miR449 Protects Airway Regeneration by Controlling AURKA/HDAC6-Mediated Ciliary Disassembly
Abstract
1. Introduction
2. Results
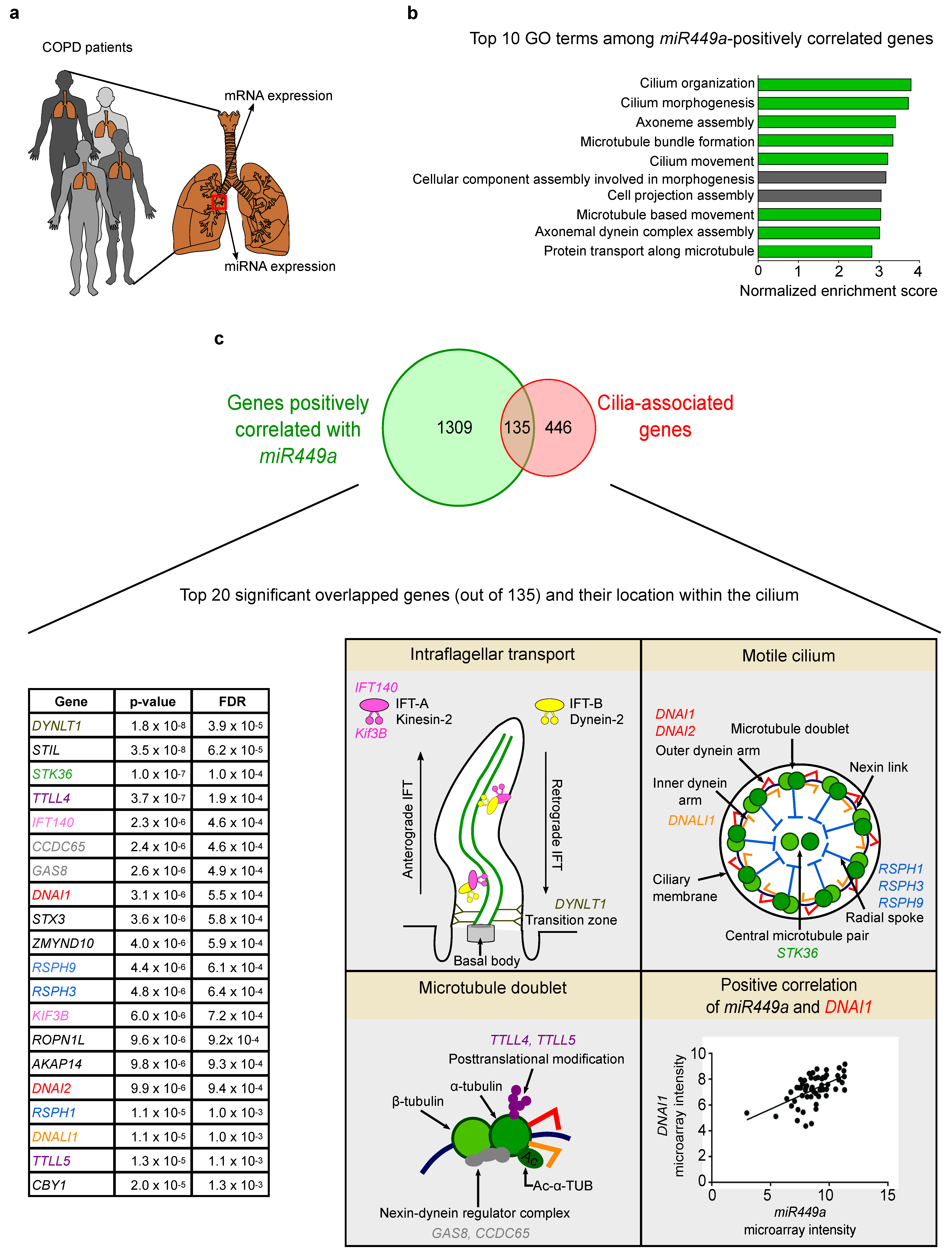
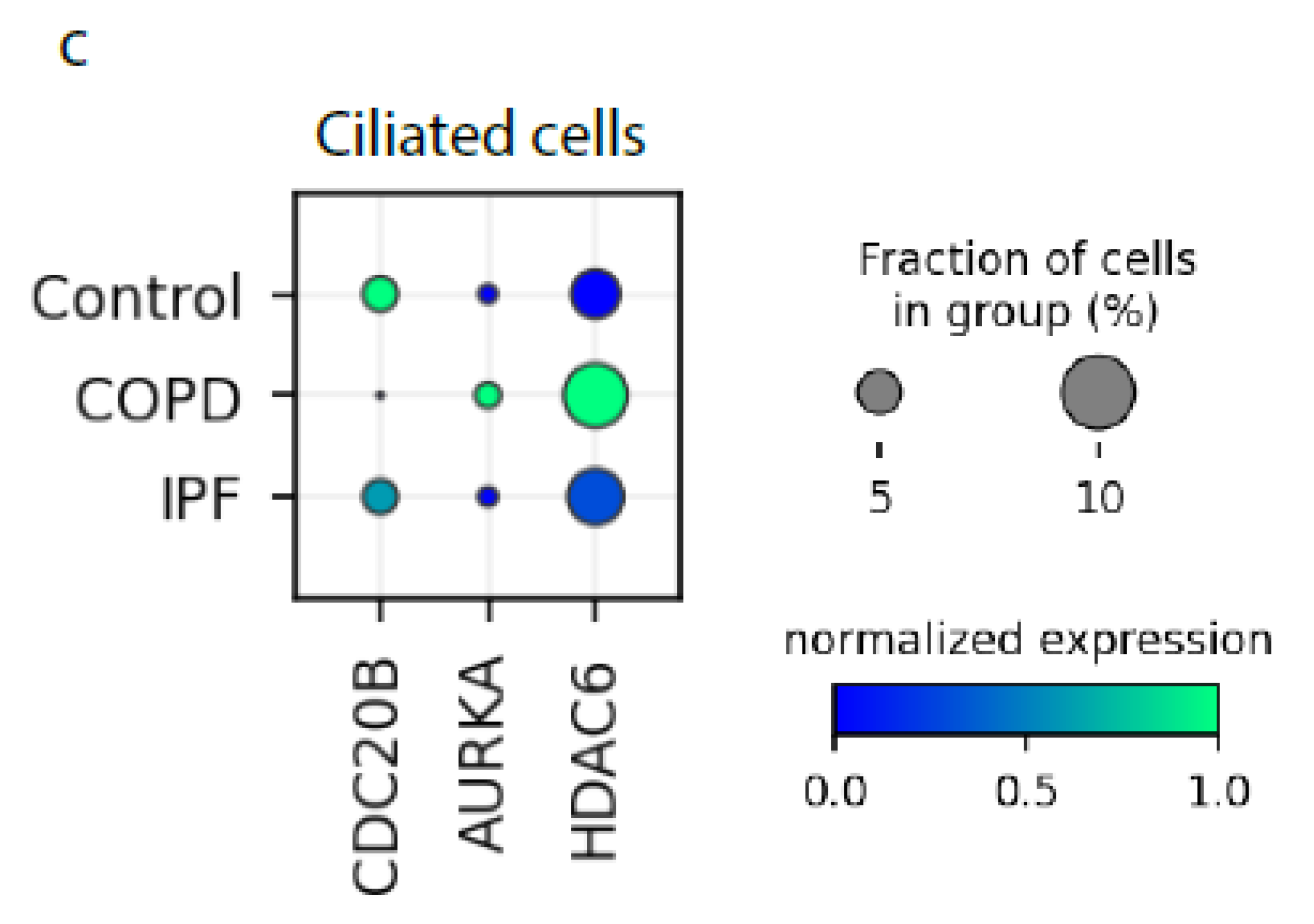
2.1. Cilia-Related Genes Positively Correlate with miR449 Expression in COPD Patients
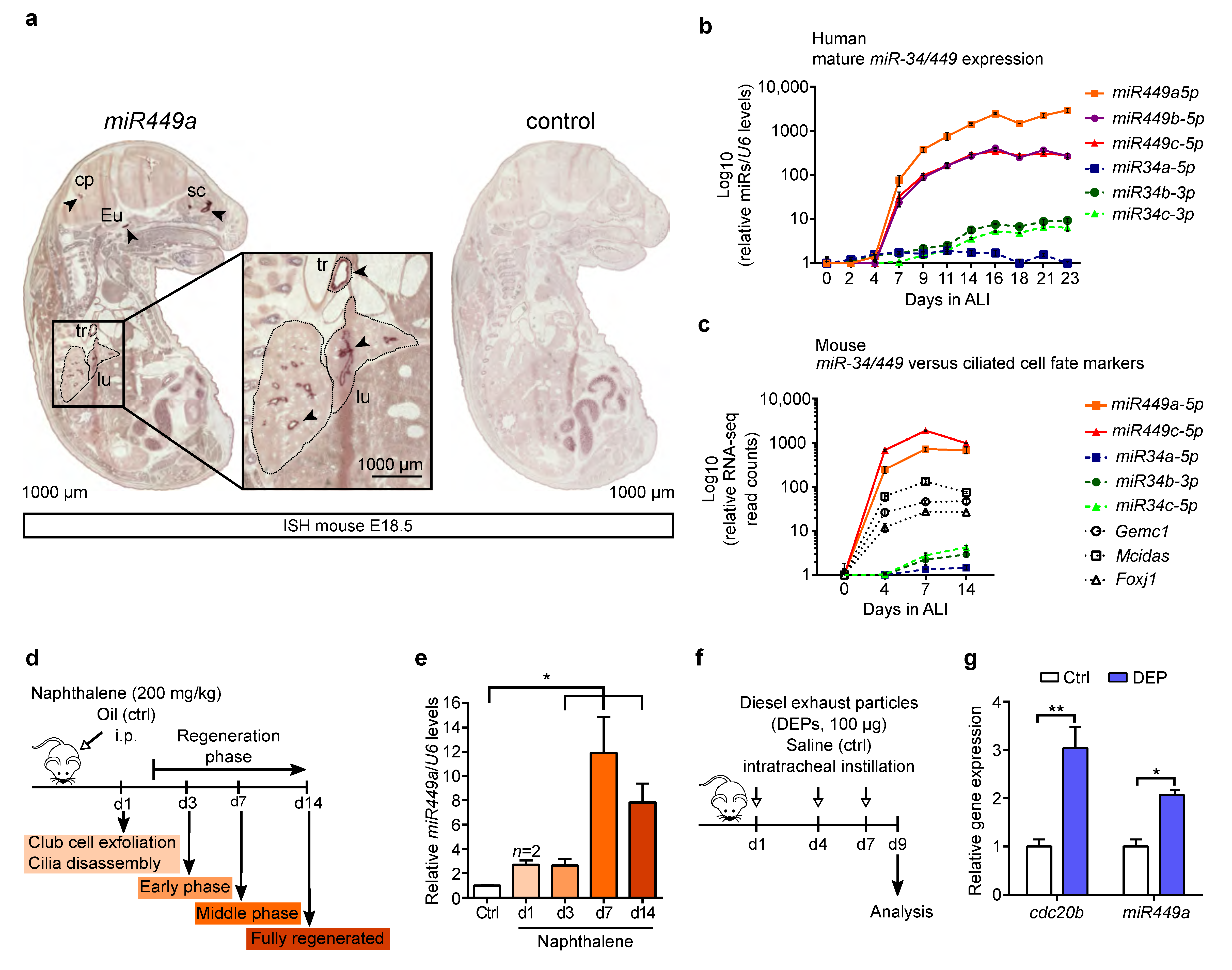
2.2. miR449 Is Induced during Airway Epithelial Differentiation and Regeneration upon Bronchial Challenges
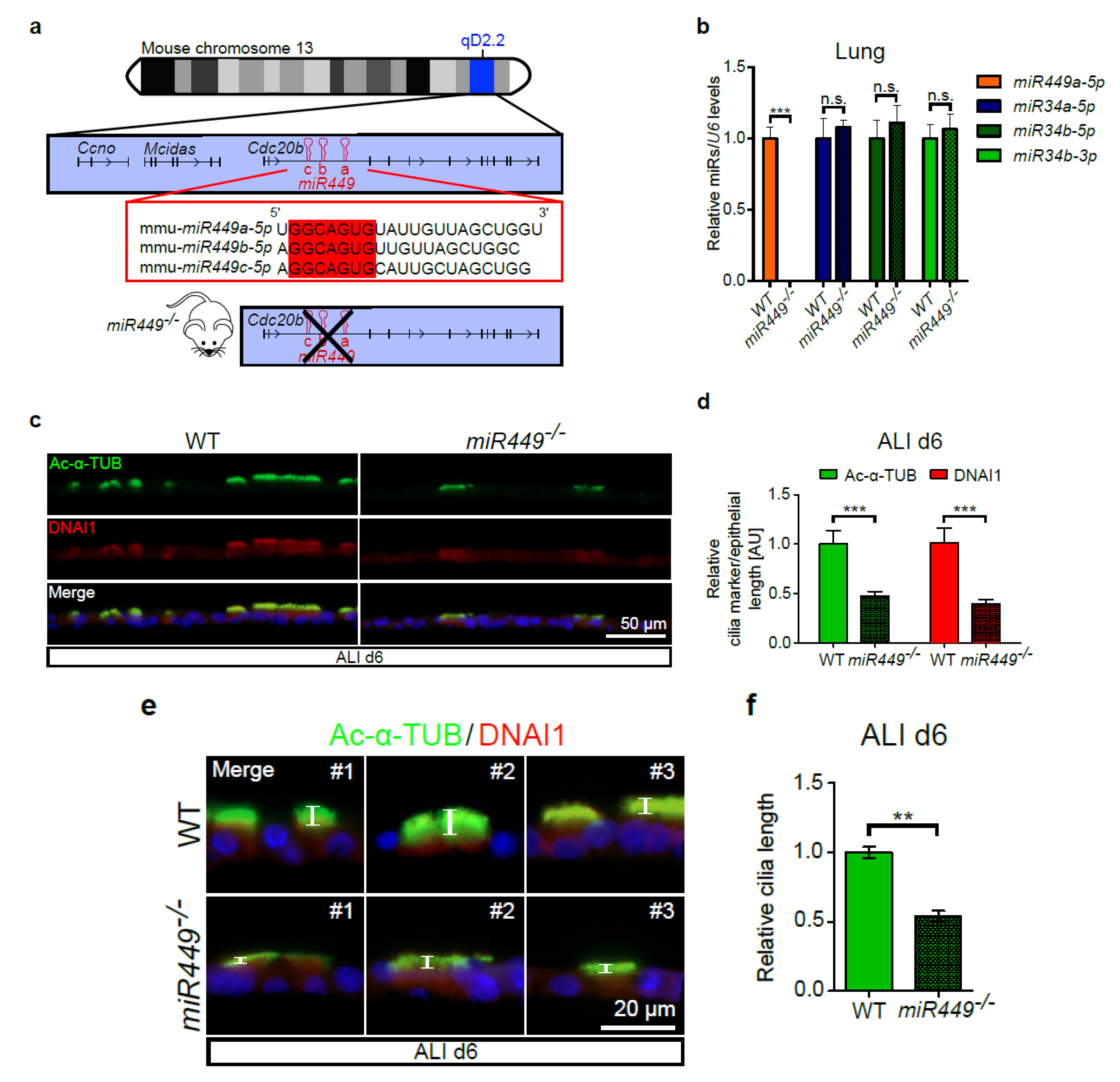
2.3. miR449 Deficiency Results in Impaired Ciliation in ALI Cultures
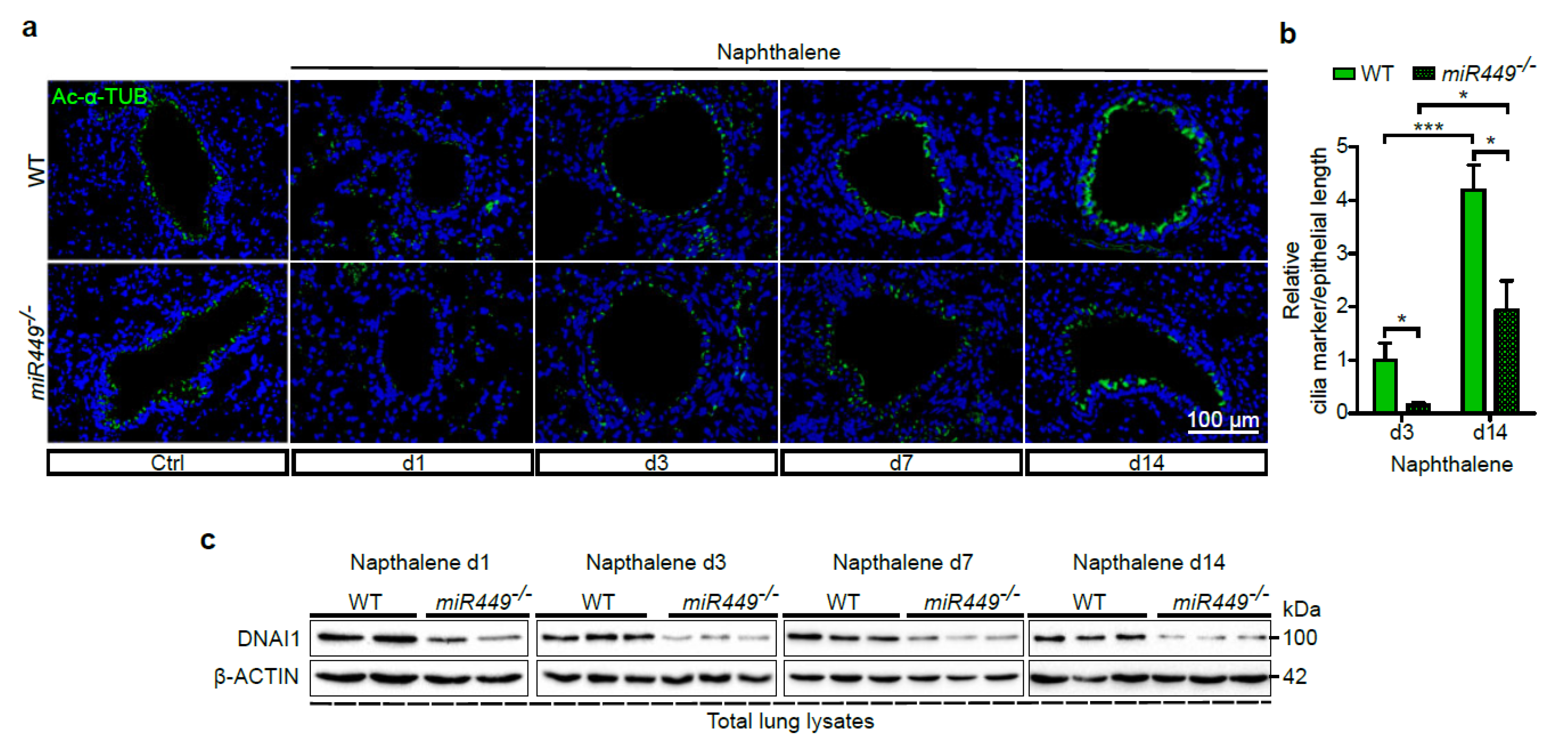
2.4. miR449 Is Essential for Bronchial Epithelial Regeneration
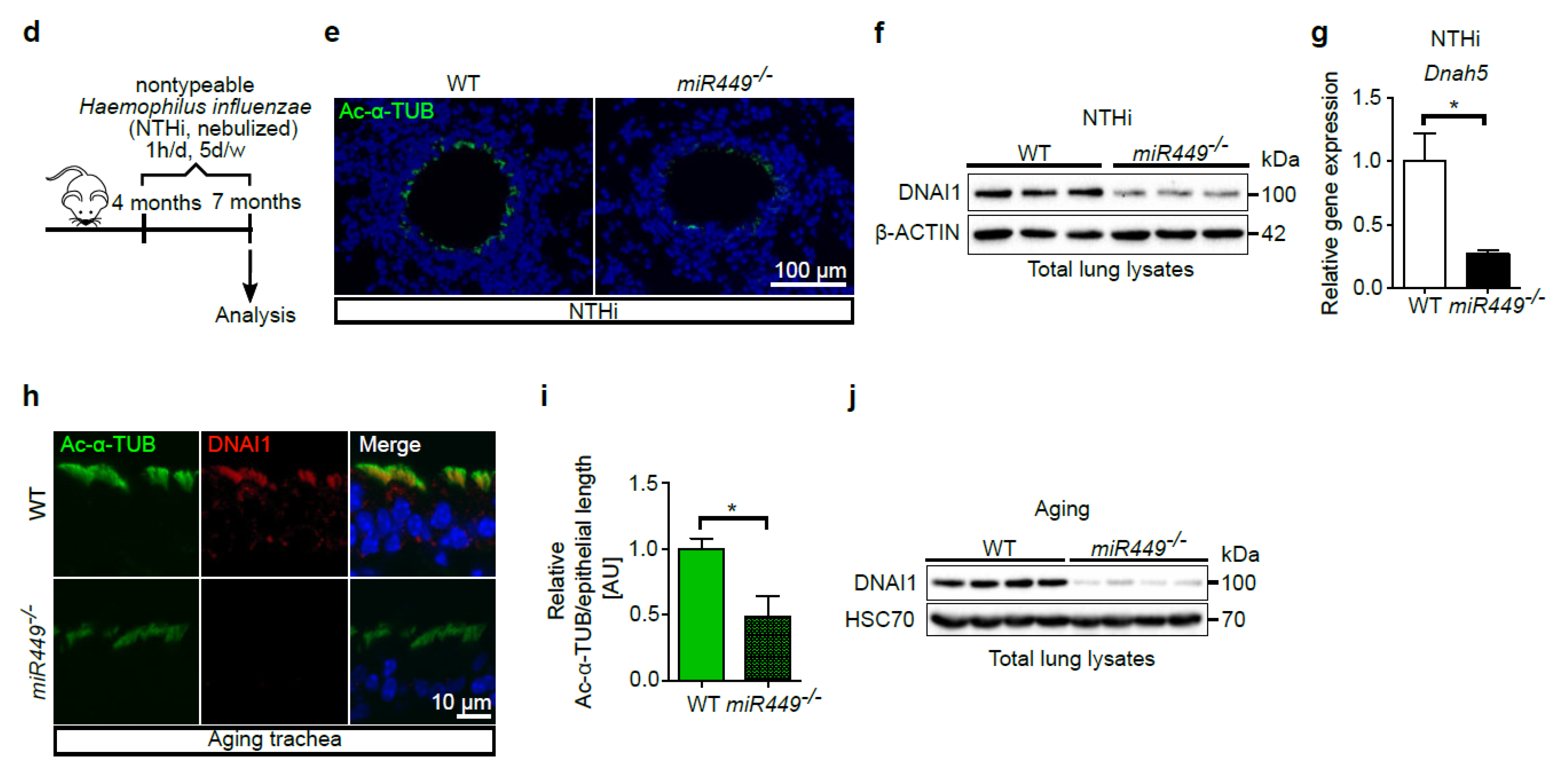
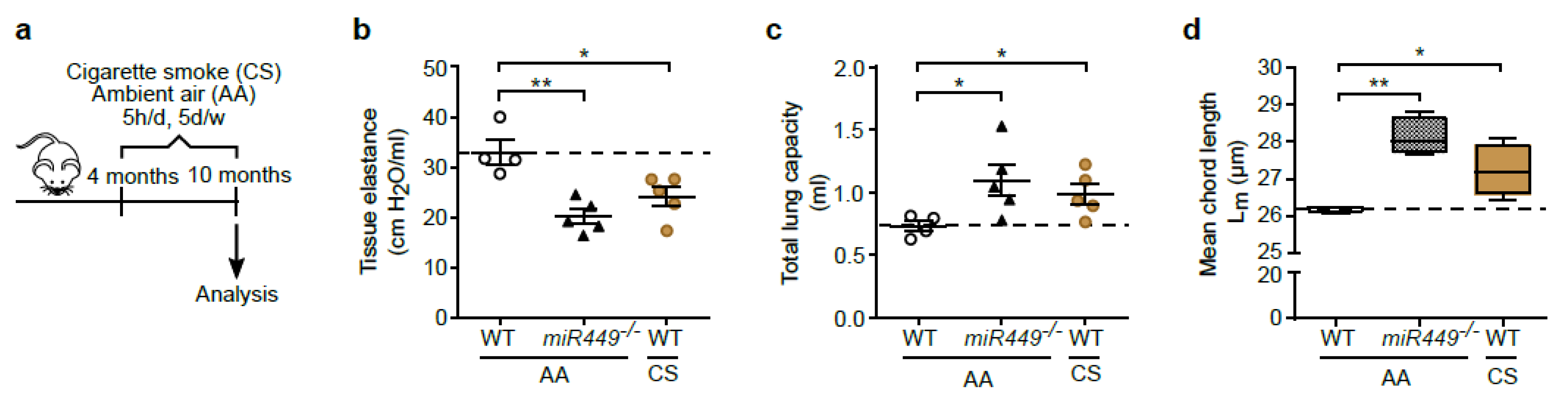
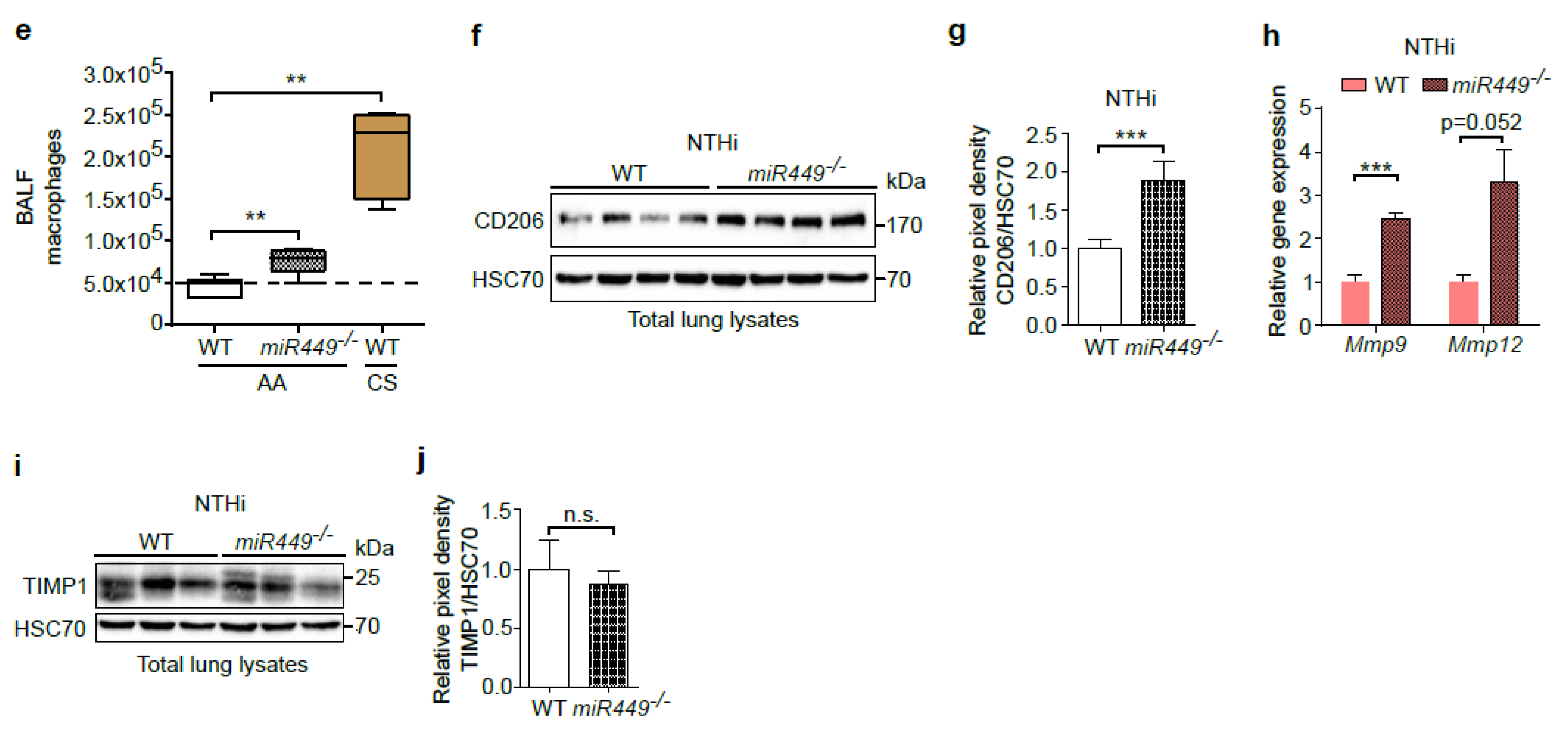
2.5. Spontaneous COPD Development, Associated with an Increased Inflammatory Reaction upon Challenge, in miR449−/− Mice
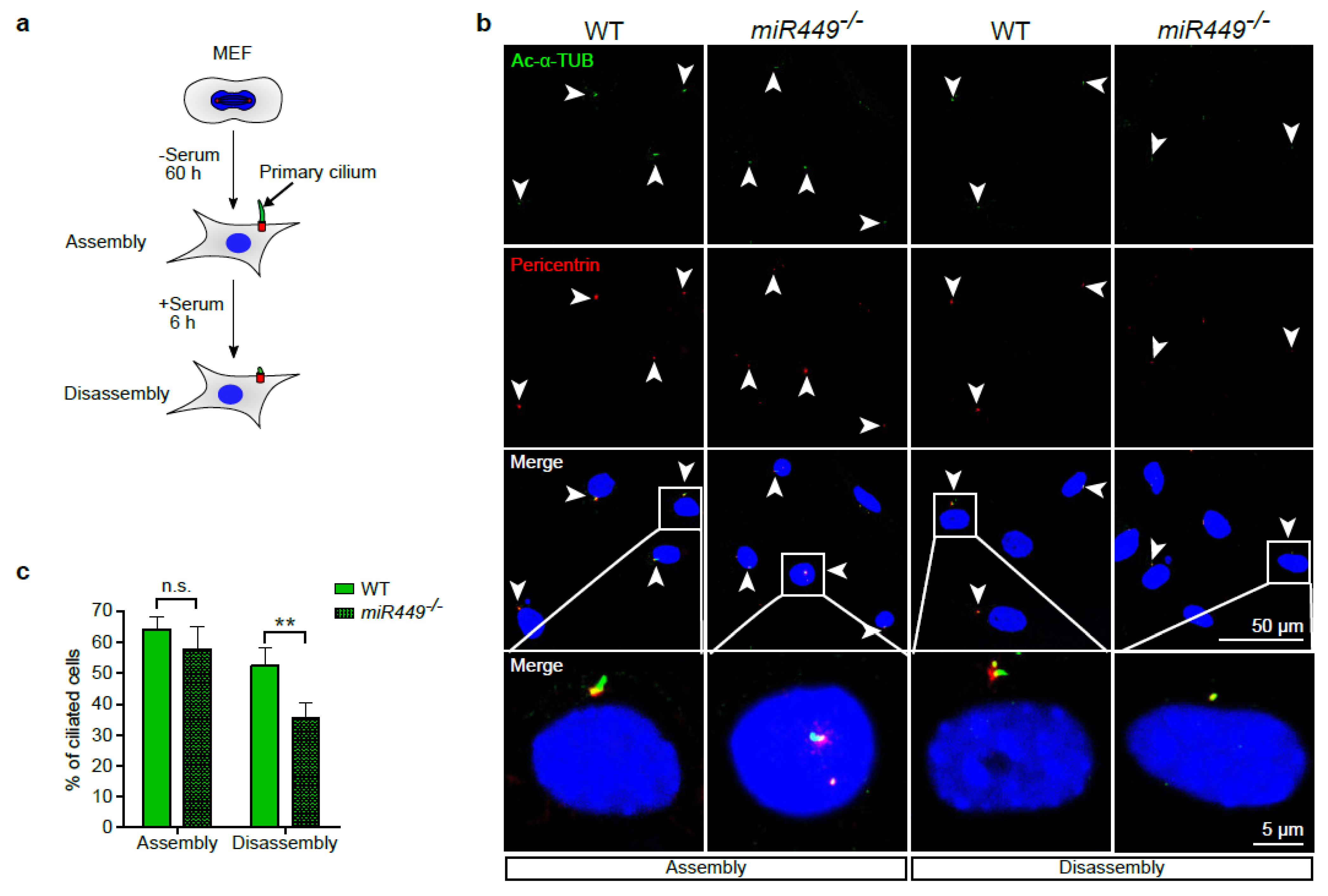
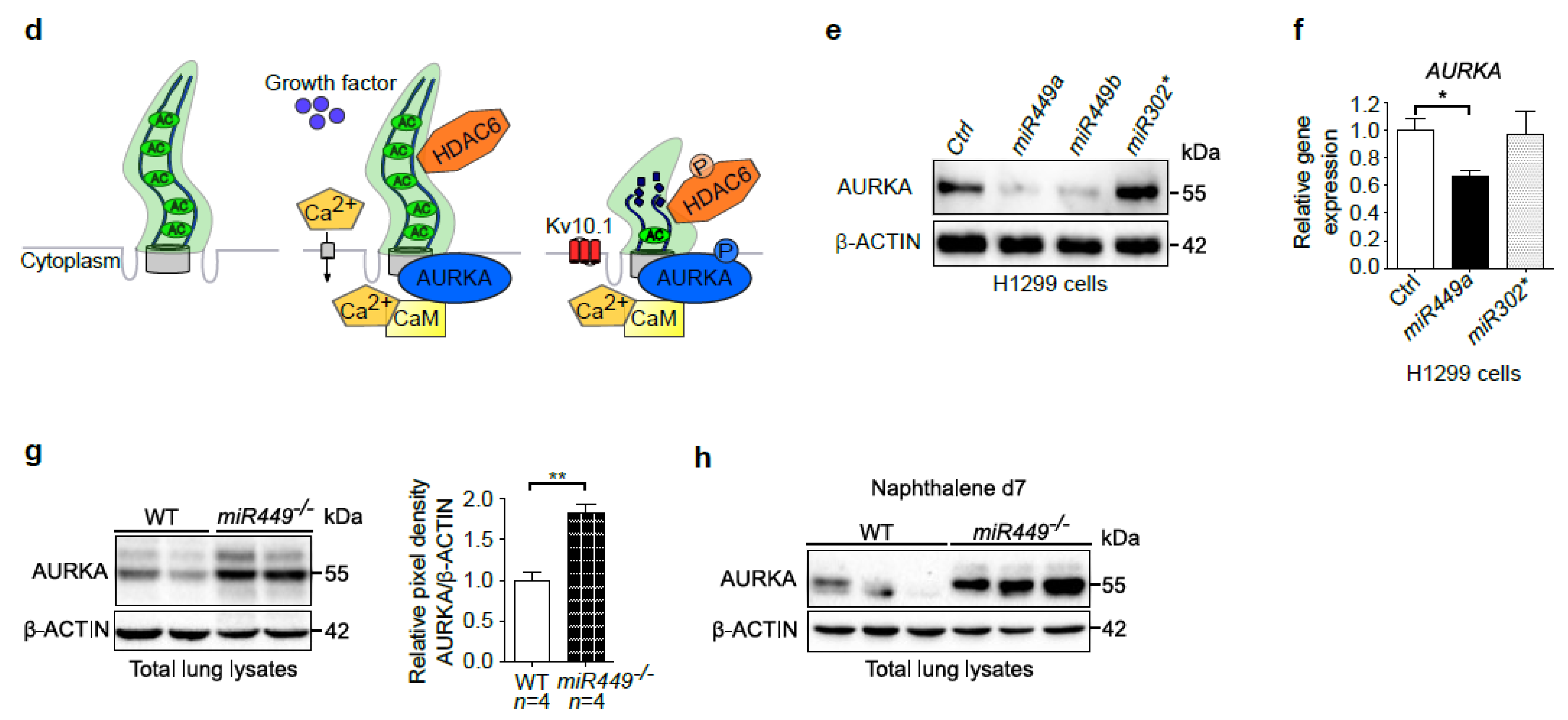
2.6. Aurora Kinase A Contributes to miR449-Driven Epithelial Regeneration Processes and Ciliary Homeostasis
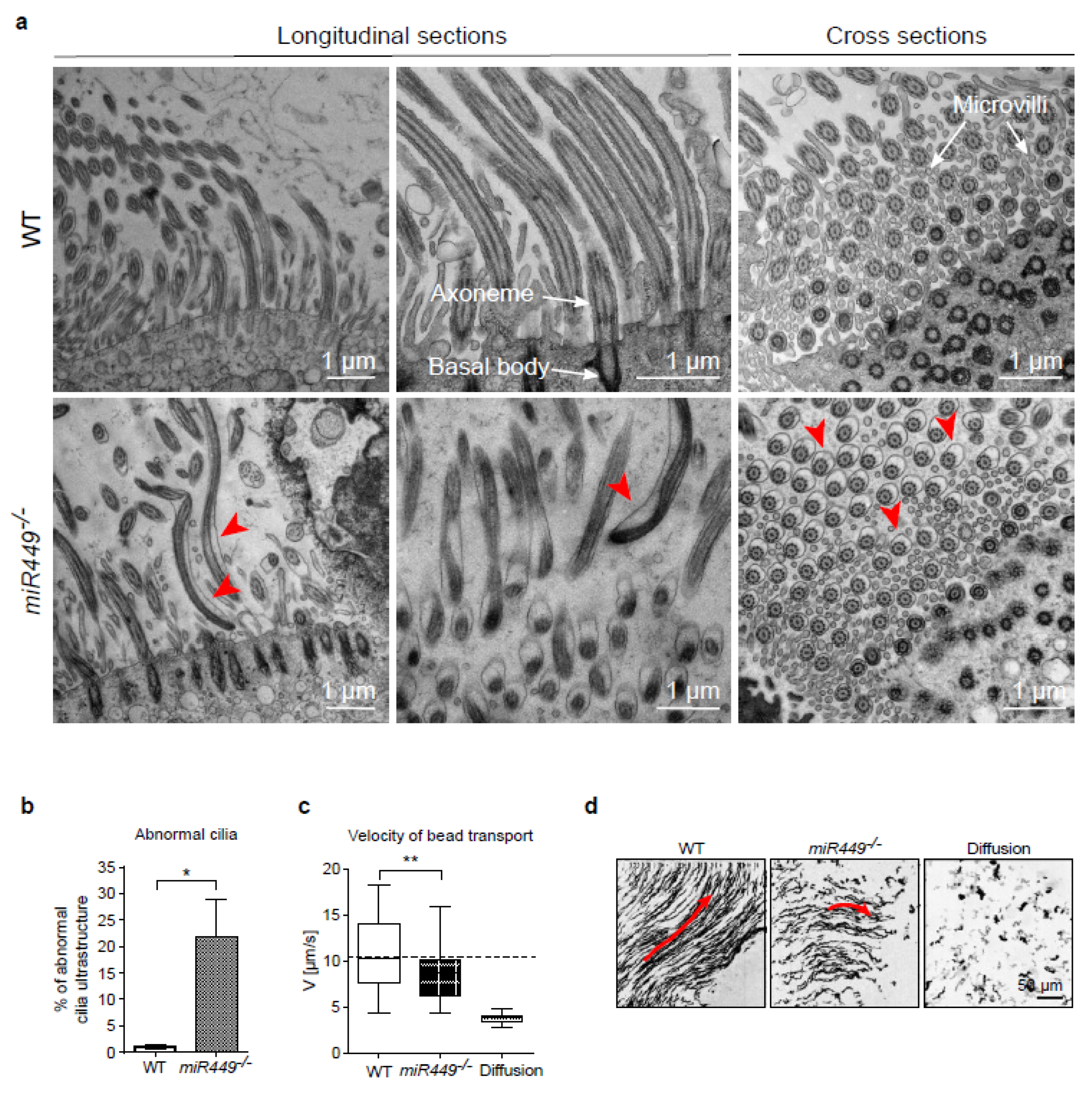
2.7. Mice Lacking miR449 Develop Ultrastructural Cilia Defects and Impaired Mucociliary Clearance
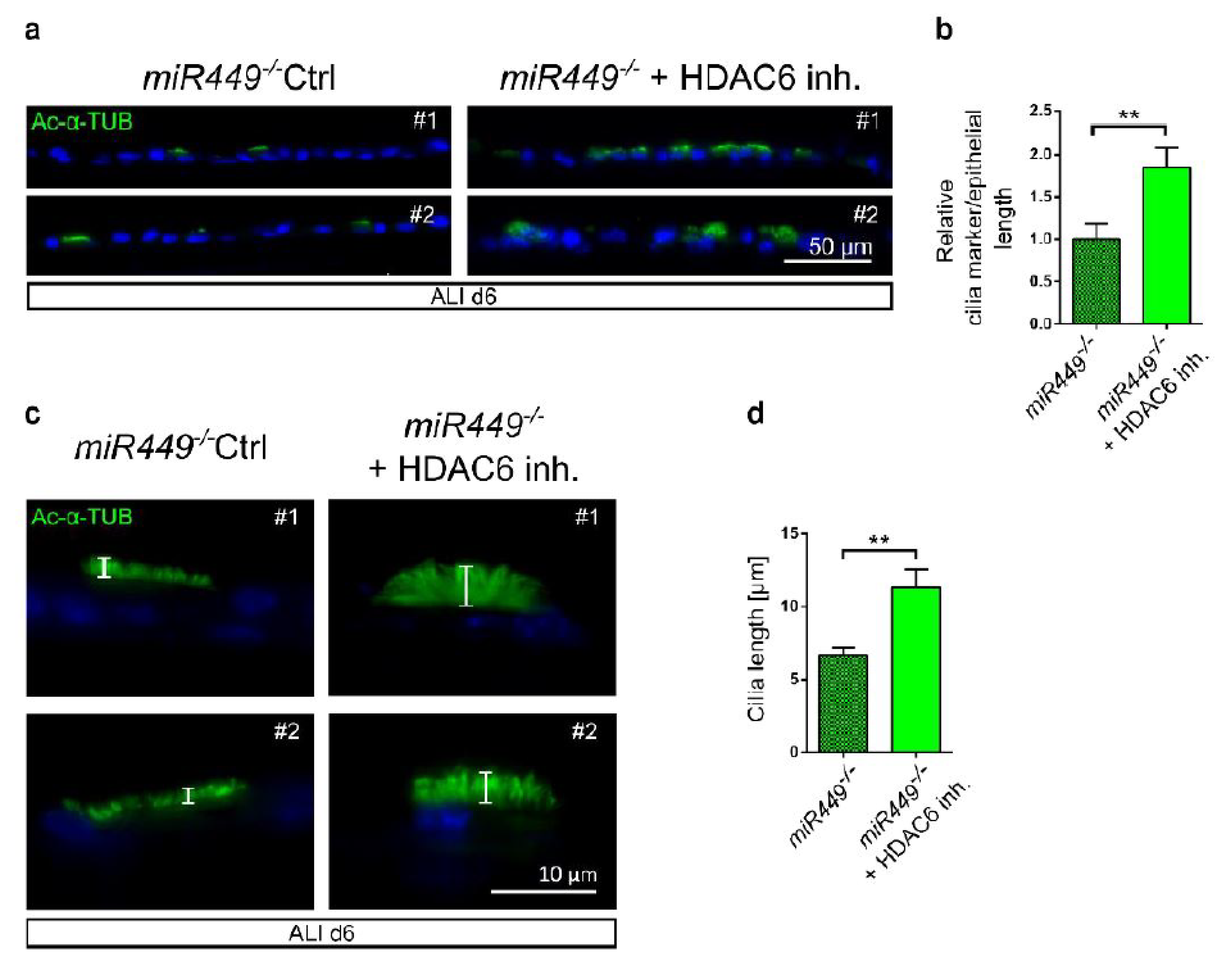
2.8. In miR449−/− Airway Cultures’ Ciliation Can Be Rescued by HDAC6 Inhibition
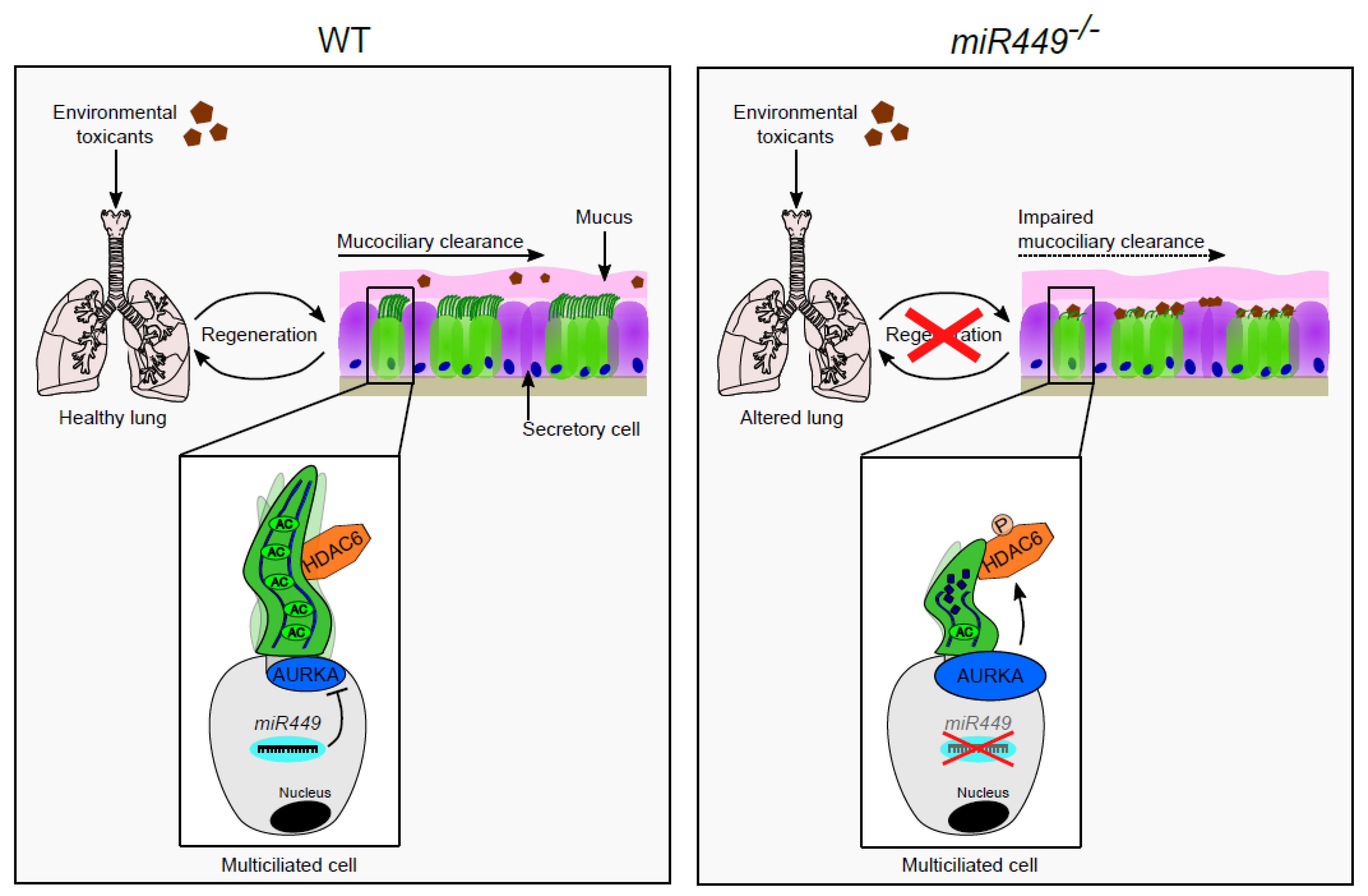
3. Discussion
4. Methods
4.1. Correlation of miR449a and mRNAs in COPD Bronchial Biopsies
4.2. Mice
4.3. Cell Culture
4.4. Transfection of Human Cells
4.5. Analysis of Primary Cilia Assembly and Disassembly
4.6. Air–Liquid Interface Cultures
4.7. In Situ Hybridization
4.8. Naphthalene-Induced Injury Model
4.9. DEP-Induced Acute Inflammation Model
4.10. NTHi-Induced Chronic Inflammation Model
4.11. Cigarette Smoke (CS)-Induced Emphysema Model
4.12. Pulmonary Function
4.13. Bronchoalveolar Lavage
4.14. Stereology
4.15. Histology and Immunostaining
4.16. Quantification of Cilia Markers
4.17. RNA Extraction and Quantitative PCR
4.18. Western Blot
4.19. Electron Microscopy
4.20. Mucociliary Transport Assay
4.21. Single Cell RNAseq Data
4.22. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Satir, P.; Christensen, S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007, 69, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.W.; Van Winkle, L.S.; Toskala, E.; Senior, R.M.; Parks, W.C.; Plopper, C.G. Mouse Strain Modulates the Role of the Ciliated Cell in Acute Tracheobronchial Airway Injury-Distal Airways. Am. J. Pathol. 2002, 160, 315–327. [Google Scholar] [CrossRef]
- Fliegauf, M.; Benzing, T.; Omran, H. When cilia go bad. Cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007, 8, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Beers, M.F.; Morrisey, E.E. The three R’s of lung health and disease: Repair, remodeling, and regeneration. J. Clin. Investig. 2011, 121, 2065–2073. [Google Scholar] [CrossRef]
- Navarro, S.; Driscoll, B. Regeneration of the Aging Lung. A Mini-Review. Gerontology 2017, 63, 270–280. [Google Scholar] [CrossRef]
- Spassky, N.; Meunier, A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs. Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Lizé, M.; Herr, C.; Klimke, A.; Bals, R.; Dobbelstein, M. MicroRNA-449a levels increase by several orders of magnitude during mucociliary differentiation of airway epithelia. Cell Cycle 2010, 9, 4579–4583. [Google Scholar] [CrossRef]
- Marcet, B.; Chevalier, B.; Luxardi, G.; Coraux, C.; Zaragosi, L.-E.; Cibois, M.; Robbe-Sermesant, K.; Jolly, T.; Cardinaud, B.; Moreilhon, C.; et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat. Cell Biol. 2011, 13, 693–699. [Google Scholar] [CrossRef]
- Song, R.; Walentek, P.; Sponer, N.; Klimke, A.; Lee, J.S.; Dixon, G.; Harland, R.; Wan, Y.; Lishko, P.; Lize, M.; et al. miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110. Nature 2014, 510, 115–120. [Google Scholar] [CrossRef]
- Wu, J.; Bao, J.; Kim, M.; Yuan, S.; Tang, C.; Zheng, H.; Mastick, G.S.; Xu, C.; Yan, W. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2851–E2857. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Candido, S.V.; Pilarz, M.S.; Sicinska, E.; Bronson, R.T.; Bowden, M.; Lachowicz, I.A.; Mulry, K.; Fassl, A.; Han, R.C.; et al. Cell cycle-targeting microRNAs promote differentiation by enforcing cell-cycle exit. Proc. Natl. Acad. Sci. USA 2017, 114, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, B.; Adamiok, A.; Mercey, O.; Revinski, D.R.; Zaragosi, L.-E.; Pasini, A.; Kodjabachian, L.; Barbry, P.; Marcet, B. miR-34/449 control apical actin network formation during multiciliogenesis through small GTPase pathways. Nat. Commun. 2015, 6, 8386. [Google Scholar] [CrossRef]
- Revinski, D.R.; Zaragosi, L.-E.; Boutin, C.; Ruiz-Garcia, S.; Deprez, M.; Thomé, V.; Rosnet, O.; Gay, A.-S.; Mercey, O.; Paquet, A.; et al. CDC20B is required for deuterosome-mediated centriole production in multiciliated cells. Nat. Commun. 2018, 9, 4668. [Google Scholar] [CrossRef]
- Stubbs, J.L.; Vladar, E.K.; Axelrod, J.D.; Kintner, C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat. Cell Biol. 2012, 14, 140–147. [Google Scholar] [CrossRef]
- Wallmeier, J.; A Al-Mutairi, D.; Chen, C.-T.; Loges, N.T.; Pennekamp, P.; Menchen, T.; Ma, L.; E Shamseldin, H.; Olbrich, H.; Dougherty, G.W.; et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Genet. 2014, 46, 646–651. [Google Scholar] [CrossRef]
- Lewis, M.; Stracker, T.H. Transcriptional regulation of multiciliated cell differentiation. Semin. Cell Dev. Biol. 2020, 110, 51–60. [Google Scholar] [CrossRef]
- Boon, M.; Wallmeier, J.; Ma, L.; Loges, N.T.; Jaspers, M.; Olbrich, H.; Dougherty, G.W.; Raidt, J.; Werner, C.; Amirav, I.; et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 2014, 5, 4418. [Google Scholar] [CrossRef]
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary health effects of air pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Martinez, C.H.; Mannino, D.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014, 44, 1055–1068. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F.M. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, A.; Dolovich, M.B. Airway Epithelial Cell Cilia and Obstructive Lung Disease. Cells 2016, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.F. Mucociliary dysfunction in COPD: Effect of current pharmacotherapeutic options. Pulm. Pharmacol. Ther. 2005, 18, 1–8. [Google Scholar] [CrossRef]
- Lapperre, T.S.; Postma, D.S.; E Gosman, M.M.; Snoeck-Stroband, J.B.; Hacken, N.H.T.T.; Hiemstra, P.S.; Timens, W.; Sterk, P.J.; Mauad, T. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax 2006, 61, 115–121. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Nemajerova, A.; Kramer, D.; Siller, S.S.; Herr, C.; Shomroni, O.; Pena, T.; Suazo, C.G.; Glaser, K.; Wildung, M.; Steffen, H.; et al. TAp73 is a central transcriptional regulator of airway multiciliogenesis. Genes Dev. 2016, 30, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Chhin, B.; Negre, D.; Merrot, O.; Pham, J.; Tourneur, Y.; Ressnikoff, D.; Jaspers, M.; Jorissen, M.; Cosset, F.-L.; Bouvagnet, P. Ciliary beating recovery in deficient human airway epithelial cells after lentivirus ex vivo gene therapy. PLoS Genet. 2009, 5, e1000422. [Google Scholar] [CrossRef]
- Van Winkle, L.S.; Buckpitt, A.R.; Nishio, S.J.; Isaac, J.M.; Plopper, C.G. Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am. J. Physiol. 1995, 269, L800–L818. [Google Scholar] [CrossRef]
- De Grove, K.C.; Provoost, S.; Brusselle, G.G.; Joos, G.F.; Maes, T. Insights in particulate matter-induced allergic airway inflammation: Focus on the epithelium. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 773–786. [Google Scholar] [CrossRef]
- Harrod, K.S.; Jaramillo, R.J.; Berger, J.A.; Gigliotti, A.P.; Seilkop, S.K.; Reed, M.D. Inhaled diesel engine emissions reduce bacterial clearance and exacerbate lung disease to Pseudomonas aeruginosa infection in vivo. Toxicol. Sci. 2005, 83, 155–165. [Google Scholar] [CrossRef]
- Bao, J.; Li, D.; Wang, L.; Wu, J.; Hu, Y.; Wang, Z.; Chen, Y.; Cao, X.; Jiang, C.; Yan, W.; et al. MicroRNA-449 and microRNA-34b/c function redundantly in murine testes by targeting E2F transcription factor-retinoblastoma protein (E2F-pRb) pathway. J. Biol. Chem. 2012, 287, 21686–21698. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Sharma, R. The Lung Immune Response to Nontypeable Haemophilus influenzae (Lung Immunity to NTHi). J. Immunol. Res. 2015, 2015, 706376. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Li, D.; Ouyang, H.; Huang, J.; Long, Z.; Liang, Z.; Chen, Y.; Chen, Y.; Zheng, Q.; Kuang, M.; et al. Comparison and evaluation of two different methods to establish the cigarette smoke exposure mouse model of COPD. Sci. Rep. 2017, 7, 15454. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015, 44–46, 167–174. [Google Scholar] [CrossRef]
- Goto, H.; Inoko, A.; Inagaki, M. Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell. Mol. Life Sci. CMLS 2013, 70, 3893–3905. [Google Scholar] [CrossRef]
- Sánchez, A.; Urrego, D.; Pardo, L.A. Cyclic expression of the voltage-gated potassium channel KV10.1 promotes disassembly of the primary cilium. EMBO Rep. 2016, 17, 708–723. [Google Scholar] [CrossRef]
- Mercey, O.; Popa, A.; Cavard, A.; Paquet, A.; Chevalier, B.; Pons, N.; Magnone, V.; Zangari, J.; Brest, P.; Zaragosi, L.; et al. Characterizing isomiR variants within the microRNA-34/449 family. FEBS Lett. 2017, 591, 693–705. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Chen, C.; Luo, X.; Xiao, J.; Dong, D.; Lu, Y.; Yang, B.; Wang, Z. Transcriptional and Post-Transcriptional Mechanisms for Oncogenic Overexpression of Ether À Go-Go K+ Channel. PLoS ONE 2011, 6, e20362. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Q.; Snell, W.J. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev. Cell 2004, 6, 445–451. [Google Scholar] [CrossRef]
- Lam, H.C.; Cloonan, S.M.; Bhashyam, A.R.; Haspel, J.A.; Singh, A.; Sathirapongsasuti, J.F.; Cervo, M.; Yao, H.; Chung, A.L.; Mizumura, K.; et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J. Clin. Investig. 2013, 123, 5212–5230. [Google Scholar] [CrossRef]
- Jacoby, M.; Cox, J.J.; Gayral, S.; Hampshire, D.; Ayub, M.; Blockmans, M.; Pernot, E.; Kisseleva, M.V.; Compère, P.; Schiffmann, S.; et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 2009, 41, 1027–1031. [Google Scholar] [CrossRef]
- Plotnikova, O.V.; Seo, S.; Cottle, D.; Conduit, S.; Hakim, S.; Dyson, J.M.; A Mitchell, C.; Smyth, I.M. INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability. J. Cell Sci. 2015, 128, 364–372. [Google Scholar] [CrossRef]
- Collins, M.; et al. in Mechanisms of lung injury and repair (European Respiratory Society09282019), PA3854.
- Yuan, S.; Liu, Y.; Peng, H.; Tang, C.; Hennig, G.W.; Wang, Z.; Wang, L.; Yu, T.; Klukovich, R.; Zhang, Y.; et al. Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc. Natl. Acad. Sci. USA 2019, 116, 3584–3593. [Google Scholar] [CrossRef]
- Wildung, M.; Esser, T.U.; Grausam, K.B.; Wiedwald, C.; Volceanov-Hahn, L.; Riedel, D.; Beuermann, S.; Li, L.; Zylla, J.; Guenther, A.-K.; et al. Transcription factor TAp73 and microRNA-449 complement each other to support multiciliogenesis. Cell Death Differ. 2019, 26, 2740–2757. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Lam, H.C.; Ryter, S.W.; Choi, A.M. “Ciliophagy”: The consumption of cilia components by autophagy. Autophagy 2014, 10, 532–534. [Google Scholar] [CrossRef]
- Sánchez, I.; Dynlacht, B.D. Cilium assembly and disassembly. Nat. Cell Biol. 2016, 18, 711–717. [Google Scholar] [CrossRef]
- De Jong, K.; Vonk, J.; Imboden, M.; LaHousse, L.; Hofman, A.; Brusselle, G.; Probst-Hensch, N.; Postma, D.; Boezen, H. Genes and pathways underlying susceptibility to impaired lung function in the context of environmental tobacco smoke exposure. Respir. Res. 2017, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.-L.T.; Schmittgen, T.D.; et al. Correction: Detection of microRNA Expression in Human Peripheral Blood Microvesicles. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Han, R.; Ji, X.; Rong, R.; Li, Y.; Yao, W.; Yuan, J.; Wu, Q.; Yang, J.; Yan, W.; Han, L.; et al. MiR-449a regulates autophagy to inhibit silica-induced pulmonary fibrosis through targeting Bcl2. J. Mol. Med. 2016, 94, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef]
- Lindon, C.; Grant, R.; Min, M. Ubiquitin-Mediated Degradation of Aurora Kinases. Front. Oncol. 2016, 5, 307. [Google Scholar] [CrossRef]
- Fu, Y.; Tong, J.; Meng, F.; Hoeltig, D.; Liu, G.; Yin, X.; Herrler, G. Ciliostasis of airway epithelial cells facilitates influenza A virus infection. Vet. Res. 2018, 49, 65. [Google Scholar] [CrossRef]
- Wilson, R.; Roberts, D.; Cole, P. Effect of bacterial products on human ciliary function in vitro. Thorax 1985, 40, 125–131. [Google Scholar] [CrossRef]
- Read, R.C.; Roberts, P.; Munro, N.; Rutman, A.; Hastie, A.; Shryock, T.; Hall, R.; McDonald-Gibson, W.; Lund, V.; Taylor, G.; et al. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J. Appl. Physiol. 1992, 72, 2271–2277. [Google Scholar] [CrossRef]
- Bailey, K.L.; LeVan, T.D.; A Yanov, D.; A Pavlik, J.; DeVasure, J.M.; Sisson, J.H.; A Wyatt, T. Non-typeable Haemophilus influenzae decreases cilia beating via protein kinase Cε. Respir. Res. 2012, 13, 49. [Google Scholar] [CrossRef]
- Janson, H.; Carlén, B.; Cervin, A.; Forsgren, A.; Magnusdottir, A.B.; Lindberg, S.; Runer, T. Effects on the ciliated epithelium of protein D-producing and -nonproducing nontypeable Haemophilus influenzae in nasopharyngeal tissue cultures. J. Infect. Dis. 1999, 180, 737–746. [Google Scholar] [CrossRef][Green Version]
- Piecková, E.; Jesenská, Z. Ciliostatic effect of fungi on the respiratory tract ciliary movement of one-day-old chickens in vitro. Folia Microbiol. 1996, 41, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; McKenna, S.; Agbetile, J.; Fairs, A.; Desai, D.; Mistry, V.; Morley, J.P.; Pancholi, M.; Pavord, I.; Wardlaw, A.; et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur. Respir. J. 2014, 43, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Stockley, R.A. COPD: Current therapeutic interventions and future approaches. Eur. Respir. J. 2005, 25, 1084–1106. [Google Scholar] [CrossRef] [PubMed]
- Hessel, J.; Heldrich, J.; Fuller, J.; Staudt, M.R.; Radisch, S.; Hollmann, C.; Harvey, B.-G.; Kaner, R.J.; Salit, J.; Yee-Levin, J.; et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS ONE 2014, 9, e85453. [Google Scholar] [CrossRef]
- Yaghi, A.; Zaman, A.; Cox, G.; Dolovich, M.B. Ciliary beating is depressed in nasal cilia from chronic obstructive pulmonary disease subjects. Respir. Med. 2012, 106, 1139–1147. [Google Scholar] [CrossRef]
- Tasena, H.; Faiz, A.; Timens, W.; Noordhoek, J.; Hylkema, M.N.; Gosens, R.; Hiemstra, P.S.; Spira, A.; Postma, D.S.; Tew, G.W.; et al. microRNA-mRNA regulatory networks underlying chronic mucus hypersecretion in COPD. Eur. Respir. J. 2018, 52, 1701556. [Google Scholar] [CrossRef]
- Vella, G.; Ritzmann, F.; Wolf, L.; Kamyschnikov, A.; Stodden, H.; Herr, C.; Slevogt, H.; Bals, R.; Beisswenger, C. IL-17C contributes to NTHi-induced inflammation and lung damage in experimental COPD and is present in sputum during acute exacerbations. PLoS ONE 2021, 16, e0243484. [Google Scholar]
- Ritzmann, F.; Borchardt, K.; Vella, G.; Chitirala, P.; Angenendt, A.; Herr, C.; Menger, M.D.; Hoth, M.; Lis, A.; Bohle, R.M.; et al. Blockade of PD-1 decreases neutrophilic inflammation and lung damage in experimental COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L958–L968. [Google Scholar] [CrossRef]
- Provoost, S.; Maes, T.; Joos, G.F.; Tournoy, K.G. Monocyte-derived dendritic cell recruitment and allergic T(H)2 responses after exposure to diesel particles are CCR2 dependent. J. Allergy Clin. Immunol. 2012, 129, 483–491. [Google Scholar] [CrossRef]
- Provoost, S.; De Grove, K.C.; Fraser, G.L.; Lannoy, V.J.; Tournoy, K.G.; Brusselle, G.; Maes, T.; Joos, G.F. Pro- and Anti-Inflammatory Role of ChemR23 Signaling in Pollutant-Induced Inflammatory Lung Responses. J. Immunol. 2016, 196, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Knust, J.; Ochs, M.; Gundersen, H.J.G.; Nyengaard, J.R. Stereological estimates of alveolar number and size and capillary length and surface area in mice lungs. Anat. Rec. 2009, 292, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Studer, D.; Michel, M.; Muller, M. High pressure freezing comes of age. Scan. Microsc. Suppl. 1989, 3, 253–268; discussion 268–269. [Google Scholar]
- Wolf, F.; Angerer, P.; Theis, F. SCANPY: Large-scale single-cell gene expression data analysis. Genome Biol. 2018, 19, 15. [Google Scholar] [CrossRef]
- Wolock, S.; Lopez, R.; Klein, A.M. Computational Identification of Cell Doublets in Single-Cell Transcriptomic Data. Cell Syst. 2019, 8, 281–291.e9. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, A.; Lopez, R.; Xing, G.; Boyeau, P.; Pour Amiri, V.V.; Hong, J.; Wu, K.; Jayasuriya, M.; Mehlman, E.; Langevin, M.; et al. A Python library for probabilistic analysis of single-cell omics data. Nat. Biotechnol. 2022, 40, 163–166. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wildung, M.; Herr, C.; Riedel, D.; Wiedwald, C.; Moiseenko, A.; Ramírez, F.; Tasena, H.; Heimerl, M.; Alevra, M.; Movsisyan, N.; et al. miR449 Protects Airway Regeneration by Controlling AURKA/HDAC6-Mediated Ciliary Disassembly. Int. J. Mol. Sci. 2022, 23, 7749. https://doi.org/10.3390/ijms23147749
Wildung M, Herr C, Riedel D, Wiedwald C, Moiseenko A, Ramírez F, Tasena H, Heimerl M, Alevra M, Movsisyan N, et al. miR449 Protects Airway Regeneration by Controlling AURKA/HDAC6-Mediated Ciliary Disassembly. International Journal of Molecular Sciences. 2022; 23(14):7749. https://doi.org/10.3390/ijms23147749
Chicago/Turabian StyleWildung, Merit, Christian Herr, Dietmar Riedel, Cornelia Wiedwald, Alena Moiseenko, Fidel Ramírez, Hataitip Tasena, Maren Heimerl, Mihai Alevra, Naira Movsisyan, and et al. 2022. "miR449 Protects Airway Regeneration by Controlling AURKA/HDAC6-Mediated Ciliary Disassembly" International Journal of Molecular Sciences 23, no. 14: 7749. https://doi.org/10.3390/ijms23147749
APA StyleWildung, M., Herr, C., Riedel, D., Wiedwald, C., Moiseenko, A., Ramírez, F., Tasena, H., Heimerl, M., Alevra, M., Movsisyan, N., Schuldt, M., Volceanov-Hahn, L., Provoost, S., Nöthe-Menchen, T., Urrego, D., Freytag, B., Wallmeier, J., Beisswenger, C., Bals, R., ... Lizé, M. (2022). miR449 Protects Airway Regeneration by Controlling AURKA/HDAC6-Mediated Ciliary Disassembly. International Journal of Molecular Sciences, 23(14), 7749. https://doi.org/10.3390/ijms23147749







