GABAergic and Glutamatergic Phenotypes of Neurons Expressing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig
Abstract
1. Introduction
2. Results
2.1. CaBPs Immunoreactivity in the Preoptic Area of the Guinea Pig
2.2. VGAT and VGLUT Immunoreactivity in the Preoptic Area of the Guinea Pig
2.3. The Coexpression Pattern of CB+, CR+ and PV+ Neurons with VGAT and/or VGLUT in the Preoptic Area of the Guinea Pig
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation
4.3. Immunofluorescence Experiments
4.4. Controls
4.5. Counts and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVPV | Anteroventral periventricular region |
| BF | Basal forebrain |
| CaBPs | Calcium binding proteins |
| CB | Calbindin |
| CB-IR | CB-immunoreactivity |
| CNS | Central nervous system |
| CR | Calretinin |
| ERs | Estrogen receptors |
| ERα | Estrogen receptors alpha |
| ERβ | Estrogen receptors beta |
| FSH | Follicle-stimulating hormone |
| GABA | Gamma-aminobutyric acid |
| GAD | Glutamic acid decarboxylase |
| Glu | Glutamate |
| GnRH | Gonadoliberin |
| LH | Luteinizing hormone |
| LHb | Lateral habenula |
| LPA | Lateral preoptic area |
| MPA | Medial preoptic area |
| MPN | Median preoptic nucleus |
| NaN3 | Sodium azide |
| NMDA | N-methyl-D-aspartate |
| NREM | Non-rapid eye movement |
| PAG | Phosphate activated glutaminase |
| PBS | Phosphate-buffered saline |
| POA | Preoptic area |
| PPN | Periventricular preoptic nucleus |
| PV | Parvalbumin |
| REM | Rapid eye movement |
| scRNA-seq | Single-cell RNA-sequencing |
| SDN-POA | Sexually dimorphic nucleus of preoptic area |
| VGAT | Vesicular GABA transporter |
| VGLUT 2 | Vesicular glutamate transporter 2 |
References
- Andressen, C.; Blümcke, I.; Celio, M.R. Calcium-Binding Proteins: Selective Markers of Nerve Cells. Cell Tissue Res. 1993, 271, 181–208. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Hattori, R.; Yui, Y. Three Distinct Subpopulations of GABAergic Neurons in Rat Frontal Agranular Cortex. Brain Res. 1994, 649, 159–173. [Google Scholar] [CrossRef]
- Miettinen, R.; Gulyás, A.I.; Baimbridge, K.G.; Jacobowitz, D.M.; Freund, T.F. Calretinin Is Present in Non-Pyramidal Cells of the Rat Hippocampus—II. Co-Existence with Other Calcium Binding Proteins and Gaba. Neuroscience 1992, 48, 29–43. [Google Scholar] [CrossRef]
- Równiak, M.; Bogus-Nowakowska, K.; Robak, A. The Densities of Calbindin and Parvalbumin, but Not Calretinin Neurons, Are Sexually Dimorphic in the Amygdala of the Guinea Pig. Brain Res. 2015, 1604, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Leranth, C.; Kiss, J. A Population of Supramammillary Area Calretinin Neurons Terminating on Medial Septal Area Cholinergic and Lateral Septal Area Calbindin-Containing Cells Are Aspartate/Glutamatergic. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 7699–7710. [Google Scholar] [CrossRef]
- Kemppainen, S.; Pitkänen, A. Distribution of Parvalbumin, Calretinin, and Calbindin-D(28k) Immunoreactivity in the Rat Amygdaloid Complex and Colocalization with Gamma-Aminobutyric Acid. J. Comp. Neurol. 2000, 426, 441–467. [Google Scholar] [CrossRef]
- Krzywkowski, P.; Jacobowitz, D.M.; Lamour, Y. Calretinin-Containing Pathways in the Rat Forebrain. Brain Res. 1995, 705, 273–294. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mascagni, F.; Zaric, V. Subpopulations of Somatostatin-Immunoreactive Non-Pyramidal Neurons in the Amygdala and Adjacent External Capsule Project to the Basal Forebrain: Evidence for the Existence of GABAergic Projection Neurons in the Cortical Nuclei and Basolateral Nuclear Complex. Front. Neural Circuits 2012, 6, 46. [Google Scholar] [CrossRef]
- Gritti, I.; Manns, I.D.; Mainville, L.; Jones, B.E. Parvalbumin, Calbindin, or Calretinin in Cortically Projecting and GABAergic, Cholinergic, or Glutamatergic Basal Forebrain Neurons of the Rat. J. Comp. Neurol. 2003, 458, 11–31. [Google Scholar] [CrossRef]
- McKenna, J.T.; Yang, C.; Franciosi, S.; Winston, S.; Abarr, K.K.; Rigby, M.S.; Yanagawa, Y.; McCarley, R.W.; Brown, R.E. Distribution and Intrinsic Membrane Properties of Basal Forebrain GABAergic and Parvalbumin Neurons in the Mouse. J. Comp. Neurol. 2013, 521, 1225–1250. [Google Scholar] [CrossRef]
- Żakowski, W.; Równiak, M.; Robak, A. Colocalization Pattern of Calbindin and Cocaine- and Amphetamine-Regulated Transcript in the Mammillary Body-Anterior Thalamic Nuclei Axis of the Guinea Pig. Neuroscience 2014, 260, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Manns, I.D.; Mainville, L.; Jones, B.E. Evidence for Glutamate, in Addition to Acetylcholine and GABA, Neurotransmitter Synthesis in Basal Forebrain Neurons Projecting to the Entorhinal Cortex. Neuroscience 2001, 107, 249–263. [Google Scholar] [CrossRef]
- Uva, L.; Grüschke, S.; Biella, G.; de Curtis, M.; Witter, M.P. Cytoarchitectonic Characterization of the Parahippocampal Region of the Guinea Pig. J. Comp. Neurol. 2004, 474, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, B.; Najdzion, J.; Równiak, M.; Bogus-Nowakowska, K.; Hermanowicz, B.; Kolenkiewicz, M.; Żakowski, W.; Robak, A. Cocaine- and Amphetamine-Regulated Transcript and Calcium Binding Proteins Immunoreactivity in the Subicular Complex of the Guinea Pig. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2016, 204, 51–62. [Google Scholar] [CrossRef]
- Bogus-Nowakowska, K. Ontogeny of Neurons Containing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig: Sexually Dimorphic Development of Calbindin. Dev. Neurobiol. 2019, 79, 175–201. [Google Scholar] [CrossRef]
- Hermanowicz-Sobieraj, B.; Robak, A. The Ontogenetic Development of Neurons Containing Calcium-Binding Proteins in the Septum of the Guinea Pig: Late Onset of Parvalbumin Immunoreactivity versus Calbindin and Calretinin. J. Chem. Neuroanat. 2017, 79, 22–31. [Google Scholar] [CrossRef]
- Zakowski, W.; Bogus-Nowakowska, K.; Robak, A. Embryonic and Postnatal Development of Calcium-Binding Proteins Immunoreactivity in the Anterior Thalamus of the Guinea Pig. J. Chem. Neuroanat. 2013, 53, 25–32. [Google Scholar] [CrossRef]
- Broadwell, R.D.; Bleier, R. A Cytoarchitectonic Atlas of the Mouse Hypothalamus. J. Comp. Neurol. 1976, 167, 315–339. [Google Scholar] [CrossRef]
- Bleier, R.; Byne, W.; Siggelkow, I. Cytoarchitectonic Sexual Dimorphisms of the Medial Preoptic and Anterior Hypothalamic Areas in Guinea Pig, Rat, Hamster, and Mouse. J. Comp. Neurol. 1982, 212, 118–130. [Google Scholar] [CrossRef]
- Bogus-Nowakowska, K.; Robak, A.; Szteyn, S.; Równiak, M.; Wasilewska, B.; Najdzion, J. A Morphometric Study of the Preoptic Area of the Guinea Pig. Folia Morphol. 2010, 69, 15–23. [Google Scholar]
- Lephart, E.D. Dimorphic Expression of Calbindin-D28K in the Medial Basal Hypothalamus from Perinatal Male and Female Rats. Brain Res. Dev. Brain Res. 1996, 96, 281–284. [Google Scholar] [CrossRef]
- Stuart, E.; Lephart, E.D. Dimorphic Expression of Medial Basal Hypothalamic–Preoptic Area Calbindin-D28K MRNA during Perinatal Development and Adult Distribution of Calbindin-D28K MRNA in Sprague–Dawley Rats. Mol. Brain Res. 1999, 73, 60–67. [Google Scholar] [CrossRef]
- Sickel, M.J.; McCarthy, M.M. Calbindin-D28k Immunoreactivity Is a Marker for a Subdivision of the Sexually Dimorphic Nucleus of the Preoptic Area of the Rat: Developmental Profile and Gonadal Steroid Modulation. J. Neuroendocrinol. 2000, 12, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Bodo, C.; Rissman, E.F. The Androgen Receptor Is Selectively Involved in Organization of Sexually Dimorphic Social Behaviors in Mice. Endocrinology 2008, 149, 4142–4150. [Google Scholar] [CrossRef]
- Orikasa, C.; Kondo, Y.; Usui, S.; Sakuma, Y. Similar Numbers of Neurons Are Generated in the Male and Female Rat Preoptic Area in Utero. Neurosci. Res. 2010, 68, 9–14. [Google Scholar] [CrossRef]
- Dominguez, J.M.; Hull, E.M. Dopamine, the Medial Preoptic Area, and Male Sexual Behavior. Physiol. Behav. 2005, 86, 356–368. [Google Scholar] [CrossRef]
- Simerly, R.B.; Swanson, L.W. Projections of the Medial Preoptic Nucleus: A Phaseolus vulgaris Leucoagglutinin Anterograde Tract-Tracing Study in the Rat. J. Comp. Neurol. 1988, 270, 209–242. [Google Scholar] [CrossRef]
- Bogus-Nowakowska, K.; Równiak, M.; Hermanowicz-Sobieraj, B.; Wasilewska, B.; Najdzion, J.; Robak, A. Tyrosine Hydroxylase-Immunoreactivity and Its Relations with Gonadotropin-Releasing Hormone and Neuropeptide Y in the Preoptic Area of the Guinea Pig. J. Chem. Neuroanat. 2016, 78, 131–139. [Google Scholar] [CrossRef]
- Belchetz, P.E.; Plant, T.M.; Nakai, Y.; Keogh, E.J.; Knobil, E. Hypophysial Responses to Continuous and Intermittent Delivery of Hypopthalamic Gonadotropin-Releasing Hormone. Science 1978, 202, 631–633. [Google Scholar] [CrossRef]
- Decavel, C.; Pol, A.N.V.D. GABA: A Dominant Neurotransmitter in the Hypothalamus. J. Comp. Neurol. 1990, 302, 1019–1037. [Google Scholar] [CrossRef]
- Van den Pol, A.N.; Wuarin, J.P.; Dudek, F.E. Glutamate, the Dominant Excitatory Transmitter in Neuroendocrine Regulation. Science 1990, 250, 1276–1278. [Google Scholar] [CrossRef]
- Petersen, S.L.; Ottem, E.N.; Carpenter, C.D. Direct and Indirect Regulation of Gonadotropin-Releasing Hormone Neurons by Estradiol1. Biol. Reprod. 2003, 69, 1771–1778. [Google Scholar] [CrossRef]
- Carbone, S.; Ponzo, O.; Szwarcfarb, B.; Rondina, D.; Reynoso, R.; Scacchi, P.; Moguilevsky, J.A. Ontogenic Modifications in the Effect of the GABAergic System on the Hypothalamic Excitatory Amino Acids: Its Relationship with GABAergic Control of Gonadotrophin Secretion during Sexual Maturation in Female Rats. Dev. Brain Res. 2002, 133, 13–18. [Google Scholar] [CrossRef]
- Ottem, E.N.; Godwin, J.G.; Krishnan, S.; Petersen, S.L. Dual-Phenotype GABA/Glutamate Neurons in Adult Preoptic Area: Sexual Dimorphism and Function. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 8097–8105. [Google Scholar] [CrossRef] [PubMed]
- Henny, P.; Brischoux, F.; Mainville, L.; Stroh, T.; Jones, B.E. Immunohistochemical Evidence for Synaptic Release of Glutamate from Orexin Terminals in the Locus Coeruleus. Neuroscience 2010, 169, 1150–1157. [Google Scholar] [CrossRef]
- Herzog, E.; Bellenchi, G.C.; Gras, C.; Bernard, V.; Ravassard, P.; Bedet, C.; Gasnier, B.; Giros, B.; Mestikawy, S.E. The Existence of a Second Vesicular Glutamate Transporter Specifies Subpopulations of Glutamatergic Neurons. J. Neurosci. 2001, 21, RC181. [Google Scholar] [CrossRef]
- Moore, A.M.; Abbott, G.; Mair, J.; Prescott, M.; Campbell, R.E. Mapping GABA and Glutamate Inputs to Gonadotrophin-Releasing Hormone Neurones in Male and Female Mice. J. Neuroendocrinol. 2018, 30, e12657. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.R.; Cullinan, W.E.; Herman, J.P. Distribution of Vesicular Glutamate Transporter MRNA in Rat Hypothalamus. J. Comp. Neurol. 2002, 448, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Hisano, S. Vesicular Glutamate Transporters in the Brain. Anat. Sci. Int. 2003, 78, 191–204. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ozawa, H.; Yamaguchi, S.; Hamaguchi, S.; Ueda, S. Calbindin-Positive Neurons Co-Express Functional Markers in a Location-Dependent Manner Within the A11 Region of the Rat Brain. Neurochem. Res. 2021, 46, 853–865. [Google Scholar] [CrossRef]
- Filippi, A.; Mueller, T.; Driever, W. Vglut2 and Gad Expression Reveal Distinct Patterns of Dual GABAergic versus Glutamatergic Cotransmitter Phenotypes of Dopaminergic and Noradrenergic Neurons in the Zebrafish Brain. J. Comp. Neurol. 2014, 522, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.; Csaba, Z.; Csáki, A.; Halász, B. Demonstration of Estrogen Receptor α Protein in Glutamatergic (Vesicular Glutamate Transporter 2 Immunoreactive) Neurons of the Female Rat Hypothalamus and Amygdala Using Double-Label Immunocytochemistry. Exp. Brain Res. 2013, 226, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; McKinney, K.; Liu, L.; Lakhlani, S.; Jennes, L. Distribution of Vesicular Glutamate Transporter-2 Messenger Ribonucleic Acid and Protein in the Septum-Hypothalamus of the Rat. Endocrinology 2003, 144, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Pompolo, S.; Pereira, A.; Scott, C.J.; Fujiyma, F.; Clarke, I.J. Evidence for Estrogenic Regulation of Gonadotropin-Releasing Hormone Neurons by Glutamatergic Neurons in the Ewe Brain: An Immunohistochemical Study Using an Antibody against Vesicular Glutamate Transporter-2. J. Comp. Neurol. 2003, 465, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Venner, A.; Anaclet, C.; Broadhurst, R.Y.; Saper, C.B.; Fuller, P.M. A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Curr. Biol. CB 2016, 26, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Centurion, C.; Bendell, E.; Zou, B.; Sun, Y.; Shiromani, P.J.; Liu, M. VGAT and VGLUT2 Expression in MCH and Orexin Neurons in Double Transgenic Reporter Mice. IBRO Rep. 2018, 4, 44–49. [Google Scholar] [CrossRef]
- Jennings, J.H.; Ung, R.L.; Resendez, S.L.; Stamatakis, A.M.; Taylor, J.G.; Huang, J.; Veleta, K.; Kantak, P.A.; Aita, M.; Shilling-Scrivo, K.; et al. Visualizing Hypothalamic Network Dynamics for Appetitive and Consummatory Behaviors. Cell 2015, 160, 516–527. [Google Scholar] [CrossRef]
- Clancy, B.; Kersh, B.; Hyde, J.; Darlington, R.B.; Anand, K.J.S.; Finlay, B.L. Web-Based Method for Translating Neurodevelopment from Laboratory Species to Humans. Neuroinformatics 2007, 5, 79–94. [Google Scholar] [CrossRef]
- Workman, A.D.; Charvet, C.J.; Clancy, B.; Darlington, R.B.; Finlay, B.L. Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. J. Neurosci. 2013, 33, 7368–7383. [Google Scholar] [CrossRef]
- Jimenez-Liñan, M.; Rubin, B.S.; King, J.C. Examination of Guinea Pig Luteinizing Hormone-Releasing Hormone Gene Reveals a Unique Decapeptide and Existence of Two Transcripts in the Brain. Endocrinology 1997, 138, 4123–4130. [Google Scholar] [CrossRef]
- Mitchell, B.F.; Taggart, M.J. Are Animal Models Relevant to Key Aspects of Human Parturition? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R525–R545. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, J.R.; Bambah-Mukku, D.; Eichhorn, S.W.; Vaughn, E.; Shekhar, K.; Perez, J.D.; Rubinstein, N.D.; Hao, J.; Regev, A.; Dulac, C.; et al. Molecular, Spatial, and Functional Single-Cell Profiling of the Hypothalamic Preoptic Region. Science 2018, 362, eaau5324. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R. Parvalbumin in Most Gamma-Aminobutyric Acid-Containing Neurons of the Rat Cerebral Cortex. Science 1986, 231, 995–997. [Google Scholar] [CrossRef]
- Demeulemeester, H.; Arckens, L.; Vandesande, F.; Orban, G.A.; Heizmann, C.W.; Pochet, R. Calcium Binding Proteins and Neuropeptides as Molecular Markers of GABAergic Interneurons in the Cat Visual Cortex. Exp. Brain Res. 1991, 84, 538–544. [Google Scholar] [CrossRef]
- Hendry, S.H.; Jones, E.G. GABA Neuronal Subpopulations in Cat Primary Auditory Cortex: Co-Localization with Calcium Binding Proteins. Brain Res. 1991, 543, 45–55. [Google Scholar] [CrossRef]
- Van Brederode, J.F.; Mulligan, K.A.; Hendrickson, A.E. Calcium-Binding Proteins as Markers for Subpopulations of GABAergic Neurons in Monkey Striate Cortex. J. Comp. Neurol. 1990, 298, 1–22. [Google Scholar] [CrossRef]
- Wouterlood, F.G.; Canto, C.B.; Aliane, V.; Boekel, A.J.; Grosche, J.; Härtig, W.; Beliën, J.A.M.; Witter, M.P. Coexpression of Vesicular Glutamate Transporters 1 and 2, Glutamic Acid Decarboxylase and Calretinin in Rat Entorhinal Cortex. Brain Struct. Funct. 2007, 212, 303–319. [Google Scholar] [CrossRef]
- DeFelipe, J.; Hendry, S.H.C.; Hashikawa, T.; Molinari, M.; Jones, E.G. A Microcolumnar Structure of Monkey Cerebral Cortex Revealed by Immunocytochemical Studies of Double Bouquet Cell Axons. Neuroscience 1990, 37, 655–673. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Gonzalez-Burgos, G.; Povysheva, N.V.; Kröner, S.; Lewis, D.A.; Krimer, L.S. Localization of Calcium-Binding Proteins in Physiologically and Morphologically Characterized Interneurons of Monkey Dorsolateral Prefrontal Cortex. Cereb. Cortex 2005, 15, 1178–1186. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kubota, Y. GABAergic Cell Subtypes and Their Synaptic Connections in Rat Frontal Cortex. Cereb. Cortex 1997, 7, 476–486. [Google Scholar] [CrossRef]
- Gonchar, Y.; Wang, Q.; Burkhalter, A. Multiple Distinct Subtypes of GABAergic Neurons in Mouse Visual Cortex Identified by Triple Immunostaining. Front. Neuroanat. 2007, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, Y.; Burkhalter, A. Three Distinct Families of GABAergic Neurons in Rat Visual Cortex. Cereb. Cortex 1997, 7, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Melchitzky, D.S.; Eggan, S.M.; Lewis, D.A. Synaptic Targets of Calretinin-Containing Axon Terminals in Macaque Monkey Prefrontal Cortex. Neuroscience 2005, 130, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Del Río, M.R.; DeFelipe, J. Colocalization of Calbindin D-28k, Calretinin, and GABA Immunoreactivities in Neurons of the Human Temporal Cortex. J. Comp. Neurol. 1996, 369, 472–482. [Google Scholar] [CrossRef]
- Moryś, J.; Berdel, B.; Kowiański, P.; Majak, K.; Tarnawski, M.; Wisniewski, H.M. Relationship of Calcium-Binding Protein Containing Neurons and Projection Neurons in the Rat Basolateral Amygdala. Neurosci. Lett. 1999, 259, 91–94. [Google Scholar] [CrossRef]
- Mcdonald, A.J. Glutamate and Aspartate Immunoreactive Neurons of the Rat Basolateral Amygdala: Colocalization of Excitatory Amino Acids and Projections to the Limbic Circuit. J. Comp. Neurol. 1996, 365, 367–379. [Google Scholar] [CrossRef]
- Swanson, L.W.; Petrovich, G.D. What Is the Amygdala? Trends Neurosci. 1998, 21, 323–331. [Google Scholar] [CrossRef]
- Saha, S.; Batten, T.F.; Henderson, Z. A GABAergic Projection from the Central Nucleus of the Amygdala to the Nucleus of the Solitary Tract: A Combined Anterograde Tracing and Electron Microscopic Immunohistochemical Study. Neuroscience 2000, 99, 613–626. [Google Scholar] [CrossRef]
- Seo, D.-O.; Funderburk, S.C.; Bhatti, D.L.; Motard, L.E.; Newbold, D.; Girven, K.S.; McCall, J.G.; Krashes, M.; Sparta, D.R.; Bruchas, M.R. A GABAergic Projection from the Centromedial Nuclei of the Amygdala to Ventromedial Prefrontal Cortex Modulates Reward Behavior. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 10831–10842. [Google Scholar] [CrossRef]
- McDonald, A.J. Immunohistochemical Identification of Gamma-Aminobutyric Acid-Containing Neurons in the Rat Basolateral Amygdala. Neurosci. Lett. 1985, 53, 203–207. [Google Scholar] [CrossRef]
- Pitkänen, A.; Amaral, D.G. The Distribution of GABAergic Cells, Fibers, and Terminals in the Monkey Amygdaloid Complex: An Immunohistochemical and in Situ Hybridization Study. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 2200–2224. [Google Scholar] [CrossRef]
- Zakowski, W.; Bogus-Nowakowska, K.; Wasilewska, B.; Hermanowicz, B.; Robak, A. Calcium-Binding Proteins in the Laterodorsal Thalamic Nucleus during Development of the Guinea Pig. J. Chem. Neuroanat. 2014, 61–62, 88–93. [Google Scholar] [CrossRef]
- Knoll, J.G.; Wolfe, C.A.; Tobet, S.A. Estrogen Modulates Neuronal Movements within the Developing Preoptic Area-Anterior Hypothalamus. Eur. J. Neurosci. 2007, 26, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Simerly, R.B.; Chang, C.; Muramatsu, M.; Swanson, L.W. Distribution of Androgen and Estrogen Receptor MRNA-Containing Cells in the Rat Brain: An In Situ Hybridization Study. J. Comp. Neurol. 1990, 294, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of Estrogen Receptor β in the Mouse Brain: Comparison with Estrogen Receptor α. Endocrinology 2003, 144, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Sendera, T.J.; Kordower, J.H.; Mufson, E.J. Estrogen Receptor Alpha Containing Neurons in the Monkey Forebrain: Lack of Association with Calcium Binding Proteins and Choline Acetyltransferase. Brain Res. 2004, 1019, 55–63. [Google Scholar] [CrossRef]
- Warembourg, M.; Leroy, D. Comparative Distribution of Estrogen Receptor α and β Immunoreactivities in the Forebrain and the Midbrain of the Female Guinea Pig. Brain Res. 2004, 1002, 55–66. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Wang, S.-R.; Jiao, Z.-L.; Zhang, W.; Lin, J.-K.; Li, X.-Y.; Li, S.-S.; Zhang, X.; Xu, X.-H. Medial Preoptic Area in Mice Is Capable of Mediating Sexually Dimorphic Behaviors Regardless of Gender. Nat. Commun. 2018, 9, 279. [Google Scholar] [CrossRef]
- Cheong, R.Y.; Czieselsky, K.; Porteous, R.; Herbison, A.E. Expression of ESR1 in Glutamatergic and GABAergic Neurons Is Essential for Normal Puberty Onset, Estrogen Feedback, and Fertility in Female Mice. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 14533–14543. [Google Scholar] [CrossRef]
- Levine, J.E.; Bauer-Dantoin, A.C.; Besecke, L.M.; Conaghan, L.A.; Legan, S.J.; Meredith, J.M.; Strobl, F.J.; Urban, J.H.; Vogelsong, K.M.; Wolfe, A.M. Neuroendocrine Regulation of the Luteinizing Hormone-Releasing Hormone Pulse Generator in the Rat. Recent Prog. Horm. Res. 1991, 47, 97–151; discussion 151–153. [Google Scholar] [CrossRef]
- Herbison, A.E.; Pape, J.R. New Evidence for Estrogen Receptors in Gonadotropin-Releasing Hormone Neurons. Front. Neuroendocrinol. 2001, 22, 292–308. [Google Scholar] [CrossRef]
- Herbison, A.E. Multimodal Influence of Estrogen upon Gonadotropin-Releasing Hormone Neurons. Endocr. Rev. 1998, 19, 302–330. [Google Scholar] [CrossRef] [PubMed]
- Hayes, F.J.; Seminara, S.B.; Decruz, S.; Boepple, P.A.; Crowley, W.F. Aromatase Inhibition in the Human Male Reveals a Hypothalamic Site of Estrogen Feedback. J. Clin. Endocrinol. Metab. 2000, 85, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Chongthammakun, S.; Terasawa, E. Negative Feedback Effects of Estrogen on Luteinizing Hormone-Releasing Hormone Release Occur in Pubertal, but Not Prepubertal, Ovariectomized Female Rhesus Monkeys. Endocrinology 1993, 132, 735–743. [Google Scholar] [CrossRef]
- Cemeroglu, A.P.; Kletter, G.B.; Guo, W.; Brown, M.B.; Kelch, R.P.; Marshall, J.C.; Padmanabhan, V.; Foster, C.M. In Pubertal Girls, Naloxone Fails to Reverse the Suppression of Luteinizing Hormone Secretion by Estradiol. J. Clin. Endocrinol. Metab. 1998, 83, 3501–3506. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, X.; Jiang, L.; Zhang, Y. Single-Cell RNA-Seq Reveals Hypothalamic Cell Diversity. Cell Rep. 2017, 18, 3227–3241. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef]
- Romanov, R.A.; Zeisel, A.; Bakker, J.; Girach, F.; Hellysaz, A.; Tomer, R.; Alpár, A.; Mulder, J.; Clotman, F.; Keimpema, E.; et al. Molecular Interrogation of Hypothalamic Organization Reveals Distinct Dopamine Neuronal Subtypes. Nat. Neurosci. 2017, 20, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Miranda-Barrientos, J.; Zhang, S.; Root, D.H.; Wang, H.-L.; Liu, B.; Calipari, E.S.; Morales, M. Lateral Preoptic Control of the Lateral Habenula through Convergent Glutamate and GABA Transmission. Cell Rep. 2017, 21, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- White, L.E.; Hodges, H.D.; Carnes, K.M.; Price, J.L.; Dubinsky, J.M. Colocalization of Excitatory and Inhibitory Neurotransmitter Markers in Striatal Projection Neurons in the Rat. J. Comp. Neurol. 1994, 339, 328–340. [Google Scholar] [CrossRef]
- Boulland, J.-L.; Qureshi, T.; Seal, R.P.; Rafiki, A.; Gundersen, V.; Bergersen, L.H.; Fremeau, R.T.; Edwards, R.H.; Storm-Mathisen, J.; Chaudhry, F.A. Expression of the Vesicular Glutamate Transporters during Development Indicates the Widespread Corelease of Multiple Neurotransmitters. J. Comp. Neurol. 2004, 480, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Chappell, P.E.; Levine, J.E. Stimulation of Gonadotropin-Releasing Hormone Surges by Estrogen. I. Role of Hypothalamic Progesterone Receptors. Endocrinology 2000, 141, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.E.; Langub, M.C.; Engle, M.G.; Maley, B.E. Estrogen-Receptive Neurons in the Anteroventral Periventricular Nucleus Are Synaptic Targets of the Suprachiasmatic Nucleus and Peri-Suprachiasmatic Region. Brain Res. 1995, 689, 254–264. [Google Scholar] [CrossRef]
- Simerly, R.B. Organization and Regulation of Sexually Dimorphic Neuroendocrine Pathways. Behav. Brain Res. 1998, 92, 195–203. [Google Scholar] [CrossRef]
- Ottem, E.N.; Godwin, J.G.; Petersen, S.L. Glutamatergic Signaling through the N-Methyl-D-Aspartate Receptor Directly Activates Medial Subpopulations of Luteinizing Hormone-Releasing Hormone (LHRH) Neurons, but Does Not Appear to Mediate the Effects of Estradiol on LHRH Gene Expression. Endocrinology 2002, 143, 4837–4845. [Google Scholar] [CrossRef][Green Version]
- Gore, A.C.; Roberts, J.L. Regulation of Gonadotropin-Releasing Hormone Gene Expression by the Excitatory Amino Acids Kainic Acid and N-Methyl-D,L-Aspartate in the Male Rat. Endocrinology 1994, 134, 2026–2031. [Google Scholar] [CrossRef]
- Brann, D.W.; Mahesh, V.B. Endogenous Excitatory Amino Acid Involvement in the Preovulatory and Steroid-Induced Surge of Gonadotropins in the Female Rat. Endocrinology 1991, 128, 1541–1547. [Google Scholar] [CrossRef]
- Chen, G.; Trombley, P.Q.; van den Pol, A.N. Excitatory Actions of GABA in Developing Rat Hypothalamic Neurones. J. Physiol. 1996, 494 Pt 2, 451–464. [Google Scholar] [CrossRef]
- Coleman, H.; Hirst, J.J.; Parkington, H.C. The GABAA Excitatory-to-Inhibitory Switch in the Hippo-Campus of Perinatal Guinea-Pigs. In Proceedings of the 40th Annual Meeting Fetal and Neonatal Physiological Society, Peurto Varas, Chile, 1–4 September 2014; p. 83. [Google Scholar]
- Herbison, A.E.; Moenter, S.M. Depolarising and Hyperpolarising Actions of GABAA Receptor Activation on GnRH Neurons: Towards an Emerging Consensus. J. Neuroendocrinol. 2011, 23, 557–569. [Google Scholar] [CrossRef]
- Moroz, L.L.; Nikitin, M.A.; Poličar, P.G.; Kohn, A.B.; Romanova, D.Y. Evolution of Glutamatergic Signaling and Synapses. Neuropharmacology 2021, 199, 108740. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Moore, R.Y. The Sexually Dimorphic Nucleus of the Hypothalamus Contains GABA Neurons in Rat and Man. Brain Res. 1996, 742, 163–171. [Google Scholar] [CrossRef]
- Conrad, L.C.; Pfaff, D.W. Efferents from Medial Basal Forebrain and Hypothalamus in the Rat. II. An Autoradiographic Study of the Anterior Hypothalamus. J. Comp. Neurol. 1976, 169, 221–261. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.W. An Autoradiographic Study of the Efferent Connections of the Preoptic Region in the Rat. J. Comp. Neurol. 1976, 167, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.W.; Mogenson, G.J.; Simerly, R.B.; Wu, M. Anatomical and Electrophysiological Evidence for a Projection from the Medial Preoptic Area to the “mesencephalic and Subthalamic Locomotor Regions” in the Rat. Brain Res. 1987, 405, 108–122. [Google Scholar] [CrossRef]
- Saito, Y.C.; Tsujino, N.; Hasegawa, E.; Akashi, K.; Abe, M.; Mieda, M.; Sakimura, K.; Sakurai, T. GABAergic Neurons in the Preoptic Area Send Direct Inhibitory Projections to Orexin Neurons. Front. Neural Circuits 2013, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.; Bogus-Nowakowska, K.; Kozłowska, A.; Równiak, M. Expression of Calbindin, a Marker of Gamma-Aminobutyric Acid Neurons, Is Reduced in the Amygdala of Oestrogen Receptor β-Deficient Female Mice. J. Clin. Med. 2022, 11, 1760. [Google Scholar] [CrossRef]
- Zimmermann, L.; Schwaller, B. Monoclonal Antibodies Recognizing Epitopes of Calretinins: Dependence on Ca2+-Binding Status and Differences in Antigen Accessibility in Colon Cancer Cells. Cell Calcium 2002, 31, 13–25. [Google Scholar] [CrossRef]
- Drexel, M.; Preidt, A.P.; Kirchmair, E.; Sperk, G. Parvalbumin Interneurons and Calretinin Fibers Arising from the Thalamic Nucleus Reuniens Degenerate in the Subiculum after Kainic Acid-Induced Seizures. Neuroscience 2011, 189, 316–329. [Google Scholar] [CrossRef]
- Mészár, Z.; Girard, F.; Saper, C.B.; Celio, M.R. The Lateral Hypothalamic Parvalbumin-Immunoreactive (PV1) Nucleus in Rodents. J. Comp. Neurol. 2012, 520, 798–815. [Google Scholar] [CrossRef]
- Berg, E.M.; Bertuzzi, M.; Ampatzis, K. Complementary Expression of Calcium Binding Proteins Delineates the Functional Organization of the Locomotor Network. Brain Struct. Funct. 2018, 223, 2181–2196. [Google Scholar] [CrossRef]
- Najdzion, J.; Wasilewska, B.; Bogus-Nowakowska, K.; Robak, A. The Cocaine- and Amphetamine-Regulated Transcript, Calbindin, Calretinin and Parvalbumin Immunoreactivity in the Medial Geniculate Body of the Guinea Pig. J. Chem. Neuroanat. 2014, 59–60, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz-Sobieraj, B.; Bogus-Nowakowska, K.; Robak, A. Calcium-Binding Proteins Expression in the Septum and Cingulate Cortex of the Adult Guinea Pig. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2018, 215, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Michalski, D.; Härtig, W.; Krügel, K.; Edwards, R.H.; Böddener, M.; Böhme, L.; Pannicke, T.; Reichenbach, A.; Grosche, A. Region-Specific Expression of Vesicular Glutamate and GABA Transporters under Various Ischaemic Conditions in Mouse Forebrain and Retina. Neuroscience 2013, 231, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Härtig, W.; Stieler, J.; Boerema, A.S.; Wolf, J.; Schmidt, U.; Weißfuß, J.; Bullmann, T.; Strijkstra, A.M.; Arendt, T. Hibernation Model of Tau Phosphorylation in Hamsters: Selective Vulnerability of Cholinergic Basal Forebrain Neurons—Implications for Alzheimer’s Disease. Eur. J. Neurosci. 2007, 25, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hassani, O.K.; Henny, P.; Lee, M.G.; Jones, B.E. GABAergic Neurons Intermingled with Orexin and MCH Neurons in the Lateral Hypothalamus Discharge Maximally during Sleep. Eur. J. Neurosci. 2010, 32, 448–457. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiao, Y.-Y.; Sun, Q.-Q. Developmental Maturation of Excitation and Inhibition Balance in Principal Neurons across Four Layers of Somatosensory Cortex. Neuroscience 2011, 174, 10–25. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Cesca, F.; Schiavo, G.; Benfenati, F.; Baldelli, P. Kidins220/ARMS Is a Novel Modulator of Short-Term Synaptic Plasticity in Hippocampal GABAergic Neurons. PLoS ONE 2012, 7, e35785. [Google Scholar] [CrossRef]
- Bleier, R. Hypothalamus of the Guinea Pig: A Cytoarchitectonic Atlas, 1st ed.; University of Wisconsin Press: Madison, WI, USA, 1984; ISBN 978-0-299-09040-1. [Google Scholar]
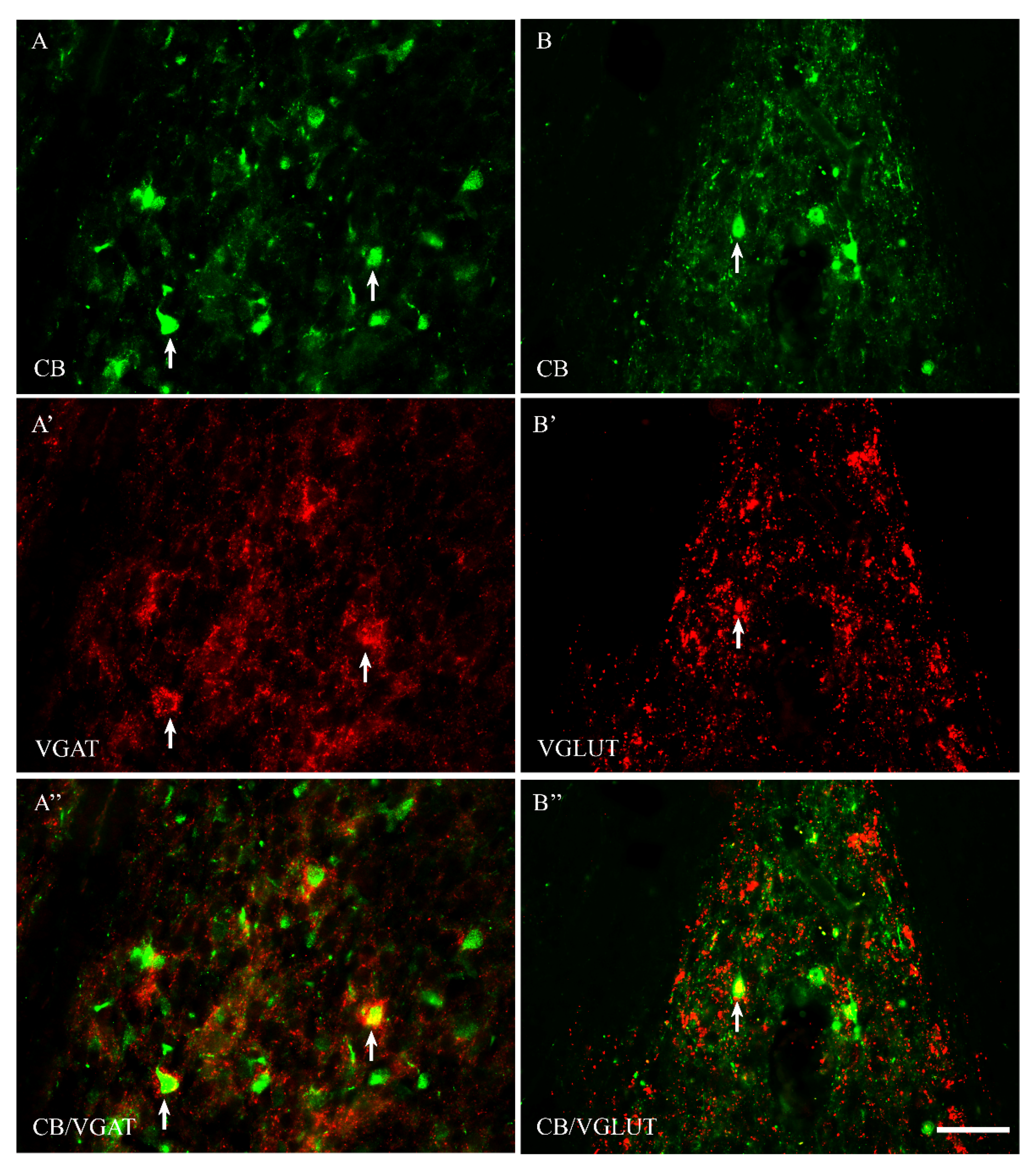
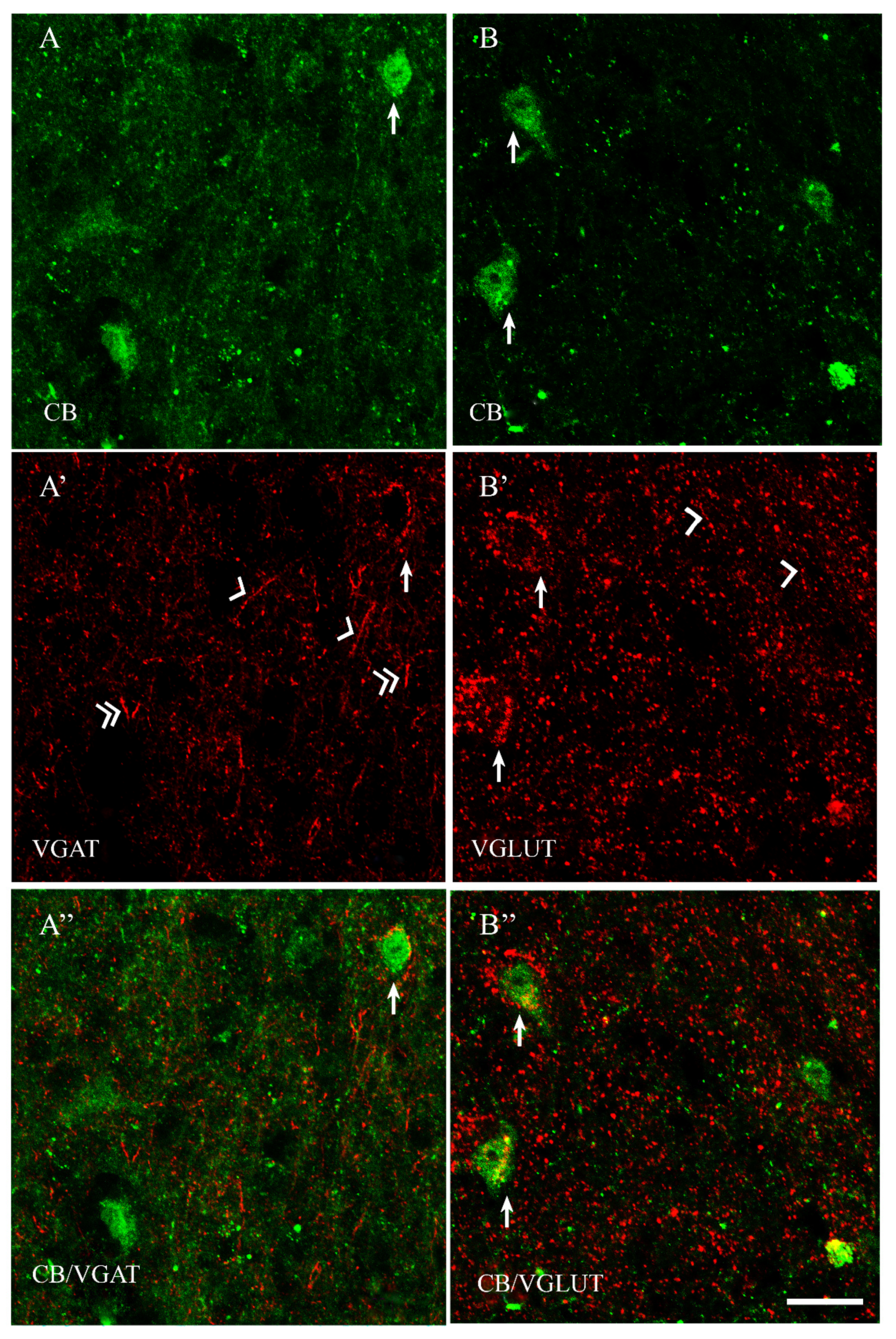

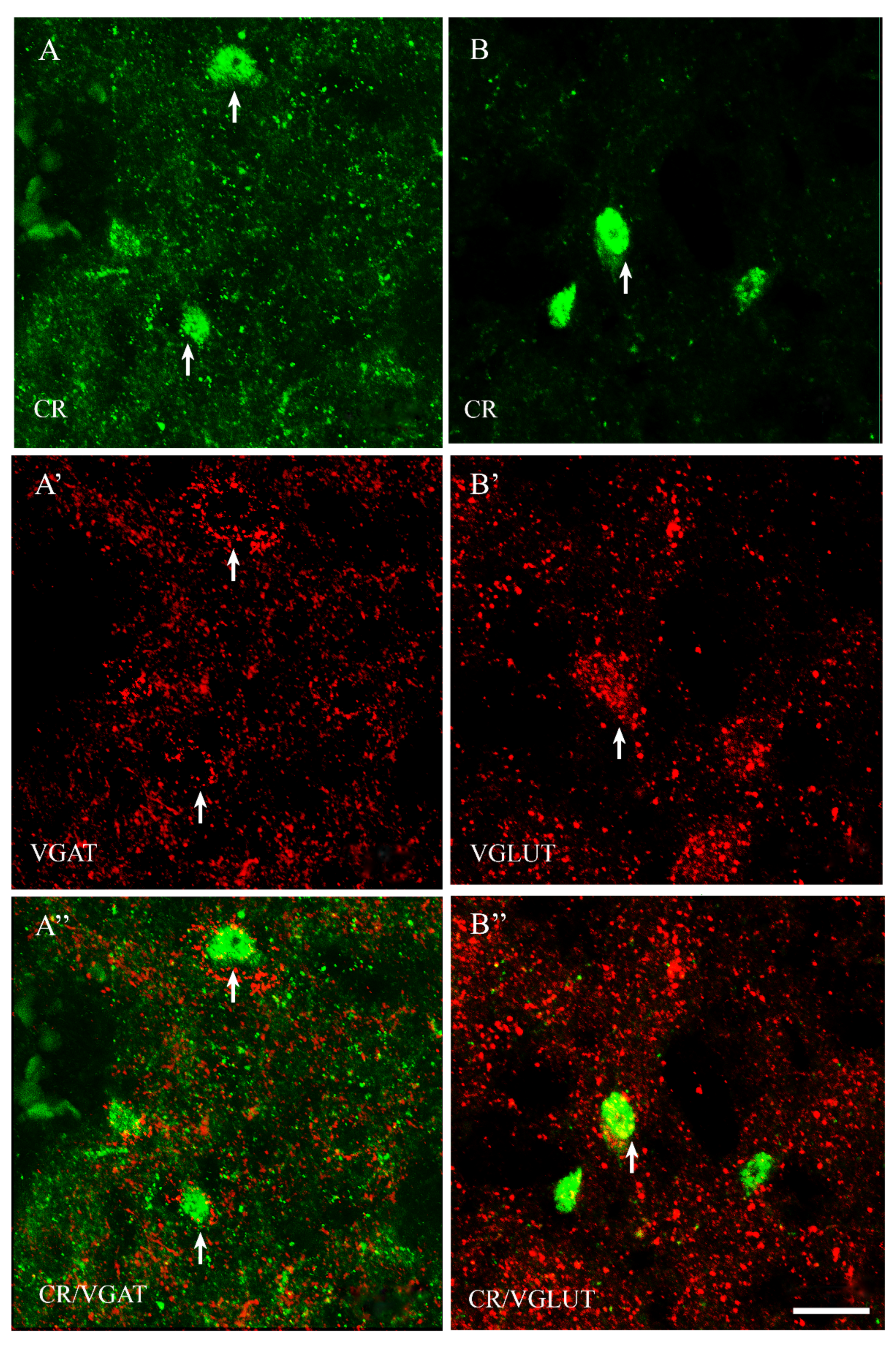

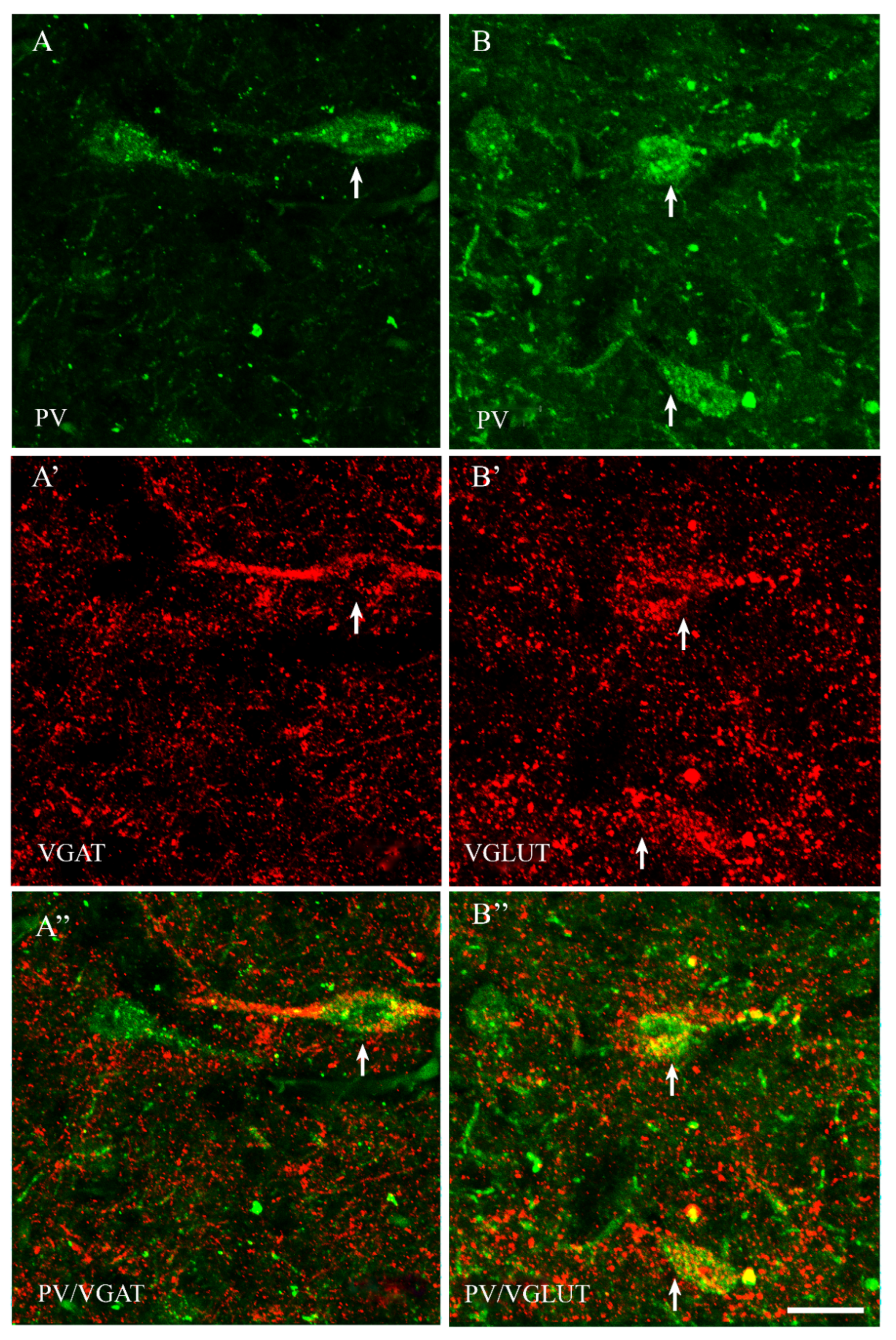
| CB+ | CB+/VGAT+ | % | CB+ | CB+/VGLUT | % | |
| Male | 262.6 ± 3.8 | 208.6 ± 4.43 | 79.4 ± 1.8 | 259.3 ± 8.3 | 49.6 ± 3.2 | 19.1 ± 1.7 |
| Female | 248 ± 11.5 | 199 ± 7.9 | 80.4 ± 6.7 | 246.6 ± 10.6 | 46.6 ± 5.2 | 18.9 ± 1.8 |
| CR+ | CR+/VGAT+ | % | CR+ | CR+/VGLUT | % | |
| Male | 157 ± 8.8 | 138 ± 4.9 | 88.2 ± 7.4 | 156.3 ± 9.9 | 50 ± 5.35 | 32.2 ± 5.09 |
| Female | 164 ± 5.5 | 145.6 ± 6.5 | 88.6 ± 6.4 | 151.6 ± 7.3 | 45.3 ± 6.1 | 30 ± 5.00 |
| PV+ | PV+/VGAT+ | % | PV+ | PV+/VGLUT | % | |
| Male | 90.3 ± 4.0 | 62 ± 3.5 | 68 ± 1.1 | 97 ± 3.5 | 64 ± 4.3 | 65.9 ± 3.2 |
| Female | 98.6 ± 3.0 | 70.3 ± 2.6 | 71.3 ± 4.2 | 93 ± 6.3 | 60 ± 2.16 | 64.9 ± 6.4 |
| Antigen | Code | Clonality | Host Species | Dilution | Supplier | Location |
|---|---|---|---|---|---|---|
| Primary antibodies | ||||||
| CB | 300 | monoclonal | Mouse | 1:4000 | Swant | Bellinzona/Switzerland |
| PV | P3088 | monoclonal | Mouse | 1:4000 | Sigma Aldrich | St. Louis, MO/USA |
| CR | 6B3 | monoclonal | Mouse | 1:4000 | Swant | Bellinzona/Switzerland |
| VGLUT | 135 402 | polyclonal | Rabbit | 1:2000 | SYSY | Göttingen/Germany |
| VGAT | AB5062P | polyclonal | Rabbit | 1:2000 | Millipore | Temecula, CA/USA |
| Secondary reagents | ||||||
| ALEXA Fluor 488 | A-21202 | polyclonal | Donkey | 1:1000 | Molecular Probes | Rockford, IL/USA |
| ALEXA Fluor 555 | A-31572 | polyclonal | Donkey | 1:1000 | Molecular Probes | Rockford, IL/USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogus-Nowakowska, K.; Robak, A.; Kalinowski, D.; Kozłowska, A.; Równiak, M. GABAergic and Glutamatergic Phenotypes of Neurons Expressing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig. Int. J. Mol. Sci. 2022, 23, 7963. https://doi.org/10.3390/ijms23147963
Bogus-Nowakowska K, Robak A, Kalinowski D, Kozłowska A, Równiak M. GABAergic and Glutamatergic Phenotypes of Neurons Expressing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig. International Journal of Molecular Sciences. 2022; 23(14):7963. https://doi.org/10.3390/ijms23147963
Chicago/Turabian StyleBogus-Nowakowska, Krystyna, Anna Robak, Daniel Kalinowski, Anna Kozłowska, and Maciej Równiak. 2022. "GABAergic and Glutamatergic Phenotypes of Neurons Expressing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig" International Journal of Molecular Sciences 23, no. 14: 7963. https://doi.org/10.3390/ijms23147963
APA StyleBogus-Nowakowska, K., Robak, A., Kalinowski, D., Kozłowska, A., & Równiak, M. (2022). GABAergic and Glutamatergic Phenotypes of Neurons Expressing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig. International Journal of Molecular Sciences, 23(14), 7963. https://doi.org/10.3390/ijms23147963







