MicroRNAs as Innovative Biomarkers for Inflammatory Bowel Disease and Prediction of Colorectal Cancer
Abstract
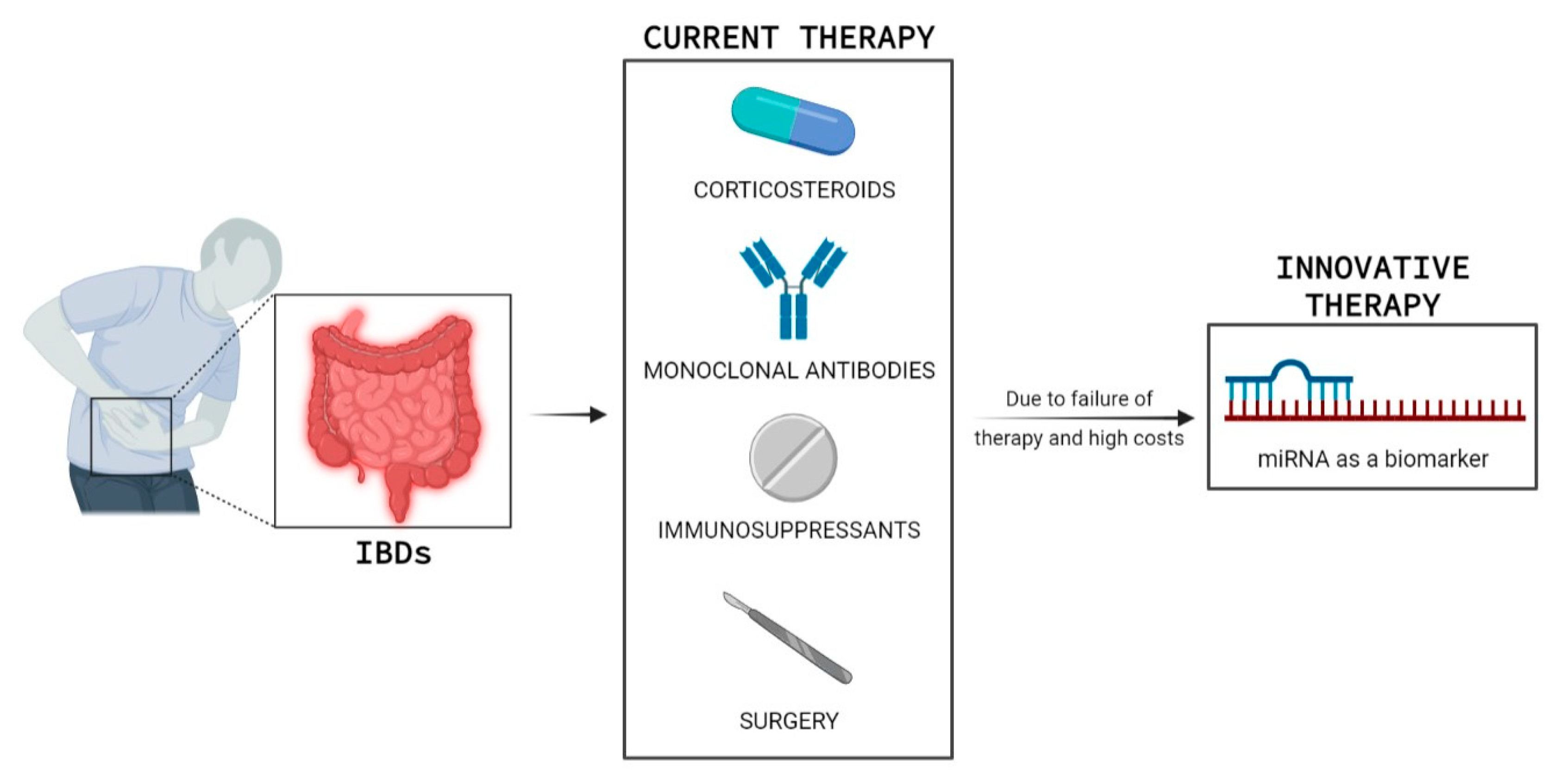
1. Introduction
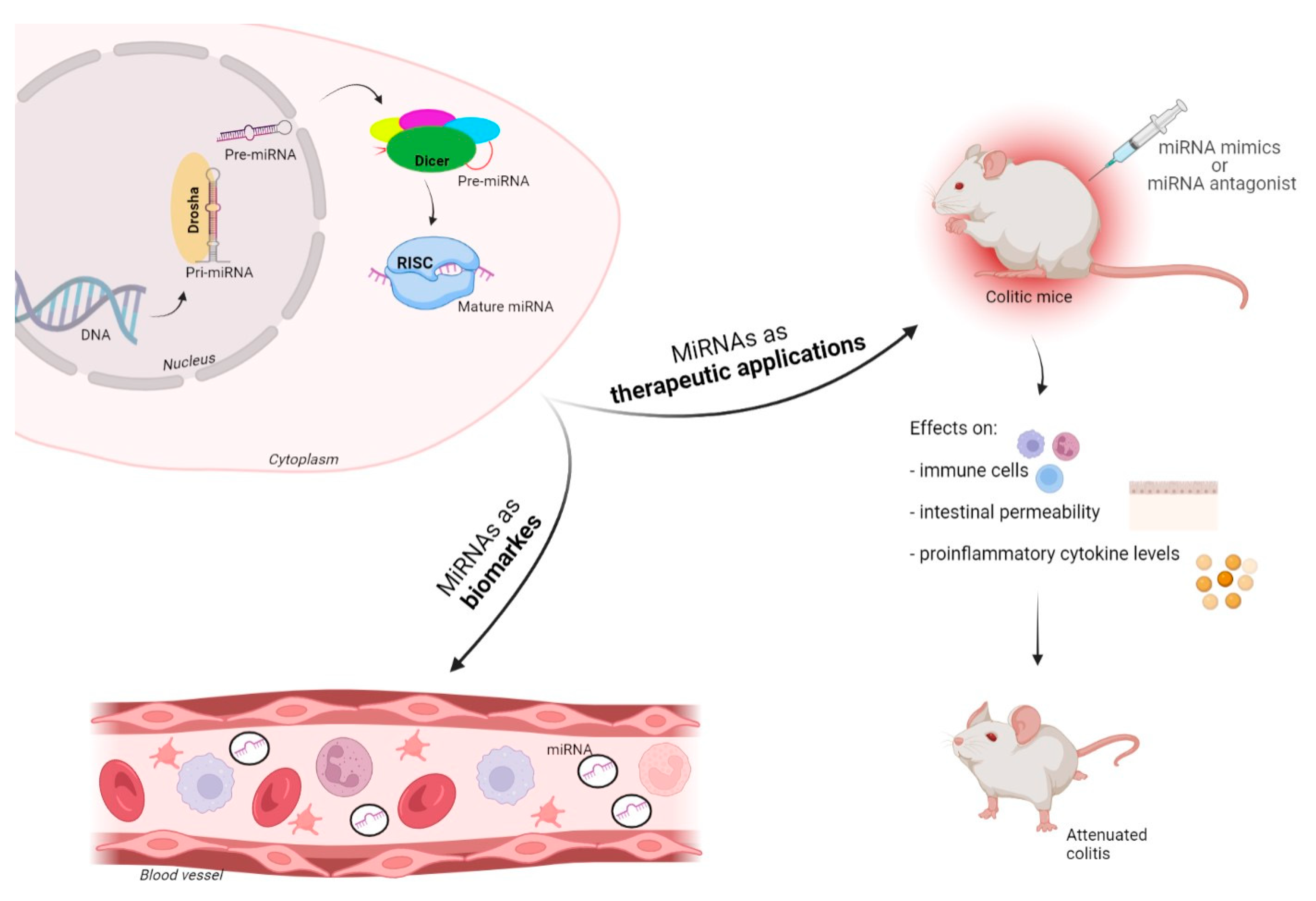
2. MicroRNAs–Biogenesis and Function
3. MiRNAs in IBD
4. Mucosal Tissue miRNAs in IBD
5. Circulating miRNAs in IBD
6. Fecal miRNAs in IBD and as Screening for Colorectal Cancer (CRC)
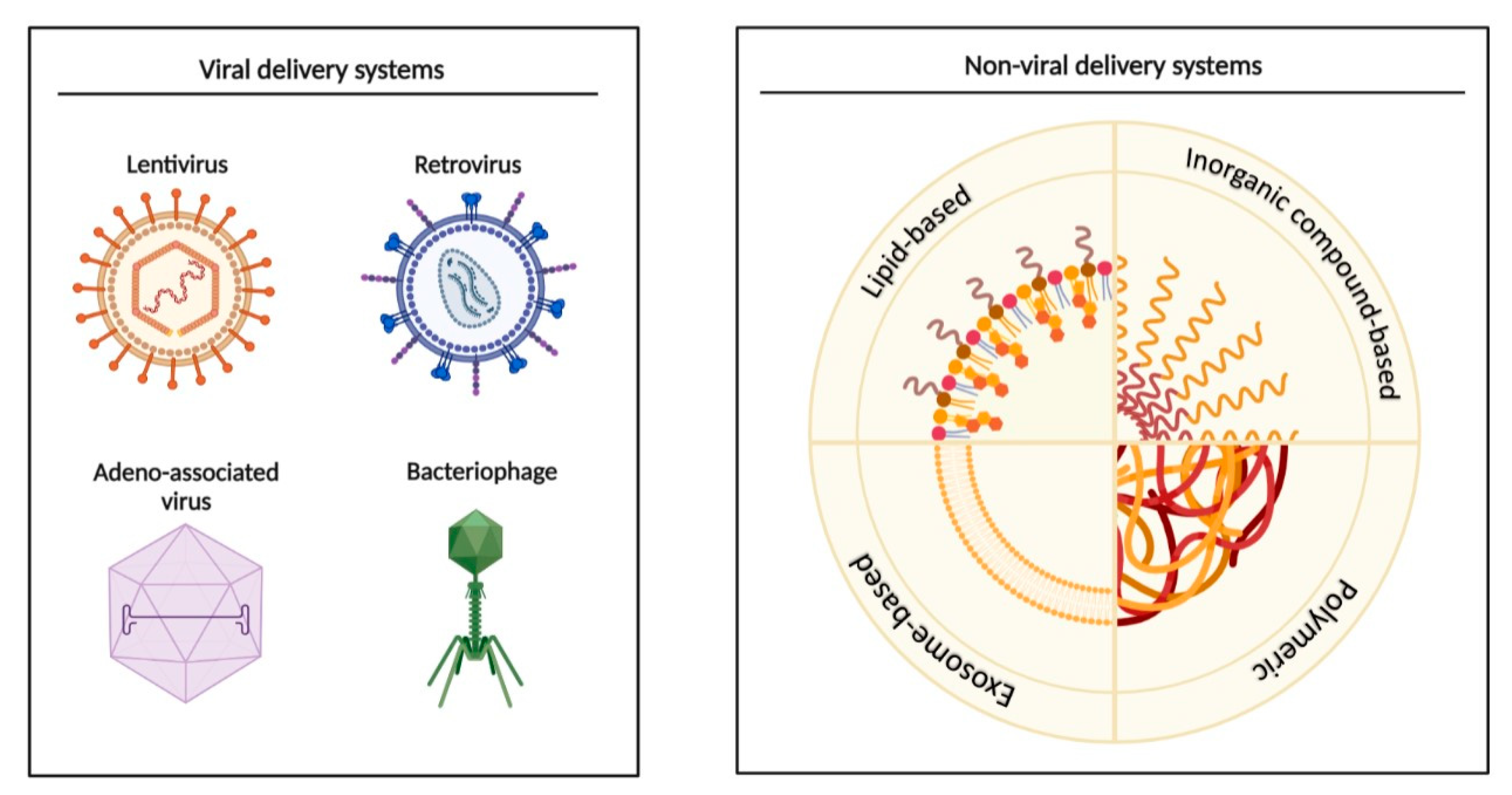
7. MiRNAs as Biomarkers, and Potential Diagnostic and Therapeutic Applications
8. Future of miRNAs in the Clinic
9. A Step Forward towards Personalized Medicine
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelsen, J.R.; Russo, P.; Sullivan, K.E. Early-Onset Inflammatory Bowel Disease. Immunol. Allergy Clin. N. Am. 2019, 39, 63–79. [Google Scholar] [CrossRef]
- Ouahed, J.; Spencer, E.; Kotlarz, D.; Shouval, D.; Kowalik, M.; Peng, K.; Field, M.; Grushkin-Lerner, L.; Pai, S.-Y.; Bousvaros, A.; et al. Very Early Onset Inflammatory Bowel Disease: A Clinical Approach With a Focus on the Role of Genetics and Underlying Immune Deficiencies. Inflamm. Bowel Dis. 2020, 26, 820–842. [Google Scholar] [CrossRef]
- Ho, S.M.; Lewis, J.D.; Mayer, E.A.; Plevy, S.E.; Chuang, E.; Rappaport, S.M.; Croitoru, K.; Korzenik, J.R.; Krischer, J.; Hyams, J.S.; et al. Challenges in IBD Research: Environmental Triggers. Inflamm. Bowel Dis. 2019, 25, S13–S23. [Google Scholar] [CrossRef]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Suri, K.; Bubier, J.A.; Wiles, M.V.; Shultz, L.D.; Amiji, M.M.; Hosur, V. Role of MicroRNA in Inflammatory Bowel Disease: Clinical Evidence and the Development of Preclinical Animal Models. Cells 2021, 10, 2204. [Google Scholar] [CrossRef]
- James, J.P.; Riis, L.B.; Malham, M.; Høgdall, E.; Langholz, E.; Nielsen, B.S. MicroRNA Biomarkers in IBD-Differential Diagnosis and Prediction of Colitis-Associated Cancer. Int. J. Mol. Sci. 2020, 21, 7893. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar]
- López-Sanromán, A.; Esplugues, J.V.; Domènech, E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol. Hepatol. 2021, 44, 39–48. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K.; et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohn’s Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef]
- Roberti, R.; Iannone, L.F.; Palleria, C.; Sarro, C.D.; Spagnuolo, R.; Barbieri, M.A.; Vero, A.; Manti, A.; Pisana, V.; Fries, W.; et al. Safety profiles of biologic agents for inflammatory bowel diseases: A prospective pharmacovigilance study in Southern Italy. Curr. Med. Res. Opin. 2020, 36, 1457–1463. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F.; Siegmund, B. Personalizing Treatment in IBD: Hype or Reality in 2020? Can We Predict Response to Anti-TNF? Front. Med. 2020, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Chong, C.A.; Chong, R.Y. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J. Crohn’s Colitis 2014, 8, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Gerardi, V.; Papa, V.; Scaldaferri, F.; Rapaccini, G.L.; Gasbarrini, A.; Papa, A. Can We Predict the Efficacy of Anti-TNF-α Agents? Int. J. Mol. Sci. 2017, 18, 1973. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cao, D.L.; Zhao, L.X.; Han, Y.; Zhang, Y.L. MicroRNA-146a-5p attenuates visceral hypersensitivity through targeting chemokine CCL8 in the spinal cord in a mouse model of colitis. Brain Res. Bull. 2018, 139, 235–242. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S.; Qiu, Y.; He, Y.; Chen, B.; Mao, R.; Cui, Y.; Zeng, Z.; Chen, M. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis. 2017, 8, e2699. [Google Scholar] [CrossRef]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-induced silencing complex: A versatile gene-silencing machine. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef]
- Correia, C.N.; Nalpas, N.C.; McLoughlin, K.E.; Browne, J.A.; Gordon, S.V.; MacHugh, D.E.; Shaughnessy, R.G. Circulating microRNAs as Potential Biomarkers of Infectious Disease. Front. Immunol. 2017, 8, 118. [Google Scholar] [CrossRef]
- Sarshar, M.; Scribano, D.; Ambrosi, C.; Palamara, A.T.; Masotti, A. Fecal microRNAs as Innovative Biomarkers of Intestinal Diseases and Effective Players in Host-Microbiome Interactions. Cancers 2020, 12, 2174. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Yang, S.; Abraham, E.; Agarwal, A.; Liu, G. Identification of a microRNA signature in renal fibrosis: Role of miR-21. Am. J. Physiol. Renal. Physiol. 2011, 301, F793–F801. [Google Scholar] [CrossRef]
- Agarwal, S.; Hanna, J.; Sherman, M.E.; Figueroa, J.; Rimm, D.L. Quantitative assessment of miR34a as an independent prognostic marker in breast cancer. Br. J. Cancer 2015, 112, 61–68. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, S.; Yanai, H.; Horev, H.S.; Elad, H.; Baram, L.; Issakov, O.; Tulchinsky, H.; Pasmanik-Chor, M.; Shomron, N.; Dotan, I. MicroRNAs Expression in the Ileal Pouch of Patients with Ulcerative Colitis Is Robustly Up-Regulated and Correlates with Disease Phenotypes. PLoS ONE 2016, 11, e0159956. [Google Scholar]
- Schaefer, J.S. MicroRNAs: How many in inflammatory bowel disease? Curr. Opin. Gastroenterol. 2016, 32, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zikusoka, M.; Trindade, A.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Brant, S.R.; Chakravarti, S.; Kwon, J.H. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 2008, 135, 1624–1635. [Google Scholar] [CrossRef]
- Fasseu, M.; Tréton, X.; Guichard, C.; Pedruzzi, E.; Cazals-Hatem, D.; Richard, C.; Aparicio, T.; Daniel, F.; Soule, J.C.; Moreau, R.; et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS ONE 2010, 5, e13160. [Google Scholar] [CrossRef]
- Zidar, N.; Boštjančič, E.; Jerala, M.; Kojc, N.; Drobne, D.; Štabuc, B.; Glavač, D. Down-regulation of microRNAs of the miR-200 family and up-regulation of Snail and Slug in inflammatory bowel diseases—Hallmark of epithelial-mesenchymal transition. J. Cell. Mol. Med. 2016, 20, 1813–1820. [Google Scholar] [CrossRef]
- Béres, N.J.; Szabó, D.; Kocsis, D.; Szűcs, D.; Kiss, Z.; Müller, K.E.; Lendvai, C.; Kiss, A.; Arató, A.; Sziksz, E.; et al. Role of Altered Expression of miR-146a, miR-155, and miR-122 in Pediatric Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 327–335. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, G.; Nielsen, B.S.; Andersen, V.; Holmstrøm, K.; Pedersen, A.E. Expression and Localization of miR-21 and miR-126 in Mucosal Tissue from Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Mizushima, K.; Hirata, I.; Yagi, N.; Tomatsuri, N.; Ando, T.; Oyamada, Y.; Isozaki, Y.; Hongo, H.; et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J. Gastroenterol. Hepatol. 2010, 25, S129–S133. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Kelly, O.B.; Smith, M.I.; Kabakchiev, B.; Silverberg, M.S. Differential miRNA Expression in Ileal and Colonic Tissues Reveals an Altered Immunoregulatory Molecular Profile in Individuals With Crohn’s Disease versus Healthy Subjects. J. Crohn’s Colitis 2019, 13, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Rieger, A.; Schildberg, F.A.; Knolle, P.A.; Schmid-Burgk, J.L.; Hornung, V. NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 2012, 189, 4175–4181. [Google Scholar] [CrossRef]
- Haneklaus, M.; Gerlic, M.; Kurowska-Stolarska, M.; Rainey, A.A.; Pich, D.; McInnes, I.B.; Hammerschmidt, W.; O’Neill, L.A.; Masters, S.L. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J. Immunol. 2012, 189, 3795–3799. [Google Scholar] [CrossRef]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef]
- Wu, F.; Guo, N.J.; Tian, H.; Marohn, M.; Gearhart, S.; Bayless, T.M.; Brant, S.R.; Kwon, J.H. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 241–250. [Google Scholar] [CrossRef]
- Paraskevi, A.; Theodoropoulos, G.; Papaconstantinou, I.; Mantzaris, G.; Nikiteas, N.; Gazouli, M. Circulating MicroRNA in inflammatory bowel disease. J. Crohn’s Colitis 2012, 6, 900–904. [Google Scholar] [CrossRef]
- Iborra, M.; Bernuzzi, F.; Correale, C.; Vetrano, S.; Fiorino, G.; Beltrán, B.; Marabita, F.; Locati, M.; Spinelli, A.; Nos, P.; et al. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin. Exp. Immunol. 2013, 173, 250–258. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Li, L.; Yu, Q.; Chao, K.; Zhou, G.; Qiu, Y.; Feng, R.; Huang, S.; He, Y.; et al. Circulating microRNA146b-5p is superior to C-reactive protein as a novel biomarker for monitoring inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 49, 733–743. [Google Scholar] [CrossRef]
- Schönauen, K.; Le, N.; Arnim, U.V.; Schulz, C.; Malfertheiner, P.; Link, A. Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2018, 24, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, Y.; Tan, S. The role of the miR-21-5p-mediated inflammatory pathway in ulcerative colitis. Exp. Ther. Med. 2020, 19, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Cordes, F.; Demmig, C.; Bokemeyer, A.; Brückner, M.; Lenze, F.; Lenz, P.; Nowacki, T.; Tepasse, P.; Schmidt, H.H.; Schmidt, M.A.; et al. MicroRNA-320a Monitors Intestinal Disease Activity in Patients With Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2020, 11, e00134. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Jeffries, C.D.; Vos, P.W.; Flake, G.; Nuovo, G.J.; Sinar, D.R.; Naziri, W.; Marcuard, S.P. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genom. Proteom. 2009, 6, 281–295. [Google Scholar]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Verdier, J.; Breunig, I.R.; Ohse, M.C.; Roubrocks, S.; Kleinfeld, S.; Roy, S.; Streetz, K.; Trautwein, C.; Roderburg, C.; Sellge, G. Faecal Micro-RNAs in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2020, 14, 110–117. [Google Scholar] [CrossRef]
- Ji, Y.; Li, X.; Zhu, Y.; Li, N.; Zhang, N.; Niu, M. Faecal microRNA as a biomarker of the activity and prognosis of inflammatory bowel diseases. Biochem. Biophys. Res. Commun. 2018, 503, 2443–2450. [Google Scholar] [CrossRef]
- Zhou, R.; Qiu, P.; Wang, H.; Yang, H.; Yang, X.; Ye, M.; Wang, F.; Zhao, Q. Identification of microRNA-16-5p and microRNA-21-5p in feces as potential noninvasive biomarkers for inflammatory bowel disease. Aging 2021, 13, 4634–4646. [Google Scholar] [CrossRef]
- Ayyadurai, S.; Charania, M.A.; Xiao, B.; Viennois, E.; Merlin, D. PepT1 expressed in immune cells has an important role in promoting the immune response during experimentally induced colitis. Lab. Investig. 2013, 93, 888–899. [Google Scholar] [CrossRef]
- Josse, C.; Bours, V. MicroRNAs and Inflammation in Colorectal Cancer. Adv. Exp. Med. Biol. 2016, 937, 53–69. [Google Scholar]
- Link, A.; Balaguer, F.; Shen, Y.; Nagasaka, T.; Lozano, J.J.; Boland, C.R.; Goel, A. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Duran-Sanchon, S.; Moreno, L.; Augé, J.M.; Serra-Burriel, M.; Cuatrecasas, M.; Moreira, L.; Martín, A.; Serradesanferm, A.; Pozo, À.; Costa, R.; et al. Identification and Validation of MicroRNA Profiles in Fecal Samples for Detection of Colorectal Cancer. Gastroenterology 2020, 158, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Duran-Sanchon, S.; Moreno, L.; Gómez-Matas, J.; Augé, J.M.; Serra-Burriel, M.; Cuatrecasas, M.; Moreira, L.; Serradesanferm, A.; Pozo, À.; Grau, J.; et al. Fecal MicroRNA-Based Algorithm Increases Effectiveness of Fecal Immunochemical Test-Based Screening for Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2021, 19, 323–330. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell. Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.G.; Pekow, J. The emerging role of miRNAs in inflammatory bowel disease: A review. Ther. Adv. Gastroenterol. 2015, 8, 4–22. [Google Scholar] [CrossRef]
- He, C.; Shi, Y.; Wu, R.; Sun, M.; Fang, L.; Wu, W.; Liu, C.; Tang, M.; Li, Z.; Wang, P. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-α in IBD. Gut 2016, 65, 1938–1950. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, T.; Chen, W.; Chen, Y.; Li, M.; Ren, L.; Chen, J.; Cao, R.; Feng, Y.; Zhang, H.; et al. MicroRNA-124 Promotes Intestinal Inflammation by Targeting Aryl Hydrocarbon Receptor in Crohn’s Disease. J. Crohn’s Colitis 2016, 10, 703–712. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Dalmasso, G.; Müller, S.; Carrière, J.; Seibold, F.; Darfeuille-Michaud, A. Crohn’s disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology 2014, 146, 508–519. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, T.; Zhou, Q.; Shi, S.; Zhao, R.; Shi, H.; Dong, L.; Zhang, C.; Zeng, K.; Chen, J.; et al. miR-141 Regulates colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human Crohn’s disease. Gut 2014, 63, 1247–1257. [Google Scholar] [CrossRef]
- Lu, Z.J.; Wu, J.J.; Jiang, W.L.; Xiao, J.H.; Tao, K.Z.; Ma, L.; Zheng, P.; Wan, R.; Wang, X.P. MicroRNA-155 promotes the pathogenesis of experimental colitis by repressing SHIP-1 expression. World J. Gastroenterol. 2017, 23, 976–985. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Engers, J.; Abdulqadir, R. Talk about micromanaging! Role of microRNAs in intestinal barrier function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 319, G170–G174. [Google Scholar] [CrossRef] [PubMed]
- Law, I.K.; Bakirtzi, K.; Polytarchou, C.; Oikonomopoulos, A.; Hommes, D.; Iliopoulos, D.; Pothoulakis, C. Neurotensin—Regulated miR-133α is involved in proinflammatory signalling in human colonic epithelial cells and in experimental colitis. Gut 2015, 64, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, D.; Zheng, R.H.; Zhang, H.; Chen, Y.P.; Xiang, Z. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J. Gastroenterol. 2017, 23, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Salvo, C.D.; Pastorelli, L.; Rana, N.; Senkfor, H.N.; Petito, V.; Di Martino, L.; Scaldaferri, F.; Gasbarrini, A.; Cominelli, F.; et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc. Natl. Acad. Sci. USA 2018, 115, E9362–E9370. [Google Scholar] [CrossRef]
- Soroosh, A.; Koutsioumpa, M.; Pothoulakis, C.; Iliopoulos, D. Functional role and therapeutic targeting of microRNAs in inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G256–G262. [Google Scholar] [CrossRef]
- Morilla, I.; Uzzan, M.; Laharie, D.; Cazals-Hatem, D.; Denost, Q.; Daniel, F.; Belleannee, G.; Bouhnik, Y.; Wainrib, G.; Panis, Y.; et al. Colonic MicroRNA Profiles, Identified by a Deep Learning Algorithm, That Predict Responses to Therapy of Patients With Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 905–913. [Google Scholar] [CrossRef]
- Batra, S.K.; Heier, C.R.; Diaz-Calderon, L.; Tully, C.B.; Fiorillo, A.A.; van den Anker, J.; Conklin, L.S. Serum miRNAs Are Pharmacodynamic Biomarkers Associated With Therapeutic Response in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1597–1606. [Google Scholar] [CrossRef]
- Hiraki, M.; Nishimura, J.; Takahashi, H.; Wu, X.; Takahashi, Y.; Miyo, M.; Nishida, N.; Uemura, M.; Hata, T.; Takemasa, I.; et al. Concurrent Targeting of KRAS and AKT by MiR-4689 Is a Novel Treatment Against Mutant KRAS Colorectal Cancer. Mol. Ther. Nucleic Acids 2015, 4, e231. [Google Scholar] [CrossRef]
- Inoue, A.; Mizushima, T.; Wu, X.; Okuzaki, D.; Kambara, N.; Ishikawa, S.; Wang, J.; Qian, Y.; Hirose, H.; Yokoyama, Y.; et al. A miR-29b Byproduct Sequence Exhibits Potent Tumor-Suppressive Activities via Inhibition of NF-κB Signaling in KRAS-Mutant Colon Cancer Cells. Mol. Cancer Ther. 2018, 17, 977–987. [Google Scholar] [CrossRef]
- Mulholland, E.J.; McErlean, E.M.; Dunne, N.; McCarthy, H.O. A Peptide/MicroRNA-31 nanomedicine within an electrospun biomaterial designed to regenerate wounds in vivo. Acta Biomater. 2022, 138, 285–300. [Google Scholar]
- Kobayashi, M.; Sawada, K.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Nakamura, K.; Kawano, M.; Kodama, M.; Hashimoto, K.; et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 527, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Keeler, G.D.; Zhang, Y.; Hoffman, B.E.; Ling, C.; Qing, K.; Srivastava, A. AAV3-miRNA vectors for growth suppression of human hepatocellular carcinoma cells in vitro and human liver tumors in a murine xenograft model in vivo. Gene Ther. 2021, 28, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Pourshafie, N.; Lee, P.R.; Chen, K.L.; Harmison, G.G.; Bott, L.C.; Fischbeck, K.H.; Rinaldi, C. Systemic Delivery of MicroRNA Using Recombinant Adeno-associated Virus Serotype 9 to Treat Neuromuscular Diseases in Rodents. J. Vis. Exp. 2018, 138, 55724. [Google Scholar] [CrossRef] [PubMed]
- Miravirsen in Combination with Telaprevir and Ribavirin in Null Responder to Pegylated-Interferon Alpha Plus Ribavirin Subjects with Chronic Hepatitis C Virus Infection. Available online: https://www.clinicaltrials.gov/ct2/show/NCT01872936 (accessed on 7 June 2013).
- Duttagupta, R.; DiRienzo, S.; Jiang, R.; Bowers, J.; Gollub, J.; Kao, J.; Kearney, K.; Rudolph, D.; Dawany, N.B.; Showe, M.K.; et al. Genome-wide maps of circulating miRNA biomarkers for ulcerative colitis. PLoS ONE. 2012, 7, e31241. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Tölle, A.; Jung, M.; Rabenhorst, S.; Kilic, E.; Jung, K.; Weikert, S. Identification of microRNAs in blood and urine as tumour markers for the detection of urinary bladder cancer. Oncol. Rep. 2013, 30, 1949–1956. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
| MiRNAs | Disease Subtype | Sample Type | Results | Reference | |
|---|---|---|---|---|---|
| 1 | miR-16, miR-21, miR-23a, miR-24, miR-29a, miR-126, miR-195, let-7f | UC, HC | Biopsy | These miRNAs were upregulated in active UC patients compared to healthy controls | [27] |
| 2 | miR-188-5p, miR-215, miR-320a, and miR-346 | UC, HC | Biopsy | These miRNAs were downregulated in active UC patients compared to healthy controls | [27] |
| 3 | miR-7, miR-26a, miR-29a, miR-29b, miR-31, miR-126*, miR-127-3p, miR-135b, and miR-324-3p | UC, HC | Biopsy | These miRNAs were upregulated in active UC patients compared to healthy controls | [28] |
| 4 | miRs-9, -21, -22, -26a, -29a, -29c, -30b, -31, -34c-5p, -106a, -126, -126*, -127-3p, -130a, -133b, -146a, -146-3p, -150, -155, 181c, -196a, -324-3p, -375 | CD, HC | Biopsy | These miRNAs were upregulated in patients with Crohn’s colitis patients compared to healthy controls | [28] |
| 5 | miR-141, miR-200a, miR-200b, miR-200c and miR-429 | CD, HC | Biopsy | These miRNAs were downregulated in CD patients compared to healthy controls | [29] |
| 6 | miR-141, miR-200b and miR-429 | UC, HC | Biopsy | These miRNAs were downregulated in active UC patients compared to healthy controls | [29] |
| 7 | miR-146a and -155 | UC, HC | Biopsy | miR-146a and -155 was higher in the inflamed mucosa of children with CD and UC than in the intact mucosa | [30] |
| 8 | miR-215-5p, miR-203a-3p, miR-223-3p, miR-194-5p, miR-192-5p, miR-10b-5p, miR-10a-5p, miR-337-5p, miR-582-5p | CD, HC | Biopsy | Nine miRNAs were differentially expressed across HC and CD, accounting for biopsy location | [33] |
| MiRNAs | Disease Subtype | Sample Type | Results | Reference | |
|---|---|---|---|---|---|
| 1 | miR-199a-5p, miR-362-3p, miR-340*, miRplus-E1271, miR-532-3p | CD, HC | Blood | These five miRNAs were significantly increased in active CD patients, as compared to healthy controls | [37] |
| 2 | miR-149* and miRplus-F1065 | CD, HC | Blood | These two miRNAs were significantly increased in active CD patients, as compared to healthy controls | [37] |
| 3 | miR-28-5p, miR-151-5p, miR-103-2*, miR-199a-5p, miR-340*, miR-362-3p, miR-532-3p, miR-505*, miRplus-E1271 | UC, HC | Blood | These twelve were significantly increased in active UC patients, as compared to healthy controls | [37] |
| 4 | miRNA-505 | UC, HC | Blood | miRNA-505 was significantly decreased in active UC patients, as compared to healthy controls | [37] |
| 5 | MiR-16, miR-23a, miR-29a, miR-106a, miR-107, miR-126, miR-191, miR-199a-5p, miR-200c, miR-362-3p and miR-532-3p | CD, HC | Blood | These miRNAs were significantly increased compared to healthy controls | [38] |
| 6 | miR-16, miR-21, miR-28-5p, miR-151-5p, miR-155 and miR-199a-5p | UC, HC | Blood | These miRNAs were significantly increased compared to healthy controls | [38] |
| 7 | miR-188-5p, miR-877, miR-140-5p, miR145. miR-18a, miR-128 | CD | Serum | Six miRNAs expressed differentially in active CD patients compared with inactive CD patients | [39] |
| 8 | miR-146b-5p | CD, UC, HC | Serum | miR-146b-5p expression was higher in patients with CD and UC, than in healthy controls. | [40] |
| 9 | miR-16, miR-21, miR-155, and miR-223 | CD, UC, HC | Serum, Feces | These miRNAs were significantly increased compared to healthy controls, and was higher in CD than in UC patients | [41] |
| 10 | miR-21-5p | UC, HC | Serum, Biopsy | miR-21-5p was downregulated in UC patients compared with healthy people and the control group | [42] |
| 11 | miR-320a | CD, UC, HC | Blood | miR-320a expression in patients with IBD follows the clinical and endoscopic disease activities | [43] |
| MiRNAs | Disease Subtype | Sample Type | Results | Reference | |
|---|---|---|---|---|---|
| 1 | miR-223 and miR-1246 | CD, UC | Feces | miR-223 and miR-1246 was increased in active IBD patients versus the control group | [46] |
| 2 | miR-16-5p and miR-21-5p | CD, UC, HC | Feces | miR-16-5p was up-regulated in UC and CD patients and miR-21-5p was up-regulated in UC patients, compared with healthy controls | [48] |
| 3 | miRNA-21 and miRNA-106a | CRC, HC | Feces | miRNA-21 and miRNA-106a was increased in patients with CRC | [51] |
| 4 | miR-21, miR-106a, miR-96, miR-203, miR-20a, miR-326, and miR-92 | CRC, HC | Feces | These miRNAs were increased in patients with CRC | [44] |
| 5 | miR-320, miR-126, miR-484-5p, miR-143, miR145, miR-16, and miR-125b | CRC, HC | Feces | These miRNAs were decreased in patients with CRC | [44] |
| 6 | miR-421, miR130b-3p miR27a-3P | CRC, HC | Feces | These miRNAs were upregulated in patients with CRC | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, L.; Capobianco, I.; Magrì, C.; Marafini, I.; Petito, V.; Scaldaferri, F. MicroRNAs as Innovative Biomarkers for Inflammatory Bowel Disease and Prediction of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 7991. https://doi.org/10.3390/ijms23147991
Masi L, Capobianco I, Magrì C, Marafini I, Petito V, Scaldaferri F. MicroRNAs as Innovative Biomarkers for Inflammatory Bowel Disease and Prediction of Colorectal Cancer. International Journal of Molecular Sciences. 2022; 23(14):7991. https://doi.org/10.3390/ijms23147991
Chicago/Turabian StyleMasi, Letizia, Ivan Capobianco, Carlotta Magrì, Irene Marafini, Valentina Petito, and Franco Scaldaferri. 2022. "MicroRNAs as Innovative Biomarkers for Inflammatory Bowel Disease and Prediction of Colorectal Cancer" International Journal of Molecular Sciences 23, no. 14: 7991. https://doi.org/10.3390/ijms23147991
APA StyleMasi, L., Capobianco, I., Magrì, C., Marafini, I., Petito, V., & Scaldaferri, F. (2022). MicroRNAs as Innovative Biomarkers for Inflammatory Bowel Disease and Prediction of Colorectal Cancer. International Journal of Molecular Sciences, 23(14), 7991. https://doi.org/10.3390/ijms23147991






