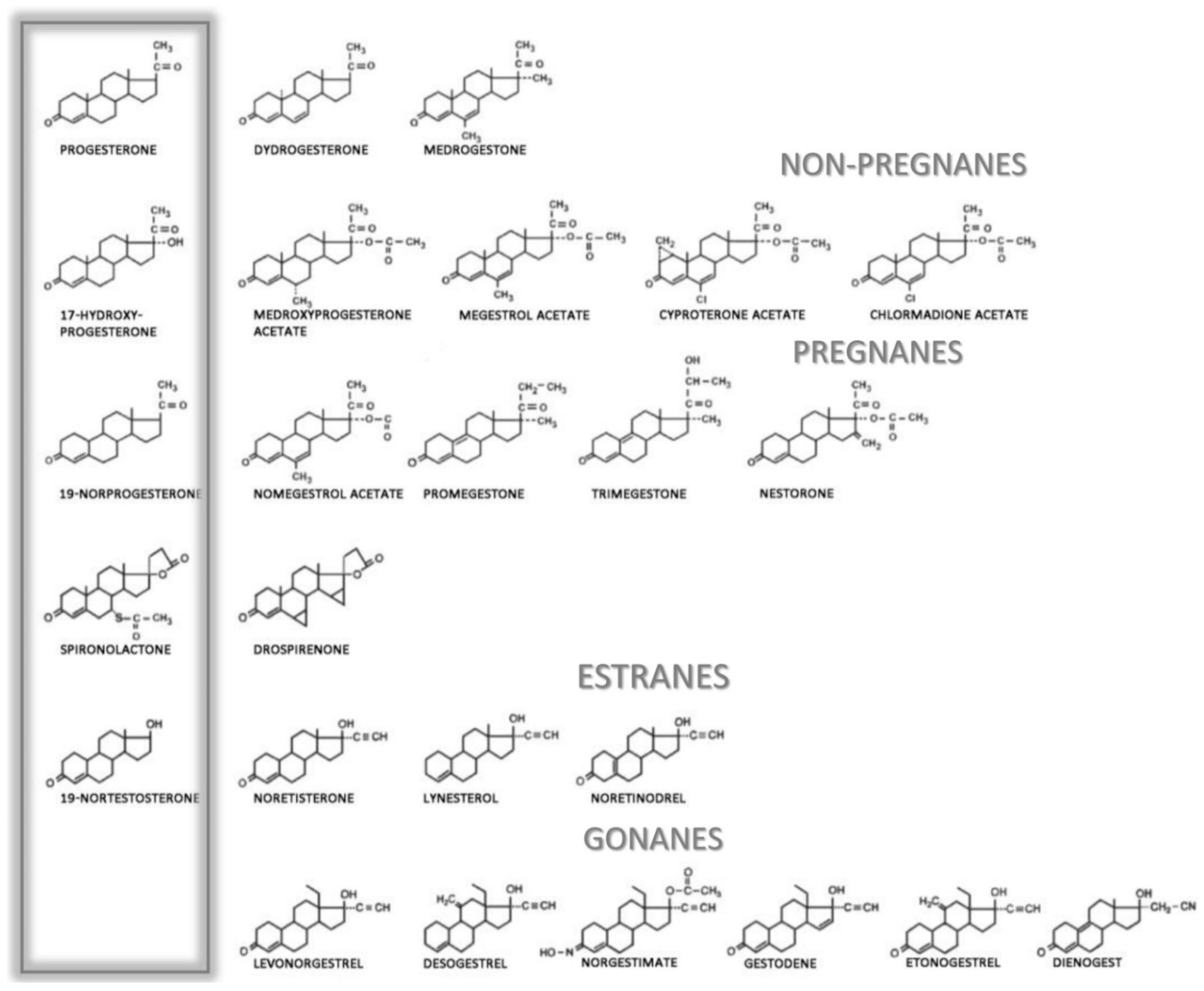
5. Clinical Routes and Applications of Progesterone
Adverse Side Effects of Gestagens and Progestins
| Progestogen Classification | Progesterone | Androgen | Estrogen | Glucocorticoid | Mineralocorticoid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor Binding Affinity | Activity | Receptor Binding Affinity | Androg. Activity | Anti-Androg. Activity | Receptor Binding Affinity | Estrogen Activity | Anti-Estrogen Activity | Receptor Binding Affinity | Activity | Receptor Binding Affinity | Anti-Mineraloc. Activity | ||
| Progesterone derivatives | |||||||||||||
| Natural progesterone | 50 | + | 0 | − | +/− | 0 | − | + | 10 | + | 100 | + | |
| Dydrogesterone | 75 | + | NA | − | +/− | NA | − | + | NA | NA | NA | +/− | |
| Medrogestone | NA | + | NA | − | +/− | NA | − | + | NA | NA | NA | − | |
| 17α-hydroxyprogesterone derivatives—Pregnanes | |||||||||||||
| Medroxyprogesterone acetate | 115 | + | 5 | +/− | − | 0 | − | + | 29 | + | 0 | − | |
| Megestrol acetate | 65 | + | 5 | +/− | + | 0 | − | + | 30 | + | 0 | − | |
| Cyproterone acetate | 90 | + | 6 | − | ++ | 0 | − | + | 6 | + | 8 | − | |
| Chlormadione acetate | 67 | + | 5 | − | + | 0 | − | + | 8 | + | 0 | − | |
| 19-norprogesterone derivatives—Non-pregnanes | |||||||||||||
| Nomegestrol acetate | 125 | + | 42 | − | +/− | 0 | − | + | 0 | − | 0 | − | |
| Promegestone | 100 | + | 0 | − | − | 0 | − | + | 5 | + | 0 | − | |
| Trimegestone | 330 | + | 1 | − | +/− | 0 | − | + | 9 | +/− | 120 | +/− | |
| Nestorone | 136 | + | 0 | − | − | 0 | − | + | 38 * | − | NA | NA | |
| Spironolactone derivative | |||||||||||||
| Drospirenone | 25 | + | 2 | − | + | 0 | − | + | 0 | − | 230 | + | |
| 19-nortestosterone derivatives—Estranes | |||||||||||||
| Noretisterone | 75 | + | 15 | + | − | 0 | + | + | 0 | − | 0 | − | |
| Lynesterol | NA | + | NA | + | − | NA | + | + | NA | − | NA | − | |
| Noretinodrel | 6 | + | 0 | +/− | − | 2 | + | + | NA | − | NA | − | |
| 19-nortestosterone derivatives—Gonanes | |||||||||||||
| Levonorgesterel | 150 | + | 45 | + | − | 0 | − | + | 1 | − | 17 | +/− | |
| Desogestrel | 1 | + | 0 | − | − | 0 | − | + | 0 | − | 0 | − | |
| Norgestimate | 15 | + | 0 | + | − | 0 | − | + | 1 | − | 0 | − | |
| Gestodene | 90 | + | 85 | + | − | 0 | − | + | 27 | + | 290 | + | |
| Etonogestrel | 150 | + | 20 | + | − | 0 | − | + | 14 | +/− | 0 | − | |
| Dienogest | 5 | + | 10 | − | + | 0 | − | + | 1 | − | 0 | − | |
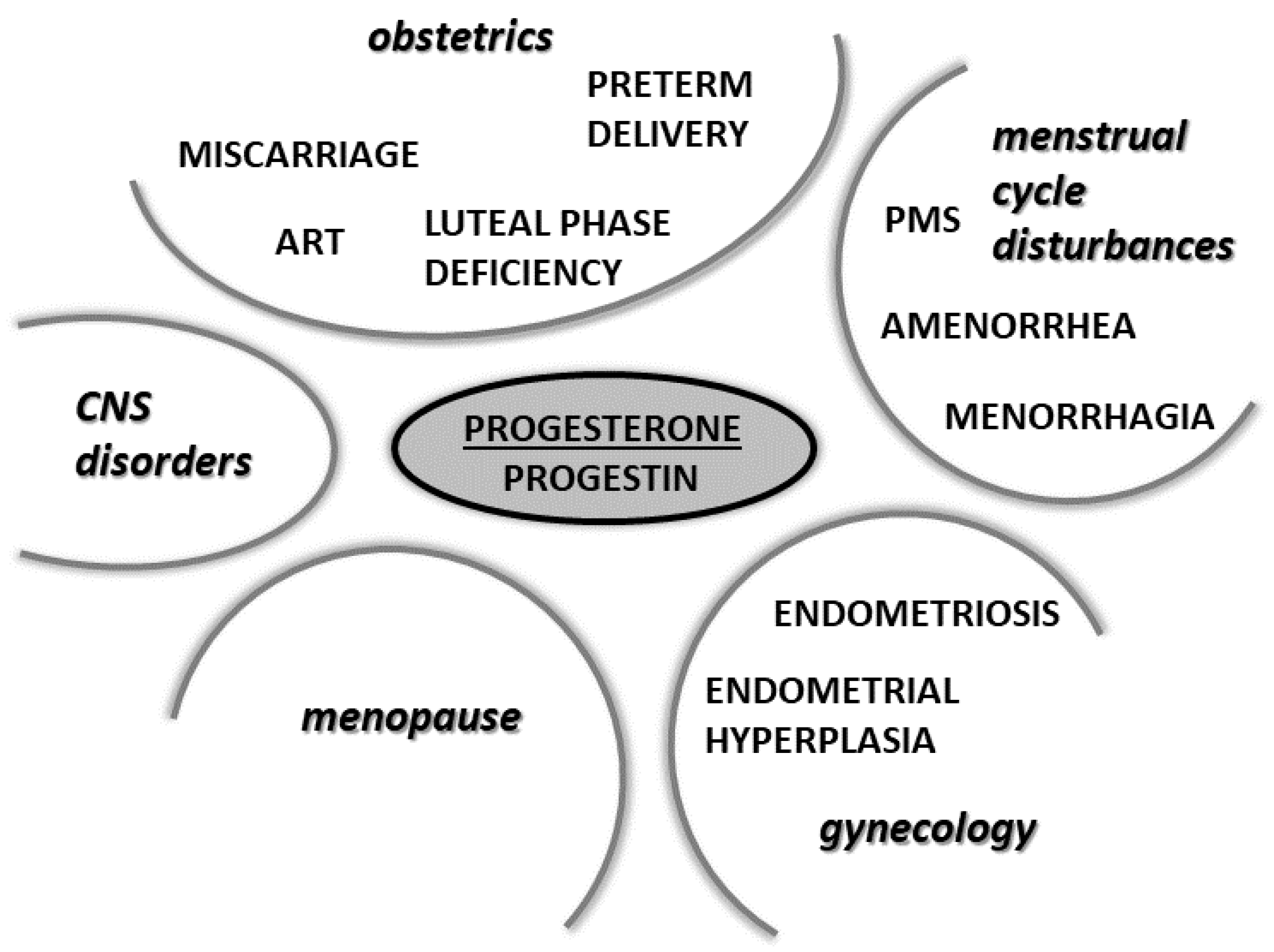
6. Physiological and Pharmacological Actions of Progesterone and Its Analogues in Selected Physiological and Pathophysiological Conditions
6.1. Progesterone in Pregnancy
6.2. Progesterone in Assisted Reproductive Technology
6.3. Progesterone in the Prevention of Miscarriage
6.4. Progesterone in the Prevention of Preterm Birth
6.5. Progesterone in Gynecological Pathologies
6.5.1. Luteal Phase Deficiency
6.5.2. Menorrhagia
6.5.3. Endometriosis
6.5.4. Endometrial Hyperplasia
6.5.5. Secondary Amenorrhea
6.5.6. Premenstrual Syndrome
6.6. Progesterone in Menopause
6.7. Progesterone in Men
6.8. Progesterone in the Treatment of CNS Disorders
6.8.1. Multiple Sclerosis
6.8.2. Amyotrophic Lateral Sclerosis
6.8.3. Spinal Cord Injury
6.8.4. Stroke
6.8.5. Carpal Tunnel Syndrome
7. Endocrine Disruption by Progestins in Wastewater
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Henderson, V.W. Progesterone and human cognition. Climacteric J. Int. Menopause Soc. 2018, 21, 333–340. [Google Scholar] [CrossRef]
- Sundstrom-Poromaa, I.; Comasco, E.; Sumner, R.; Luders, E. Progesterone—Friend or foe? Front. Neuroendocrinol. 2020, 59, 100856. [Google Scholar] [CrossRef]
- Zhang, Y.; Nadeau, M.; Faucher, F.; Lescelleur, O.; Biron, S.; Daris, M.; Rheaume, C.; Luu-The, V.; Tchernof, A. Progesterone metabolism in adipose cells. Mol. Cell. Endocrinol. 2009, 298, 76–83. [Google Scholar] [CrossRef]
- Rossato, M.; Nogara, A.; Merico, M.; Ferlin, A.; Foresta, C. Identification of functional binding sites for progesterone in rat Leydig cell plasma membrane. Steroids 1999, 64, 168–175. [Google Scholar] [CrossRef]
- Stoffel-Wagner, B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001, 145, 669–679. [Google Scholar] [CrossRef]
- Kuhl, H. Pharmacology of progestogens. J. Für Reprod. Und Endokrinol.-J. Reprod. Med. Endocrinol. 2011, 8, 157–177. [Google Scholar]
- Leonhardt, S.A.; Boonyaratanakornkit, V.; Edwards, D.P. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids 2003, 68, 761–770. [Google Scholar] [CrossRef]
- Varticovski, L.; Stavreva, D.A.; McGowan, A.; Raziuddin, R.; Hager, G.L. Endocrine disruptors of sex hormone activities. Mol. Cell. Endocrinol. 2022, 539, 111415. [Google Scholar] [CrossRef]
- Condon, J.C.; Hardy, D.B.; Kovaric, K.; Mendelson, C.R. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 2006, 20, 764–775. [Google Scholar] [CrossRef]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Gynecol. Scand. 2015, 94 (Suppl. 161), 8–16. [Google Scholar] [CrossRef]
- Singh, M.; Su, C.; Ng, S. Non-genomic mechanisms of progesterone action in the brain. Front. Neurosci. 2013, 7, 159. [Google Scholar] [CrossRef]
- Gellersen, B.; Fernandes, M.S.; Brosens, J.J. Non-genomic progesterone actions in female reproduction. Hum. Reprod. Update 2009, 15, 119–138. [Google Scholar] [CrossRef]
- Luconi, M.; Francavilla, F.; Porazzi, I.; Macerola, B.; Forti, G.; Baldi, E. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids 2004, 69, 553–559. [Google Scholar] [CrossRef]
- Blackmore, P.F.; Neulen, J.; Lattanzio, F.; Beebe, S.J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J. Biol. Chem. 1991, 266, 18655–18659. [Google Scholar] [CrossRef]
- Kirkman-Brown, J.C.; Bray, C.; Stewart, P.M.; Barratt, C.L.; Publicover, S.J. Biphasic elevation of [Ca(2+)](i) in individual human spermatozoa exposed to progesterone. Dev. Biol. 2000, 222, 326–335. [Google Scholar] [CrossRef][Green Version]
- El-Hefnawy, T.; Huhtaniemi, I. Progesterone can participate in down-regulation of the luteinizing hormone receptor gene expression and function in cultured murine Leydig cells. Mol. Cell. Endocrinol. 1998, 137, 127–138. [Google Scholar] [CrossRef]
- Huhtaniemi, I.T.; Aittomaki, K. Mutations of follicle-stimulating hormone and its receptor: Effects on gonadal function. Eur. J. Endocrinol. 1998, 138, 473–481. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.; Rice, C.D.; Pang, Y.; Pace, M.; Thomas, P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 2231–2236. [Google Scholar] [CrossRef]
- Thomas, P.; Zhu, Y.; Pace, M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: A review with some new findings. Steroids 2002, 67, 511–517. [Google Scholar] [CrossRef]
- Maller, J.L. The elusive progesterone receptor in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 8–10. [Google Scholar] [CrossRef]
- Bagowski, C.P.; Myers, J.W.; Ferrell, J.E., Jr. The classical progesterone receptor associates with p42 MAPK and is involved in phosphatidylinositol 3-kinase signaling in Xenopus oocytes. J. Biol. Chem. 2001, 276, 37708–37714. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.; Romo, X.; Grandy, R.; Soto, X.; Montecino, M.; Hinrichs, M.; Olate, J. A Gbetagamma stimulated adenylyl cyclase is involved in Xenopus laevis oocyte maturation. J. Cell. Physiol. 2005, 202, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Evaul, K.; Jamnongjit, M.; Bhagavath, B.; Hammes, S.R. Testosterone and progesterone rapidly attenuate plasma membrane Gbetagamma-mediated signaling in Xenopus laevis oocytes by signaling through classical steroid receptors. Mol. Endocrinol. 2007, 21, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehoshua, L.J.; Lewellyn, A.L.; Thomas, P.; Maller, J.L. The role of Xenopus membrane progesterone receptor beta in mediating the effect of progesterone on oocyte maturation. Mol. Endocrinol. 2007, 21, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, W.J.; Pinto, L.H.; O’Connor, C.M.; Smith, L.D. Progesterone induces a rapid increase in [Ca2+]in of Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 1980, 77, 1534–1536. [Google Scholar] [CrossRef]
- Dosiou, C.; Hamilton, A.E.; Pang, Y.; Overgaard, M.T.; Tulac, S.; Dong, J.; Thomas, P.; Giudice, L.C. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J. Endocrinol. 2008, 196, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ehring, G.R.; Kerschbaum, H.H.; Eder, C.; Neben, A.L.; Fanger, C.M.; Khoury, R.M.; Negulescu, P.A.; Cahalan, M.D. A nongenomic mechanism for progesterone-mediated immunosuppression: Inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J. Exp. Med. 1998, 188, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.; Lahav, J.; Hod, M.; Ben-Rafael, Z.; Weinberger, I.; Brosens, J. Regulation of platelet aggregation and adenosine triphosphate release in vitro by 17beta-estradiol and medroxyprogesterone acetate in postmenopausal women. Thromb. Haemost. 2000, 84, 695–700. [Google Scholar]
- Blackmore, P.F. Extragenomic actions of progesterone in human sperm and progesterone metabolites in human platelets. Steroids 1999, 64, 149–156. [Google Scholar] [CrossRef]
- Blackmore, P.F. Progesterone metabolites rapidly stimulate calcium influx in human platelets by a src-dependent pathway. Steroids 2008, 73, 738–750. [Google Scholar] [CrossRef]
- Peluso, J.J.; Pappalardo, A. Progesterone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 14-3-3sigma. Biol. Reprod. 2004, 71, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Peluso, J.J.; Fernandez, G.; Pappalardo, A.; White, B.A. Characterization of a putative membrane receptor for progesterone in rat granulosa cells. Biol. Reprod. 2001, 65, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J.; Licata, G.; Shan, J.; Bing, L.; Karpinski, E.; Pang, P.K.; Resnick, L.M. Vascular Effects of Progesterone: Role of Cellular Calcium Regulation. Hypertension 2001, 37, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.D.; Giretti, M.S.; Baldacci, C.; Garibaldi, S.; Flamini, M.; Sanchez, A.M.; Gadducci, A.; Genazzani, A.R.; Simoncini, T. Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. PLoS ONE 2008, 3, e2790. [Google Scholar] [CrossRef]
- Fu, X.D.; Flamini, M.; Sanchez, A.M.; Goglia, L.; Giretti, M.S.; Genazzani, A.R.; Simoncini, T. Progestogens regulate endothelial actin cytoskeleton and cell movement via the actin-binding protein moesin. Mol. Hum. Reprod. 2008, 14, 225–234. [Google Scholar] [CrossRef][Green Version]
- Bielefeldt, K.; Waite, L.; Abboud, F.M.; Conklin, J.L. Nongenomic effects of progesterone on human intestinal smooth muscle cells. Am. J. Physiol. 1996, 271 Pt 1, G370–G376. [Google Scholar] [CrossRef]
- Hsu, S.P.; Chen, T.H.; Chou, Y.P.; Chen, L.C.; Kuo, C.T.; Lee, T.S.; Lin, J.J.; Chang, N.C.; Lee, W.S. Extra-nuclear activation of progesterone receptor in regulating arterial smooth muscle cell migration. Atherosclerosis 2011, 217, 83–89. [Google Scholar] [CrossRef]
- Verikouki, C.H.; Hatzoglou, C.H.; Gourgoulianis, K.I.; Molyvdas, P.A.; Kallitsaris, A.; Messinis, I.E. Rapid effect of progesterone on transepithelial resistance of human fetal membranes: Evidence for non-genomic action. Clin. Exp. Pharmacol. Physiol. 2008, 35, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Ohmichi, M.; Takahashi, K.; Kawagoe, J.; Ohta, T.; Doshida, M.; Takahashi, T.; Igarashi, H.; Mori-Abe, A.; Du, B.; et al. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in human breast cancer cells. Endocrinology 2005, 146, 4917–4925. [Google Scholar] [CrossRef]
- Kaur, P.; Jodhka, P.K.; Underwood, W.A.; Bowles, C.A.; de Fiebre, N.C.; de Fiebre, C.M.; Singh, M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J. Neurosci. Res. 2007, 85, 2441–2449. [Google Scholar] [CrossRef]
- Nilsen, J.; Brinton, R.D. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 10506–10511. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhu, Y.; Furuya, K.; Li, Z.; Sokabe, M.; Chen, L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology 2008, 55, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Zhao, L.; Nilsen, J.; McClure, K.; Wong, K.; Brinton, R.D. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology 2009, 150, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Cunningham, R.L.; Rybalchenko, N.; Singh, M. Progesterone increases the release of brain-derived neurotrophic factor from glia via progesterone receptor membrane component 1 (Pgrmc1)-dependent ERK5 signaling. Endocrinology 2012, 153, 4389–4400. [Google Scholar] [CrossRef]
- Koulen, P.; Madry, C.; Duncan, R.S.; Hwang, J.Y.; Nixon, E.; McClung, N.; Gregg, E.V.; Singh, M. Progesterone potentiates IP(3)-mediated calcium signaling through Akt/PKB. Cell. Physiol. Biochem. 2008, 21, 161–172. [Google Scholar] [CrossRef]
- Sleiter, N.; Pang, Y.; Park, C.; Horton, T.H.; Dong, J.; Thomas, P.; Levine, J.E. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 2009, 150, 3833–3844. [Google Scholar] [CrossRef]
- Frye, C.A.; Sumida, K.; Lydon, J.P.; O’Malley, B.W.; Pfaff, D.W. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3alpha,5alpha-THP-facilitated lordosis. Psychopharmacology 2006, 185, 423–432. [Google Scholar] [CrossRef]
- Anderson, G.D.; Odegard, P.S. Pharmacokinetics of estrogen and progesterone in chronic kidney disease. Adv. Chronic Kidney Dis. 2004, 11, 357–360. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Mamsen, L.S.; Jeppesen, J.V.; Botkjaer, J.A.; Pors, S.E.; Borgbo, T.; Ernst, E.; Macklon, K.T.; Andersen, C.Y. Hallmarks of Human Small Antral Follicle Development: Implications for Regulation of Ovarian Steroidogenesis and Selection of the Dominant Follicle. Front. Endocrinol 2017, 8, 376. [Google Scholar] [CrossRef]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum. Reprod. Update 2012, 18, 73–91. [Google Scholar] [CrossRef]
- Sykes, L.; Bennett, P.R. Efficacy of progesterone for prevention of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.; Gomez, E.C.; Weinstein, G.D.; Lamas, J.; Hsia, S.L. Metabolism of progesterone-4-14C in vitro in human skin and vaginal mucosa. Biochemistry 1969, 8, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.; Yabe, R.; Kurihara, T.; Saegusa, H.; Zong, S.; Tanabe, T. Progesterone receptor antagonist is effective in relieving neuropathic pain. Eur. J. Pharmcol. 2006, 541, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, B.; Rosyidi, R.M.; Islam, A.A.; Turchan, A.; Pintaningrum, Y. The effect of progesteron for expression delta (delta) opioid receptor spinal cord through peripheral nerve injury. Ann. Med. Surg. 2022, 75, 103376. [Google Scholar] [CrossRef]
- Petersen, S.L.; LaFlamme, K.D. Progesterone increases levels of mu-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Brain Res. Mol. Brain Res. 1997, 52, 32–37. [Google Scholar] [CrossRef]
- Selye, H. Anesthetic effect of steroid hormones. Proc. Soc. Exp. Biol. Med. 1941, 46, 116–121. [Google Scholar] [CrossRef]
- Selye, H. Acquired adaptation to the anesthetic effect of steroid hormones. J. Immunol. 1941, 41, 259–268. [Google Scholar]
- Kuba, T.; Wu, H.B.; Nazarian, A.; Festa, E.D.; Barr, G.A.; Jenab, S.; Inturrisi, C.E.; Quinones-Jenab, V. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm. Behav. 2006, 49, 441–449. [Google Scholar] [CrossRef]
- Vincent, K.; Stagg, C.J.; Warnaby, C.E.; Moore, J.; Kennedy, S.; Tracey, I. “Luteal Analgesia”: Progesterone Dissociates Pain Intensity and Unpleasantness by Influencing Emotion Regulation Networks. Front. Endocrinol. 2018, 9, 413. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z. Sex differences in pain perception. Gend. Med. 2005, 2, 137–145. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Ness, T.J. Sex-related hormonal influences on pain and analgesic responses. Neurosci. Biobehav. Rev. 2000, 24, 485–501. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Berkley, K.J.; Iezzi, S.; de Bigontina, P.; Vecchiet, L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain 1997, 71, 187–197. [Google Scholar] [CrossRef]
- Hapidou, E.G.; De Catanzaro, D. Sensitivity to cold pressor pain in dysmenorrheic and non-dysmenorrheic women as a function of menstrual cycle phase. Pain 1988, 34, 277–283. [Google Scholar] [CrossRef]
- Frolich, M.A.; Banks, C.; Warren, W.; Robbins, M.; Ness, T. The Association Between Progesterone, Estradiol, and Oxytocin and Heat Pain Measures in Pregnancy: An Observational Cohort Study. Anesth. Analg. 2016, 123, 396–401. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Ko, S. The relationship between serum progesterone concentration and anesthetic and analgesic requirements: A prospective observational study of parturients undergoing cesarean delivery. Anesth. Analg. 2014, 119, 901–905. [Google Scholar] [CrossRef]
- Kashanian, M.; Dadkhah, F.; Zarei, S.; Sheikhansari, N.; Javanmanesh, F. Evaluation the relationship between serum progesterone level and pain perception after cesarean delivery. J. Matern.-Fetal Neonatal Med. 2019, 32, 3548–3551. [Google Scholar] [CrossRef]
- Gyermek, L.; Soyka, L.F. Steroid anesthetics. Anesthesiology 1975, 42, 331–344. [Google Scholar] [CrossRef]
- Lawrence, D.K.; Gill, E.W. Structurally specific effects of some steroid anesthetics on spin-labeled liposomes. Mol. Pharmcol. 1975, 11, 280–286. [Google Scholar]
- Majewska, M.D.; Harrison, N.L.; Schwartz, R.D.; Barker, J.L.; Paul, S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986, 232, 1004–1007. [Google Scholar] [CrossRef]
- Demirgoren, S.; Majewska, M.D.; Spivak, C.E.; London, E.D. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience 1991, 45, 127–135. [Google Scholar] [CrossRef]
- Majewska, M.D.; Demirgoren, S.; Spivak, C.E.; London, E.D. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990, 526, 143–146. [Google Scholar] [CrossRef]
- Paul, S.M.; Purdy, R.H. Neuroactive steroids. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1992, 6, 2311–2322. [Google Scholar] [CrossRef]
- Hauser, C.A.; Chesnoy-Marchais, D.; Robel, P.; Baulieu, E.E. Modulation of recombinant alpha 6 beta 2 gamma 2 GABAA receptors by neuroactive steroids. Eur. J. Pharmcol. 1995, 289, 249–257. [Google Scholar] [CrossRef]
- Backstrom, T.; Das, R.; Bixo, M. Positive GABAA receptor modulating steroids and their antagonists: Implications for clinical treatments. J. Neuroendocrinol. 2022, 34, e13013. [Google Scholar] [CrossRef]
- Backstrom, T.; Bixo, M.; Stromberg, J. GABAA Receptor-Modulating Steroids in Relation to Women’s Behavioral Health. Curr. Psychiatry Rep. 2015, 17, 92. [Google Scholar] [CrossRef]
- Wu, F.S.; Gibbs, T.T.; Farb, D.H. Inverse modulation of gamma-aminobutyric acid- and glycine-induced currents by progesterone. Mol. Pharmcol. 1990, 37, 597–602. [Google Scholar]
- Weaver, C.E.; Land, M.B.; Purdy, R.H.; Richards, K.G.; Gibbs, T.T.; Farb, D.H. Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca(2+) accumulation and cell death. J. Pharmcol. Exp. Ther. 2000, 293, 747–754. [Google Scholar]
- Park-Chung, M.; Wu, F.S.; Farb, D.H. 3 alpha-Hydroxy-5 beta-pregnan-20-one sulfate: A negative modulator of the NMDA-induced current in cultured neurons. Mol. Pharmcol. 1994, 46, 146–150. [Google Scholar]
- Valera, S.; Ballivet, M.; Bertrand, D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 9949–9953. [Google Scholar] [CrossRef]
- Grazzini, E.; Guillon, G.; Mouillac, B.; Zingg, H.H. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 1998, 392, 509–512. [Google Scholar] [CrossRef]
- Schreiber, V. Neuropeptides and neurosteroids (author’s transl). Cas. Lek. Ceskych 1980, 119, 656–659. [Google Scholar]
- Corpechot, C.; Robel, P.; Axelson, M.; Sjovall, J.; Baulieu, E.E. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci. USA 1981, 78, 4704–4707. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.F.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef]
- Kancheva, R.; Hill, M.; Cibula, D.; Vcelakova, H.; Kancheva, L.; Vrbikova, J.; Fait, T.; Parizek, A.; Starka, L. Relationships of circulating pregnanolone isomers and their polar conjugates to the status of sex, menstrual cycle, and pregnancy. J. Endocrinol. 2007, 195, 67–78. [Google Scholar] [CrossRef]
- Hill, M.; Cibula, D.; Havlikova, H.; Kancheva, L.; Fait, T.; Kancheva, R.; Parizek, A.; Starka, L. Circulating levels of pregnanolone isomers during the third trimester of human pregnancy. J. Steroid Biochem. Mol. Biol. 2007, 105, 166–175. [Google Scholar] [CrossRef]
- Hirst, J.J.; Kelleher, M.A.; Walker, D.W.; Palliser, H.K. Neuroactive steroids in pregnancy: Key regulatory and protective roles in the foetal brain. J. Steroid Biochem. Mol. Biol. 2014, 139, 144–153. [Google Scholar] [CrossRef]
- Hill, M.; Popov, P.; Havlikova, H.; Kancheva, L.; Vrbikova, J.; Kancheva, R.; Pouzar, V.; Cerny, I.; Starka, L. Altered profiles of serum neuroactive steroids in premenopausal women treated for alcohol addiction. Steroids 2005, 70, 515–524. [Google Scholar] [CrossRef]
- Bixo, M.; Andersson, A.; Winblad, B.; Purdy, R.H.; Backstrom, T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997, 764, 173–178. [Google Scholar] [CrossRef]
- Hill, M.; Parizek, A.; Cibula, D.; Kancheva, R.; Jirasek, J.E.; Jirkovska, M.; Velikova, M.; Kubatova, J.; Klimkova, M.; Paskova, A.; et al. Steroid metabolome in fetal and maternal body fluids in human late pregnancy. J. Steroid Biochem. Mol. Biol. 2010, 122, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Bicikova, M.; Sosvorova, L. Hormones and the blood-brain barrier. Horm. Mol. Biol. Clin. Investig. 2015, 21, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Purdy, R.H.; Morrow, A.L.; Moore, P.H., Jr.; Paul, S.M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4553–4557. [Google Scholar] [CrossRef]
- Droogleever Fortuyn, H.A.; van Broekhoven, F.; Span, P.N.; Backstrom, T.; Zitman, F.G.; Verkes, R.J. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptor density. Psychoneuroendocrinology 2004, 29, 1341–1344. [Google Scholar] [CrossRef]
- Wang, M.; Seippel, L.; Purdy, R.H.; Backstrom, T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J. Clin. Endocrinol. Metab. 1996, 81, 1076–1082. [Google Scholar]
- Genazzani, A.D.; Luisi, M.; Malavasi, B.; Strucchi, C.; Luisi, S.; Casarosa, E.; Bernardi, F.; Genazzani, A.R.; Petraglia, F. Pulsatile secretory characteristics of allopregnanolone, a neuroactive steroid, during the menstrual cycle and in amenorrheic subjects. Eur. J. Endocrinol. 2002, 146, 347–356. [Google Scholar] [CrossRef][Green Version]
- Brunton, P.J.; Russell, J.A.; Hirst, J.J. Allopregnanolone in the brain: Protecting pregnancy and birth outcomes. Prog. Neurobiol. 2014, 113, 106–136. [Google Scholar] [CrossRef]
- Hill, M.; Hana, V., Jr.; Velikova, M.; Parizek, A.; Kolatorova, L.; Vitku, J.; Skodova, T.; Simkova, M.; Simjak, P.; Kancheva, R.; et al. A method for determination of one hundred endogenous steroids in human serum by gas chromatography-tandem mass spectrometry. Physiol. Res. 2019, 68, 179–207. [Google Scholar] [CrossRef]
- Hill, M.; Parizek, A.; Kancheva, R.; Jirasek, J.E. Reduced progesterone metabolites in human late pregnancy. Physiol. Res. 2011, 60, 225–241. [Google Scholar] [CrossRef]
- Hill, M.; Parizek, A.; Kancheva, R.; Duskova, M.; Velikova, M.; Kriz, L.; Klimkova, M.; Paskova, A.; Zizka, Z.; Matucha, P.; et al. Steroid metabolome in plasma from the umbilical artery, umbilical vein, maternal cubital vein and in amniotic fluid in normal and preterm labor. J. Steroid Biochem. Mol. Biol. 2010, 121, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Wahlstrom, G.; Backstrom, T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J. Steroid Biochem. Mol. Biol. 1997, 62, 299–306. [Google Scholar] [CrossRef]
- Yoshihara, S.; Morimoto, H.; Ohori, M.; Yamada, Y.; Abe, T.; Arisaka, O. A neuroactive steroid, allotetrahydrocorticosterone inhibits sensory nerves activation in guinea-pig airways. Neurosci. Res. 2005, 53, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Panzica, G.; Garcia-Segura, L.M. Neuroactive steroids: Focus on human brain. Neuroscience 2011, 191, 1–5. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Kurogi, K.; Shimohira, T.; Teramoto, T.; Liu, M.C.; Suiko, M.; Sakakibara, Y. Delta(4)-3-ketosteroids as a new class of substrates for the cytosolic sulfotransferases. Biochim. Biophys. Acta Gen. Subj. 2017, 1861 Pt A, 2883–2890. [Google Scholar] [CrossRef]
- Rubin, G.L.; Harrold, A.J.; Mills, J.A.; Falany, C.N.; Coughtrie, M.W. Regulation of sulphotransferase expression in the endometrium during the menstrual cycle, by oral contraceptives and during early pregnancy. Mol. Hum. Reprod. 1999, 5, 995–1002. [Google Scholar] [CrossRef]
- Lindsay, J.; Wang, L.L.; Li, Y.; Zhou, S.F. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr. Drug Metab. 2008, 9, 99–105. [Google Scholar]
- Brussaard, A.B.; Koksma, J.J. Conditional regulation of neurosteroid sensitivity of GABAA receptors. Ann. N. Y. Acad. Sci. 2003, 1007, 29–36. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Bethea, C.L.; Lu, N.Z.; Gundlah, C.; Streicher, J.M. Diverse actions of ovarian steroids in the serotonin neural system. Front. Neuroendocrinol. 2002, 23, 41–100. [Google Scholar] [CrossRef]
- Brean, A.; Fredo, H.L.; Sollid, S.; Muller, T.; Sundstrom, T.; Eide, P.K. Five-year incidence of surgery for idiopathic normal pressure hydrocephalus in Norway. Acta Neurol. Scand. 2009, 120, 314–316. [Google Scholar] [CrossRef]
- Gundlah, C.; Lu, N.Z.; Bethea, C.L. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology 2002, 160, 271–282. [Google Scholar] [CrossRef]
- Luine, V.N.; Rhodes, J.C. Gonadal hormone regulation of MAO and other enzymes in hypothalamic areas. Neuroendocrinology 1983, 36, 235–241. [Google Scholar] [CrossRef]
- Benmansour, S.; Weaver, R.S.; Barton, A.K.; Adeniji, O.S.; Frazer, A. Comparison of the effects of estradiol and progesterone on serotonergic function. Biol. Psychiatry 2012, 71, 633–641. [Google Scholar] [CrossRef]
- Perrotti, L.I.; Beck, K.D.; Luine, V.N.; Quinones, V. Progesterone and cocaine administration affect serotonin in the medial prefrontal cortex of ovariectomized rats. Neurosci. Lett. 2000, 291, 155–158. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.J.; Amor, J.C.; Ramos, J.A. Time-dependent effects of estradiol and progesterone on the number of striatal dopaminergic D2-receptors. Brain Res. 1989, 476, 388–395. [Google Scholar] [CrossRef]
- Lolier, M.; Wagner, C.K. Sex differences in dopamine innervation and microglia are altered by synthetic progestin in neonatal medial prefrontal cortex. J. Neuroendocrinol. 2021, 33, e12962. [Google Scholar] [CrossRef]
- Druckmann, R.; Druckmann, M.A. Progesterone and the immunology of pregnancy. J. Steroid Biochem. Mol. Biol. 2005, 97, 389–396. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Barakonyi, A.; Par, G.; Polgar, B.; Palkovics, T.; Szereday, L. Progesterone as an immunomodulatory molecule. Int. Immunopharmacol. 2001, 1, 1037–1048. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Reznikoff-Etievant, M.F.; Varga, P.; Pichon, M.F.; Varga, Z.; Chaouat, G. Lymphocytic progesterone receptors in normal and pathological human pregnancy. J. Reprod. Immunol. 1989, 16, 239–247. [Google Scholar] [CrossRef]
- Shah, N.M.; Lai, P.F.; Imami, N.; Johnson, M.R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Polgar, B.; Kozma, N.; Miko, E.; Par, G.; Szereday, L.; Barakonyi, A.; Palkovics, T.; Papp, O.; Varga, P. Progesterone-Dependent Immunomodulation. In Immunology of Pregnancy; Markert, U.R., Ed.; Karger: Jena, Germany, 2005. [Google Scholar]
- Buyon, J.P.; Korchak, H.M.; Rutherford, L.E.; Ganguly, M.; Weissmann, G. Female hormones reduce neutrophil responsiveness in vitro. Arthritis Rheum. 1984, 27, 623–630. [Google Scholar] [CrossRef]
- Nadkarni, S.; Smith, J.; Sferruzzi-Perri, A.N.; Ledwozyw, A.; Kishore, M.; Haas, R.; Mauro, C.; Williams, D.J.; Farsky, S.H.; Marelli-Berg, F.M.; et al. Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc. Natl. Acad. Sci. USA 2016, 113, E8415–E8424. [Google Scholar] [CrossRef]
- Arck, P.; Hansen, P.J.; Mulac Jericevic, B.; Piccinni, M.P.; Szekeres-Bartho, J. Progesterone during pregnancy: Endocrine-immune cross talk in mammalian species and the role of stress. Am. J. Reprod. Immunol. 2007, 58, 268–279. [Google Scholar] [CrossRef]
- Hall, O.J.; Nachbagauer, R.; Vermillion, M.S.; Fink, A.L.; Phuong, V.; Krammer, F.; Klein, S.L. Progesterone-Based Contraceptives Reduce Adaptive Immune Responses and Protection against Sequential Influenza A Virus Infections. J. Virol. 2017, 91, e02160-16. [Google Scholar] [CrossRef]
- Lincová, D.; Farghali, H. Základní a Aplikovaná Farmakologie; druhé, doplnûné a pfiepracované vydání; GalénPublishing: Prague, Czech Republic, 2007. [Google Scholar]
- Simon, J.A. Micronized progesterone: Vaginal and oral uses. Clin. Obstet. Gynecol. 1995, 38, 902–914. [Google Scholar] [CrossRef]
- Gomes, L.G.; Huang, N.; Agrawal, V.; Mendonca, B.B.; Bachega, T.A.; Miller, W.L. Extraadrenal 21-hydroxylation by CYP2C19 and CYP3A4: Effect on 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2009, 94, 89–95. [Google Scholar] [CrossRef]
- Niwa, T.; Narita, K.; Okamoto, A.; Murayama, N.; Yamazaki, H. Comparison of Steroid Hormone Hydroxylations by and Docking to Human Cytochromes P450 3A4 and 3A5. J. Pharm. Pharm. Sci. 2019, 22, 332–339. [Google Scholar] [CrossRef]
- Niwa, T.; Toyota, M.; Kawasaki, H.; Ishii, R.; Sasaki, S. Comparison of the Stimulatory and Inhibitory Effects of Steroid Hormones and alpha-Naphthoflavone on Steroid Hormone Hydroxylation Catalyzed by Human Cytochrome P450 3A Subfamilies. Biol. Pharm. Bull. 2021, 44, 579–584. [Google Scholar] [CrossRef]
- Patil, A.S.; Swamy, G.K.; Murtha, A.P.; Heine, R.P.; Zheng, X.; Grotegut, C.A. Progesterone Metabolites Produced by Cytochrome P450 3A Modulate Uterine Contractility in a Murine Model. Reprod. Sci. 2015, 22, 1577–1586. [Google Scholar] [CrossRef][Green Version]
- Quinney, S.K.; Benjamin, T.; Zheng, X.; Patil, A.S. Characterization of Maternal and Fetal CYP3A-Mediated Progesterone Metabolism. Fetal Pediatr. Pathol. 2017, 36, 400–411. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Tosto, V.; Tsibizova, V. Progesterone: History, facts, and artifacts. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 2–12. [Google Scholar] [CrossRef]
- Spark, M.J.; Willis, J. Systematic review of progesterone use by midlife and menopausal women. Maturitas 2012, 72, 192–202. [Google Scholar] [CrossRef]
- Wambach, G.; Higgins, J.R.; Kem, D.C.; Kaufmann, W. Interaction of synthetic progestagens with renal mineralocorticoid receptors. Acta Endocrinol. 1979, 92, 560–567. [Google Scholar] [CrossRef]
- Rylance, P.B.; Brincat, M.; Lafferty, K.; De Trafford, J.C.; Brincat, S.; Parsons, V.; Studd, J.W. Natural progesterone and antihypertensive action. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 13–14. [Google Scholar] [CrossRef]
- Piette, P.C.M. The pharmacodynamics and safety of progesterone. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 13–29. [Google Scholar] [CrossRef]
- McCann, M.F.; Potter, L.S. Progestin-only oral contraception: A comprehensive review. Contraception 1994, 50 (Suppl. 1), S1–S195. [Google Scholar] [CrossRef]
- de Lignieres, B.; Dennerstein, L.; Backstrom, T. Influence of route of administration on progesterone metabolism. Maturitas 1995, 21, 251–257. [Google Scholar] [CrossRef]
- Prior, J.C. Progesterone for treatment of symptomatic menopausal women. Climacteric J. Int. Menopause Soc. 2018, 21, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Seifert-Klauss, V.; Prior, J.C. Progesterone and bone: Actions promoting bone health in women. J. Osteoporos. 2010, 2010, 845180. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric J. Int. Menopause Soc. 2005, 8 (Suppl. 1), 3–63. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H. Comparative pharmacology of newer progestogens. Drugs 1996, 51, 188–215. [Google Scholar] [CrossRef]
- Schindler, A.E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J.R.; Schweppe, K.W.; Thijssen, J.H. Classification and pharmacology of progestins. Maturitas 2003, 46 (Suppl. 1), S7–S16. [Google Scholar] [CrossRef]
- Sitruk-Ware, R. Pharmacological profile of progestins. Maturitas 2004, 47, 277–283. [Google Scholar] [CrossRef]
- Wiegratz, I.; Kuhl, H. Progestogen therapies: Differences in clinical effects? Trends Endocrinol. Metab. TEM 2004, 15, 277–285. [Google Scholar] [CrossRef]
- Africander, D.; Louw, R.; Hapgood, J.P. Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochem. Biophys. Res. Commun. 2013, 433, 305–310. [Google Scholar] [CrossRef]
- Winneker, R.C.; Bitran, D.; Zhang, Z. The preclinical biology of a new potent and selective progestin: Trimegestone. Steroids 2003, 68, 915–920. [Google Scholar] [CrossRef]
- Ruan, X.; Seeger, H.; Mueck, A.O. The pharmacology of nomegestrol acetate. Maturitas 2012, 71, 345–353. [Google Scholar] [CrossRef]
- Kumar, N.; Koide, S.S.; Tsong, Y.; Sundaram, K. Nestorone: A progestin with a unique pharmacological profile. Steroids 2000, 65, 629–636. [Google Scholar] [CrossRef]
- Schneider, M.A.; Davies, M.C.; Honour, J.W. The timing of placental competence in pregnancy after oocyte donation. Fertil. Steril. 1993, 59, 1059–1064. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Giardina, I.; Clerici, G.; Brillo, E.; Gerli, S. Progesterone in normal and pathological pregnancy. Horm. Mol. Biol. Clin. Investig. 2016, 27, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Zakar, T.; Mesiano, S. How does progesterone relax the uterus in pregnancy? N. Engl. J. Med. 2011, 364, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Walch, K.T.; Huber, J.C. Progesterone for recurrent miscarriage: Truth and deceptions. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 375–389. [Google Scholar] [CrossRef]
- Csapo, A.I.; Pulkkinen, M. Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstet. Gynecol. Surv. 1978, 33, 69–81. [Google Scholar] [CrossRef]
- Peyron, R.; Aubeny, E.; Targosz, V.; Silvestre, L.; Renault, M.; Elkik, F.; Leclerc, P.; Ulmann, A.; Baulieu, E.E. Early termination of pregnancy with mifepristone (RU 486) and the orally active prostaglandin misoprostol. N. Engl. J. Med. 1993, 328, 1509–1513. [Google Scholar] [CrossRef]
- Parizek, A.; Koucky, M.; Duskova, M. Progesterone, inflammation and preterm labor. J. Steroid Biochem. Mol. Biol. 2014, 139, 159–165. [Google Scholar] [CrossRef]
- Norman, J.E. Progesterone and preterm birth. Int. J. Gynaecol. Obstet. 2020, 150, 24–30. [Google Scholar] [CrossRef]
- Pieber, D.; Allport, V.C.; Hills, F.; Johnson, M.; Bennett, P.R. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol. Hum. Reprod. 2001, 7, 875–879. [Google Scholar] [CrossRef]
- Mesiano, S. Myometrial progesterone responsiveness and the control of human parturition. J. Soc. Gynecol. Investig. 2004, 11, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Stjernholm-Vladic, Y.; Wang, H.; Stygar, D.; Ekman, G.; Sahlin, L. Differential regulation of the progesterone receptor A and B in the human uterine cervix at parturition. Gynecol. Endocrinol. 2004, 18, 41–46. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, C.J.; Park, I.; Romero, R.; Sohn, Y.K.; Moon, K.C.; Yoon, B.H. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am. J. Obstet. Gynecol. 2005, 193 Pt 2, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Tsui, P.; Dorogin, A.; Lye, S.J. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J. Immunol. 2008, 181, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.; Purohit, P.; Kunde, K.N.N. Progesterone in Assisted Reproduction: Classification, Pharmacology and its clinical coorelation: A Commentary. Women’s Health Gynecol. 2020, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Groenewoud, E.R.; Cantineau, A.E.; Kollen, B.J.; Macklon, N.S.; Cohlen, B.J. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum. Reprod. Updat. 2013, 19, 458–470. [Google Scholar] [CrossRef]
- Labarta, E.; Rodríguez, C. Progesterone use in assisted reproductive technology. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 74–84. [Google Scholar] [CrossRef]
- Labarta, E. Relationship between serum progesterone (P) levels and pregnancy outcome: Lessons from artificial cycles when using vaginal natural micronized progesterone. J. Assist. Reprod. Genet. 2020, 37, 2047–2048. [Google Scholar] [CrossRef]
- Haas, D.M.; Ramsey, P.S. Progestogen for preventing miscarriage. Cochrane Database Syst. Rev. 2013, CD003511. [Google Scholar] [CrossRef]
- Wahabi, H.A.; Fayed, A.A.; Esmaeil, S.A.; Bahkali, K.H. Progestogen for treating threatened miscarriage. Cochrane Database Syst. Rev. 2018, CD005943. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Tan, H.; Bai, Y.; Fang, F.; Faramand, A.; Chong, W.; Hai, Y. Effect of progestogen for women with threatened miscarriage: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Khakwani, M.; Tabassum, S.; Masood, S. Oral versus Vaginal Micronized Progesterone for the treatment of threatened miscarriage. Pak. J. Med. Sci. 2021, 37, 628. [Google Scholar] [CrossRef] [PubMed]
- Coomarasamy, A.; Devall, A.J.; Brosens, J.J.; Quenby, S.; Stephenson, M.D.; Sierra, S.; Christiansen, O.B.; Small, R.; Brewin, J.; Roberts, T.E. Micronized vaginal progesterone to prevent miscarriage: A critical evaluation of randomized evidence. Am. J. Obstet. Gynecol. 2020, 223, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Devall, A.J.; Melo, P.; Coomarasamy, A. Progesterone for the prevention of threatened miscarriage. Obstet. Gynaecol. Reprod. Med. 2022, 2, 44–47. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Z.; Yang, Y.; Zheng, X.; Zou, M.; Cheng, G.; Yuan, Z. Efficacy of progesterone on threatened miscarriage: An updated meta-analysis of randomized trials. Arch. Gynecol. Obstet. 2021, 303, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Ku, C.W.; Kwek, L.K.; Lee, K.W.; Zhang, X.; Allen, J.C.; Zhang, V.R.-Y.; Tan, N.S. Novel approach using serum progesterone as a triage to guide management of patients with threatened miscarriage: A prospective cohort study. Sci. Rep. 2020, 10, 9153. [Google Scholar] [CrossRef]
- Ku, C.W.; Allen, J.C., Jr.; Lek, S.M.; Chia, M.L.; Tan, N.S.; Tan, T.C. Serum progesterone distribution in normal pregnancies compared to pregnancies complicated by threatened miscarriage from 5 to 13 weeks gestation: A prospective cohort study. BMC Pregnancy Childbirth 2018, 18, 360. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. National Institute for Health and Care Excellence: Guidelines. In Ectopic Pregnancy and Miscarriage: Diagnosis and Initial Management; National Institute for Health and Care Excellence (NICE): London, UK, 2021. [Google Scholar]
- Jarde, A.; Lutsiv, O.; Beyene, J.; McDonald, S.D. Vaginal progesterone, oral progesterone, 17-OHPC, cerclage, and pessary for preventing preterm birth in at-risk singleton pregnancies: An updated systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 556–567. [Google Scholar] [CrossRef]
- Romero, R.; Conde-Agudelo, A.; Da Fonseca, E.; O’Brien, J.M.; Cetingoz, E.; Creasy, G.W.; Hassan, S.S.; Nicolaides, K.H. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: A meta-analysis of individual patient data. Am. J. Obstet. Gynecol. 2018, 218, 161–180. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R.; Da Fonseca, E.; O’Brien, J.M.; Cetingoz, E.; Creasy, G.W.; Hassan, S.S.; Erez, O.; Pacora, P.; Nicolaides, K.H. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: Updated indirect comparison meta-analysis. Am. J. Obstet. Gynecol. 2018, 219, 10–25. [Google Scholar] [CrossRef]
- Boelig, R.C.; Della Corte, L.; Ashoush, S.; McKenna, D.; Saccone, G.; Rajaram, S.; Berghella, V. Oral progesterone for the prevention of recurrent preterm birth: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. MFM 2019, 1, 50–62. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, E.B.; Damião, R.; Moreira, D.A. Preterm birth prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.A.; Simmonds, M.; Duley, L.; Llewellyn, A.; Sharif, S.; Walker, R.A.; Beresford, L.; Wright, K.; Aboulghar, M.M.; Alfirevic, Z. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): Meta-analysis of individual participant data from randomised controlled trials. Lancet 2021, 397, 1183–1194. [Google Scholar] [CrossRef]
- Boelig, R.C.; Locci, M.; Saccone, G.; Gragnano, E.; Berghella, V. Vaginal progesterone compared with intramuscular 17-alpha-hydroxyprogesterone caproate for prevention of recurrent preterm birth in singleton gestations: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2022, 4, 100658. [Google Scholar] [CrossRef] [PubMed]
- Boelig, R.C.; Schoen, C.N.; Frey, H.; Gimovsky, A.C.; Springel, E.; Backley, S.; Berghella, V. Vaginal progesterone vs intramuscular 17-hydroxyprogesterone caproate for prevention of recurrent preterm birth: A randomized controlled trial. Am. J. Obstet. Gynecol. 2022, 226, 722.e1–722.e12. [Google Scholar] [CrossRef]
- Gillen-Goldstein, J.; Roque, H.; Young, B.K. Steroidogenesis patterns in common trisomies. J. Perinat. Med. 2002, 30, 132–136. [Google Scholar] [CrossRef]
- Kratzer, P.G.; Golbus, M.S.; Monroe, S.E.; Finkelstein, D.E.; Taylor, R.N. First-trimester aneuploidy screening using serum human chorionic gonadotropin (hCG), free ahCG, and progesterone. Prenat. Diagn. 1991, 11, 751–763. [Google Scholar] [CrossRef]
- Jewson, M.; Purohit, P.; Lumsden, M.A. Progesterone and abnormal uterine bleeding/menstrual disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 62–73. [Google Scholar] [CrossRef]
- Kadir, R.A. Menorrhagia: Treatment options. Thromb. Res. 2009, 123 (Suppl. 2), S21–S29. [Google Scholar] [CrossRef]
- Li, Y.; Adur, M.K.; Kannan, A.; Davila, J.; Zhao, Y.; Nowak, R.A.; Bagchi, M.K.; Bagchi, I.C.; Li, Q. Progesterone Alleviates Endometriosis via Inhibition of Uterine Cell Proliferation, Inflammation and Angiogenesis in an Immunocompetent Mouse Model. PLoS ONE 2016, 11, e0165347. [Google Scholar] [CrossRef]
- Casper, R.F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 2017, 107, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Poulos, C.; Soliman, A.M.; Renz, C.L.; Posner, J.; Agarwal, S.K. Patient Preferences for Endometriosis Pain Treatments in the United States. Value Health 2019, 22, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Kim, J.J.; Benbrook, D.M.; Dwivedi, A.; Rai, R. Therapeutic options for management of endometrial hyperplasia. J. Gynecol. Oncol. 2016, 27, e8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef]
- Gompel, A. Progesterone and endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 95–107. [Google Scholar] [CrossRef]
- Master-Hunter, T.; Heiman, D.L. Amenorrhea: Evaluation and treatment. Am. Fam. Physician 2006, 73, 1374–1382. [Google Scholar]
- McIver, B.; Romanski, S.A.; Nippoldt, T.B. Evaluation and Management of Amenorrhea. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 1997; pp. 1161–1169. [Google Scholar]
- Kiningham, R.B.; Apgar, B.S.; Schwenk, T.L. Evaluation of amenorrhea. Am. Fam. Physician 1996, 53, 1185–1194. [Google Scholar]
- Klein, D.A.; Paradise, S.L.; Reeder, R.M. Amenorrhea: A Systematic Approach to Diagnosis and Management. Am. Fam. Physician 2019, 100, 39–48. [Google Scholar]
- Ford, O.; Lethaby, A.; Roberts, H.; Mol, B.W. Progesterone for premenstrual syndrome. Cochrane Database Syst. Rev. 2012, CD003415. [Google Scholar] [CrossRef]
- Itriyeva, K. Premenstrual syndrome and premenstrual dysphoric disorder in adolescents. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101187. [Google Scholar] [CrossRef]
- Burger, H.G. Physiology and endocrinology of the menopause. Medicine 2006, 34, 27–30. [Google Scholar] [CrossRef]
- Deliveliotou, A.E. What is menopause? An overview of physiological changes. In Skin, Mucosa and Menopause; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–14. [Google Scholar]
- Hall, J.E. Endocrinology of the Menopause. Endocrinol. Metab. Clin. N. Am. 2015, 44, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, C.; Clavel-Chapelon, F.; Kaaks, R.; Peris, C.; Berrino, F. Progestins and progesterone in hormone replacement therapy and the risk of breast cancer. J. Steroid Biochem. Mol. Biol. 2005, 96, 95–108. [Google Scholar] [CrossRef]
- Vigneswaran, K.; Hamoda, H. Hormone replacement therapy—Current recommendations. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 81, 8–21. [Google Scholar] [CrossRef]
- Oettel, M.; Mukhopadhyay, A.K. Progesterone: The forgotten hormone in men? Aging Male 2004, 7, 236–257. [Google Scholar] [CrossRef]
- Matthiesson, K.L.; McLachlan, R.I. Male hormonal contraception: Concept proven, product in sight? Hum. Reprod. Update 2006, 12, 463–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McLachlan, R.I.; Robertson, D.M.; Pruysers, E.; Ugoni, A.; Matsumoto, A.M.; Anawalt, B.D.; Bremner, W.J.; Meriggiola, C. Relationship between serum gonadotropins and spermatogenic suppression in men undergoing steroidal contraceptive treatment. J. Clin. Endocrinol. Metab. 2004, 89, 142–149. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Cui, Y.G.; Wang, X.H.; Jia, Y.; Sinha Hikim, A.; Lue, Y.H.; Tong, J.S.; Qian, L.X.; Sha, J.H.; Zhou, Z.M.; et al. Transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J. Clin. Endocrinol. Metab. 2007, 92, 3292–3304. [Google Scholar] [CrossRef]
- Falsetti, C.; Baldi, E.; Krausz, C.; Casano, R.; Failli, P.; Forti, G. Decreased responsiveness to progesterone of spermatozoa in oligozoospermic patients. J. Androl. 1993, 14, 17–22. [Google Scholar]
- Oehninger, S.; Blackmore, P.; Morshedi, M.; Sueldo, C.; Acosta, A.A.; Alexander, N.J. Defective calcium influx and acrosome reaction (spontaneous and progesterone-induced) in spermatozoa of infertile men with severe teratozoospermia. Fertil. Steril. 1994, 61, 349–354. [Google Scholar] [CrossRef]
- Abid, S.; Gokral, J.; Maitra, A.; Meherji, P.; Kadam, S.; Pires, E.; Modi, D. Altered expression of progesterone receptors in testis of infertile men. Reprod. Biomed. Online 2008, 17, 175–184. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C. Defective function of a nongenomic progesterone receptor as a sole sperm anomaly in infertile patients. Fertil. Steril. 1992, 58, 793–797. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Bonsack, B.; Brinton, R.; Schumacher, M.; Kumar, N.; Lee, J.Y.; Castelli, V.; Corey, S.; Coats, A.; Sadanandan, N.; et al. Progress in progestin-based therapies for neurological disorders. Neurosci. Biobehav. Rev. 2021, 122, 38–65. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.; Gonzalez Deniselle, M.C.; Lima, A.; Roig, P.; De Nicola, A.F. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J. Steroid Biochem. Mol. Biol. 2007, 107, 228–237. [Google Scholar] [CrossRef]
- Del Rio, J.P.; Alliende, M.I.; Molina, N.; Serrano, F.G.; Molina, S.; Vigil, P. Steroid Hormones and Their Action in Women’s Brains: The Importance of Hormonal Balance. Front. Public Health 2018, 6, 141. [Google Scholar] [CrossRef]
- Sparaco, M.; Bonavita, S. The role of sex hormones in women with multiple sclerosis: From puberty to assisted reproductive techniques. Front. Neuroendocrinol. 2021, 60, 100889. [Google Scholar] [CrossRef]
- Theis, V.; Theiss, C. Progesterone Effects in the Nervous System. Anat. Rec. 2019, 302, 1276–1286. [Google Scholar] [CrossRef]
- Mancino, D.N.; Leicaj, M.L.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F.; Garay, L.I. Developmental expression of genes involved in progesterone synthesis, metabolism and action during the post-natal cerebellar myelination. J. Steroid Biochem. Mol. Biol. 2021, 207, 105820. [Google Scholar] [CrossRef]
- Koenig, H.L.; Schumacher, M.; Ferzaz, B.; Thi, A.N.; Ressouches, A.; Guennoun, R.; Jung-Testas, I.; Robel, P.; Akwa, Y.; Baulieu, E.E. Progesterone synthesis and myelin formation by Schwann cells. Science 1995, 268, 1500–1503. [Google Scholar] [CrossRef]
- Acs, P.; Kipp, M.; Norkute, A.; Johann, S.; Clarner, T.; Braun, A.; Berente, Z.; Komoly, S.; Beyer, C. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia 2009, 57, 807–814. [Google Scholar] [CrossRef]
- Garay, L.; Gonzalez Deniselle, M.C.; Gierman, L.; Meyer, M.; Lima, A.; Roig, P.; De Nicola, A.F. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation 2008, 15, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, I.; Stein, D.G. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain Res. 2009, 175, 219–237. [Google Scholar] [PubMed]
- Ghoumari, A.M.; Ibanez, C.; El-Etr, M.; Leclerc, P.; Eychenne, B.; O’Malley, B.W.; Baulieu, E.E.; Schumacher, M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J. Neurochem. 2003, 86, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Ghoumari, A.M.; Baulieu, E.E.; Schumacher, M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience 2005, 135, 47–58. [Google Scholar] [CrossRef]
- Kipp, M.; Amor, S.; Krauth, R.; Beyer, C. Multiple sclerosis: Neuroprotective alliance of estrogen-progesterone and gender. Front. Neuroendocrinol. 2012, 33, 1–16. [Google Scholar] [CrossRef]
- Schumacher, M.; Guennoun, R.; Robert, F.; Carelli, C.; Gago, N.; Ghoumari, A.; Gonzalez Deniselle, M.C.; Gonzalez, S.L.; Ibanez, C.; Labombarda, F.; et al. Local synthesis and dual actions of progesterone in the nervous system: Neuroprotection and myelination. Growth Horm. IGF Res. 2004, 14 (Suppl. A), S18–S33. [Google Scholar] [CrossRef]
- Ibanez, C.; Shields, S.A.; El-Etr, M.; Baulieu, E.E.; Schumacher, M.; Franklin, R.J. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol. Appl. Neurobiol. 2004, 30, 80–89. [Google Scholar] [CrossRef]
- Labombarda, F.; Gonzalez, S.; Gonzalez Deniselle, M.C.; Garay, L.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J. Neurotrauma 2006, 23, 181–192. [Google Scholar] [CrossRef]
- Hughes, M.D. Multiple sclerosis and pregnancy. Neurol. Clin. 2004, 22, 757–769. [Google Scholar] [CrossRef]
- Kipp, M.; Hochstrasser, T.; Schmitz, C.; Beyer, C. Female sex steroids and glia cells: Impact on multiple sclerosis lesion formation and fine tuning of the local neurodegenerative cellular network. Neurosci. Biobehav. Rev. 2016, 67, 125–136. [Google Scholar] [CrossRef]
- Gargiulo-Monachelli, G.; Meyer, M.; Lara, A.; Garay, L.; Lima, A.; Roig, P.; De Nicola, A.F.; Gonzalez Deniselle, M.C. Comparative effects of progesterone and the synthetic progestin norethindrone on neuroprotection in a model of spontaneous motoneuron degeneration. J. Steroid Biochem. Mol. Biol. 2019, 192, 105385. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Garay, L.I.; Kruse, M.S.; Lara, A.; Gargiulo-Monachelli, G.; Schumacher, M.; Guennoun, R.; Coirini, H.; De Nicola, A.F.; Gonzalez Deniselle, M.C. Protective effects of the neurosteroid allopregnanolone in a mouse model of spontaneous motoneuron degeneration. J. Steroid Biochem. Mol. Biol. 2017, 174, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Gonzalez Deniselle, M.C.; Garay, L.I.; Monachelli, G.G.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Stage dependent effects of progesterone on motoneurons and glial cells of wobbler mouse spinal cord degeneration. Cell. Mol. Neurobiol. 2010, 30, 123–135. [Google Scholar] [CrossRef]
- Gonzalez Deniselle, M.C.; Carreras, M.C.; Garay, L.; Gargiulo-Monachelli, G.; Meyer, M.; Poderoso, J.J.; De Nicola, A.F. Progesterone prevents mitochondrial dysfunction in the spinal cord of wobbler mice. J. Neurochem. 2012, 122, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, P.E.; Patil, A.A.; Chamczuk, A.J.; Agrawal, D.K. Hormonal therapy in traumatic spinal cord injury. Am. J. Transl. Res. 2017, 9, 3881–3895. [Google Scholar] [PubMed]
- De Nicola, A.F.; Gonzalez, S.L.; Labombarda, F.; Gonzalez Deniselle, M.C.; Garay, L.; Guennoun, R.; Schumacher, M. Progesterone treatment of spinal cord injury: Effects on receptors, neurotrophins, and myelination. J. Mol. Neurosci. MN 2006, 28, 3–15. [Google Scholar] [CrossRef]
- Aminmansour, B.; Asnaashari, A.; Rezvani, M.; Ghaffarpasand, F.; Amin Noorian, S.M.; Saboori, M.; Abdollahzadeh, P. Effects of progesterone and vitamin D on outcome of patients with acute traumatic spinal cord injury; a randomized, double-blind, placebo controlled study. J. Spinal Cord Med. 2016, 39, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Frechou, M.; Zhang, S.; Liere, P.; Delespierre, B.; Soyed, N.; Pianos, A.; Schumacher, M.; Mattern, C.; Guennoun, R. Intranasal delivery of progesterone after transient ischemic stroke decreases mortality and provides neuroprotection. Neuropharmacology 2015, 97, 394–403. [Google Scholar] [CrossRef]
- Won, S.; Lee, J.H.; Wali, B.; Stein, D.G.; Sayeed, I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: Involvement of the VEGF-MMP pathway. J. Cereb. Blood Flow Metab. 2014, 34, 72–80. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, J.; Li, X.; Liu, C.; Chen, N.; Hao, Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm. Res. 2009, 58, 619–624. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Sayeed, I.; Wang, J.; Stein, D.G. Neuroprotection by progesterone after transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Horm. Behav. 2016, 84, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Medhi, B.; Pathak, A.; Dhawan, V.; Chakrabarti, A. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J. Pharm. Pharmacol. 2008, 60, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Mondelli, M.; Ginanneschi, F.; Mazzocchio, R.; Rossi, A. Progesterone—New therapy in mild carpal tunnel syndrome? Study design of a randomized clinical trial for local therapy. J. Brachial Plex. Peripher. Nerve Inj. 2010, 5, 11. [Google Scholar] [CrossRef][Green Version]
- Ginanneschi, F.; Milani, P.; Filippou, G.; Mondelli, M.; Frediani, B.; Melcangi, R.C.; Rossi, A. Evidences for antinociceptive effect of 17-alpha-hydroxyprogesterone caproate in carpal tunnel syndrome. J. Mol. Neurosci. MN 2012, 47, 59–66. [Google Scholar] [CrossRef]
- Bahrami, M.H.; Shahraeeni, S.; Raeissadat, S.A. Comparison between the effects of progesterone versus corticosteroid local injections in mild and moderate carpal tunnel syndrome: A randomized clinical trial. BMC Musculoskelet. Disord. 2015, 16, 322. [Google Scholar] [CrossRef] [PubMed]
- Raeissadat, S.A.; Shahraeeni, S.; Sedighipour, L.; Vahdatpour, B. Randomized controlled trial of local progesterone vs corticosteroid injection for carpal tunnel syndrome. Acta Neurol. Scand. 2017, 136, 365–371. [Google Scholar] [CrossRef]
- Fent, K. Progestins as endocrine disrupters in aquatic ecosystems: Concentrations, effects and risk assessment. Environ. Int. 2015, 84, 115–130. [Google Scholar] [CrossRef]
- Zucchi, S.; Castiglioni, S.; Fent, K. Progestins and antiprogestins affect gene expression in early development in zebrafish (Danio rerio) at environmental concentrations. Environ. Sci. Technol. 2012, 46, 5183–5192. [Google Scholar] [CrossRef]
- Zucchi, S.; Castiglioni, S.; Fent, K. Progesterone alters global transcription profiles at environmental concentrations in brain and ovary of female zebrafish (Danio rerio). Environ. Sci. Technol. 2013, 47, 12548–12556. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Wu, S.; Fan, Z.; Hu, J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 732–740. [Google Scholar] [CrossRef]
- Sauer, P.; Stara, A.; Golovko, O.; Valentova, O.; Borik, A.; Grabic, R.; Kroupova, H.K. Two synthetic progestins and natural progesterone are responsible for most of the progestagenic activities in municipal wastewater treatment plant effluents in the Czech and Slovak republics. Water Res. 2018, 137, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ying, G.G.; Zhang, R.Q.; Zhou, L.J.; Lai, H.J.; Chen, Z.F. Fate and occurrence of steroids in swine and dairy cattle farms with different farming scales and wastes disposal systems. Environ. Pollut. 2012, 170, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Majumder, C.B.; Roy, P. Development of a yeast-based assay to determine the (anti)androgenic contaminants from pulp and paper mill effluents in India. Environ. Toxicol. Pharmacol. 2007, 24, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, E.K.; Jayasinghe, B.S.; Pine, W.E.; Wilkinson, K.A.; Denslow, N.D. Exposure to paper mill effluent at a site in North Central Florida elicits molecular-level changes in gene expression indicative of progesterone and androgen exposure. PLoS ONE 2014, 9, e106644. [Google Scholar]
- Kroupova, H.K.; Trubiroha, A.; Lorenz, C.; Contardo-Jara, V.; Lutz, I.; Grabic, R.; Kocour, M.; Kloas, W. The progestin levonorgestrel disrupts gonadotropin expression and sex steroid levels in pubertal roach (Rutilus rutilus). Aquat. Toxicol. 2014, 154, 154–162. [Google Scholar] [CrossRef]
- Kumar, V.; Johnson, A.C.; Trubiroha, A.; Tumova, J.; Ihara, M.; Grabic, R.; Kloas, W.; Tanaka, H.; Kroupova, H.K. The challenge presented by progestins in ecotoxicological research: A critical review. Environ. Sci. Technol. 2015, 49, 2625–2638. [Google Scholar] [CrossRef]
- Raghavan, R.; Romano, M.E.; Karagas, M.R.; Penna, F.J. Pharmacologic and Environmental Endocrine Disruptors in the Pathogenesis of Hypospadias: A Review. Curr. Environ. Health Rep. 2018, 5, 499–511. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Xu, W.; Liang, X.; Jing, Z.; Pan, C.G.; Tian, F. The synthetic progestin norethindrone causes thyroid endocrine disruption in adult zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 236, 108819. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Huang, G.Y.; Liu, S.S.; Zhao, J.L.; Yang, Y.Y.; Chen, X.W.; Tian, F.; Jiang, Y.X.; Ying, G.G. Long-term exposure to environmentally relevant concentrations of progesterone and norgestrel affects sex differentiation in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 160, 172–179. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Huang, G.Y.; Ying, G.G.; Liu, S.S.; Jiang, Y.X.; Liu, S.; Peng, F.J. A time-course transcriptional kinetics of the hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal axes in zebrafish eleutheroembryos after exposure to norgestrel. Environ. Toxicol. Chem. 2015, 34, 112–119. [Google Scholar] [CrossRef]
- Runnalls, T.J.; Beresford, N.; Losty, E.; Scott, A.P.; Sumpter, J.P. Several synthetic progestins with different potencies adversely affect reproduction of fish. Environ. Sci. Technol. 2013, 47, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, J.; Steger-Hartmann, T.; Maser, E.; Goller, S.; Vonk, R.; Lange, R. Effects of synthetic gestagens on fish reproduction. Environ. Toxicol. Chem. 2009, 28, 2663–2670. [Google Scholar] [CrossRef]
- Svensson, J.; Fick, J.; Brandt, I.; Brunstrom, B. The synthetic progestin levonorgestrel is a potent androgen in the three-spined stickleback (Gasterosteus aculeatus). Environ. Sci. Technol. 2013, 47, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, H.; Xu, X.R.; Liu, S.S.; Sun, K.F.; Zhao, J.L.; Ying, G.G. Steroids in marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioconcentration, and human dietary exposure. Sci. Total Environ. 2015, 502, 400–407. [Google Scholar] [CrossRef]
- Sauer, P.; Tumova, J.; Steinbach, C.; Golovko, O.; Komen, H.; Maillot-Marechal, E.; Machova, J.; Grabic, R.; Ait-Aissa, S.; Kocour Kroupova, H. Chronic simultaneous exposure of common carp (Cyprinus carpio) from embryonic to juvenile stage to drospirenone and gestodene at low ng/L level caused intersex. Ecotoxicol. Environ. Saf. 2020, 188, 109912. [Google Scholar] [CrossRef]
- Silva, E.; Rajapakse, N.; Kortenkamp, A. Something from “nothing”—Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 2002, 36, 1751–1756. [Google Scholar] [CrossRef]



| Action | Cell/Tissue | Receptor | Signaling Pathway | References |
|---|---|---|---|---|
| Acrosome reaction/capacitation | Human spermatozoa | mPRs | Ca2+, cAMP, G/adenylyl cyclase, mitogen activated protein kinase | [13,14,15] |
| Steroidogenesis, luteinizing hormone action | Rodent Leydig cells | mPRs | Na+ | [4,16,17] |
| Oocyte maturation | Amphibian and fish oocytes | mPRs | G-protein, extracellular signal-regulated kinases, phosphoinositide-3-kinase, cAMP | [18,19,20,21,22,23,24,25] |
| Immunoregulation | Human T-lymphocytes | mPRs | G-protein, K+ channel | [26,27] |
| Platelet aggregation | Human platelets | mPRs | Ca2+, Src-dependent pathway | [28,29,30] |
| Anti-apoptotic effect | Rat granulosa cells | mPRs | mitogen-activated protein kinase, Ca2+, protein kinase G | [31,32] |
| Vasoreaction | Rat vascular smooth muscle cells | Ca2+, cAMP | [33] | |
| Actin cytoskeleton remodeling/cell movement | Human umbilical vein endothelial cells | mPRs | G-protein, phosphoinositide 3-kinase, Rho-associated kinase | [34,35] |
| Muscle contraction | Human intestinal smooth muscle cells | mPRs | Ca2+ | [36] |
| Inhibition of proliferation | Smooth muscle cells | mPRs | Src/RhoA—kinases | [37] |
| Transepithelial resistance | Human fetal membranes | Not determined | not determined | [38] |
| Activation of transcription factors | Breast cancer | mPRs | extracellular signal regulated kinases, Src/Akt-kinases, phosphoinositide-3-kinases | [34,39] |
| Neuroprotection | Mouse cerebral cortex, rat hippocampal neurons | mPRs, σ1 receptor | Phosphoinositide 3-kinase, extracellular receptor kinase, Ca2+, | [40,41,42,43] |
| Brain-derived neurotropic factor (BDNP) release | Glia | mPRs | Extracellular signal regulated kinases | [44] |
| Retinal neuronal activity | Mouse rod bipolar cells | Inositol-triphosphate receptor type 1 | Phosphoinositide 3-kinase, Ca2+ | [45] |
| Gonadotropin-releasing hormone (GnRH) release | Hypothalamic neurons | mPRs | Not determined | [46] |
| Lordosis | Ventral tegmental area, mid-brain | GABAA/benzodiazepine receptor complexes | Not determined | [47] |
| Steroid (nmol/L) | Follicular Phase | Luteal Phase | Pregnancy |
|---|---|---|---|
| Progesterone | 1.3 | 36.2 | 320 |
| Allopregnanolone | 0.51 | 1.59 | 32 |
| Isopregnanolone | 0.27 | 0.9 | 18 |
| Pregnanolone | 0.134 | 0.375 | 20 |
| Epipregnanolone | 0.062 | 0.168 | 1.4 |
| Receptor | Modulation | Pregnane Isomers | Steroids | Action |
|---|---|---|---|---|
| GABAA | Positive | 3α-isomers | Allopregnanolone, pregnanolone | Neuroinhibition |
| Negative | 3β-isomers | Isopregnanolone, epipregnanolone | Neuroactivation | |
| Conjugates of all preg. isomers | ||||
| PregS, DHEAS | ||||
| NMDA | Positive | Conjugates of 5α-isomers | Allopregnanolone, pregnanolone | Neuroactivation |
| PregS, DHEAS | ||||
| Negative | Conjugates of 5β-isomers | Isopregnanolone, epipregnanolone | Neuroinhibition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolatorova, L.; Vitku, J.; Suchopar, J.; Hill, M.; Parizek, A. Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine. Int. J. Mol. Sci. 2022, 23, 7989. https://doi.org/10.3390/ijms23147989
Kolatorova L, Vitku J, Suchopar J, Hill M, Parizek A. Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine. International Journal of Molecular Sciences. 2022; 23(14):7989. https://doi.org/10.3390/ijms23147989
Chicago/Turabian StyleKolatorova, Lucie, Jana Vitku, Josef Suchopar, Martin Hill, and Antonin Parizek. 2022. "Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine" International Journal of Molecular Sciences 23, no. 14: 7989. https://doi.org/10.3390/ijms23147989
APA StyleKolatorova, L., Vitku, J., Suchopar, J., Hill, M., & Parizek, A. (2022). Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine. International Journal of Molecular Sciences, 23(14), 7989. https://doi.org/10.3390/ijms23147989








