Lipophilic Bioactive Compounds Transported in Triglyceride-Rich Lipoproteins Modulate Microglial Inflammatory Response
Abstract
1. Introduction
2. Results
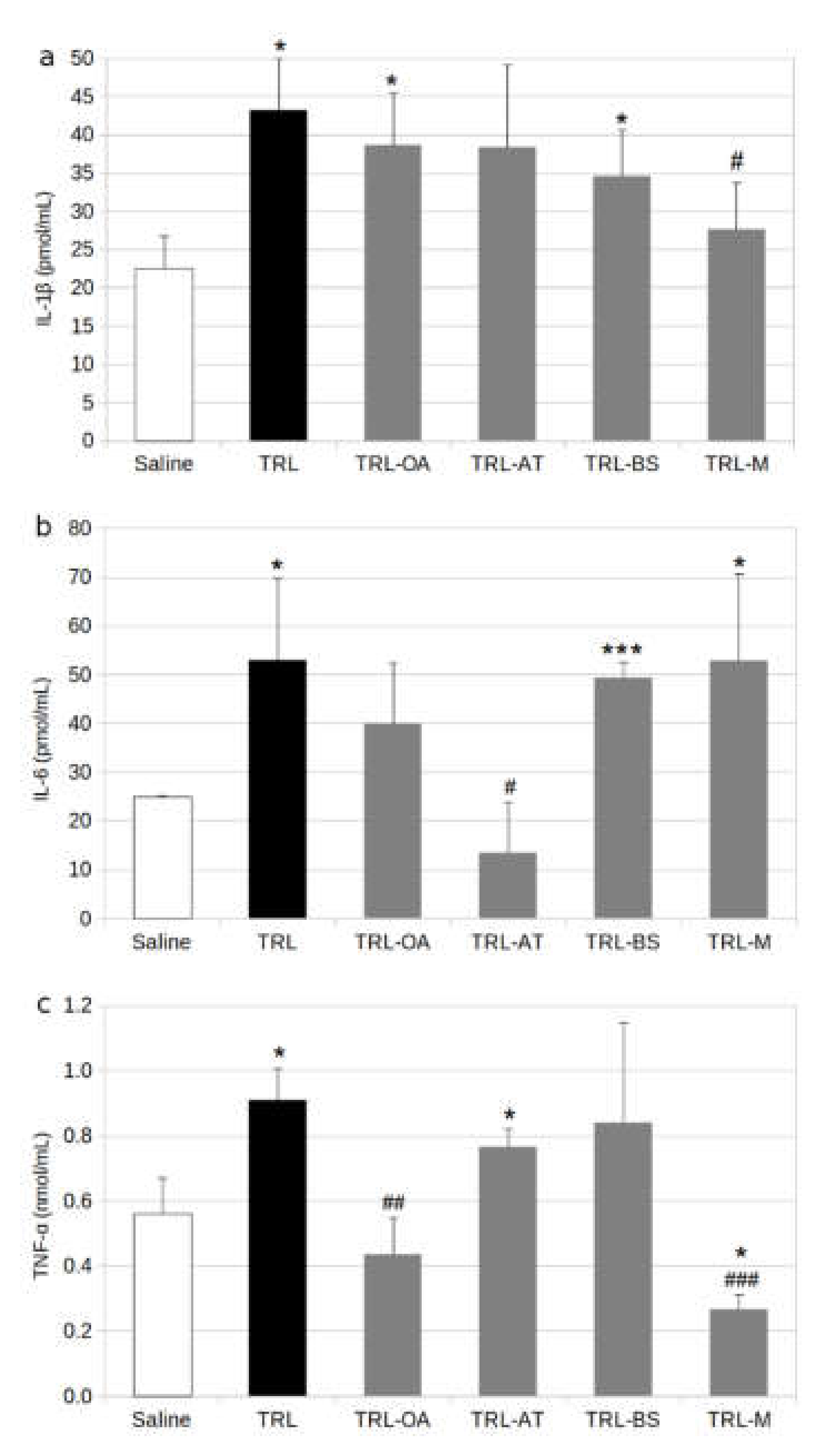
2.1. Protein and Gene Expression of Proinflammatory Cytokines in BV-2 Cells
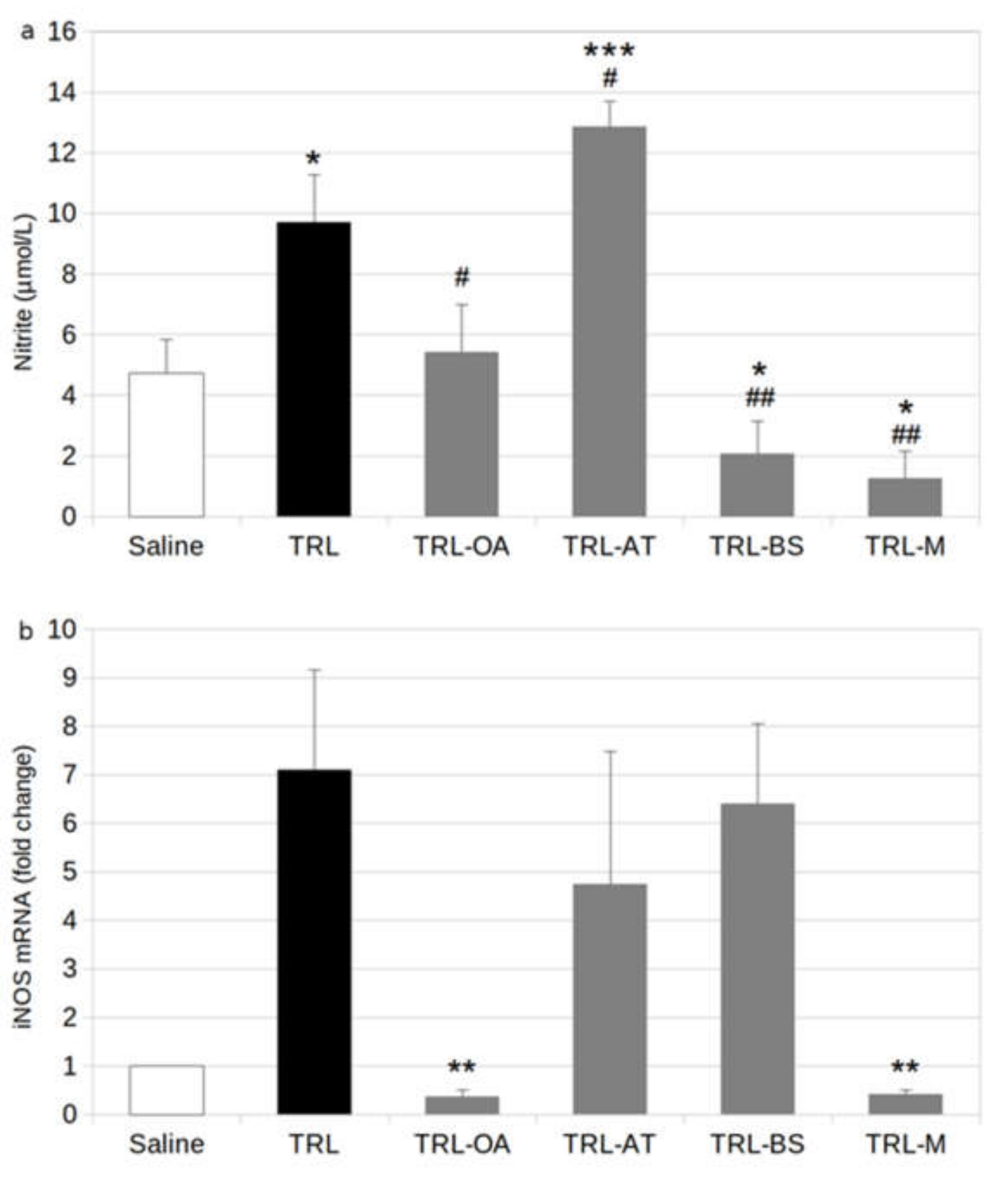
2.2. NO Release and Expression of iNOS in BV-2 Cells
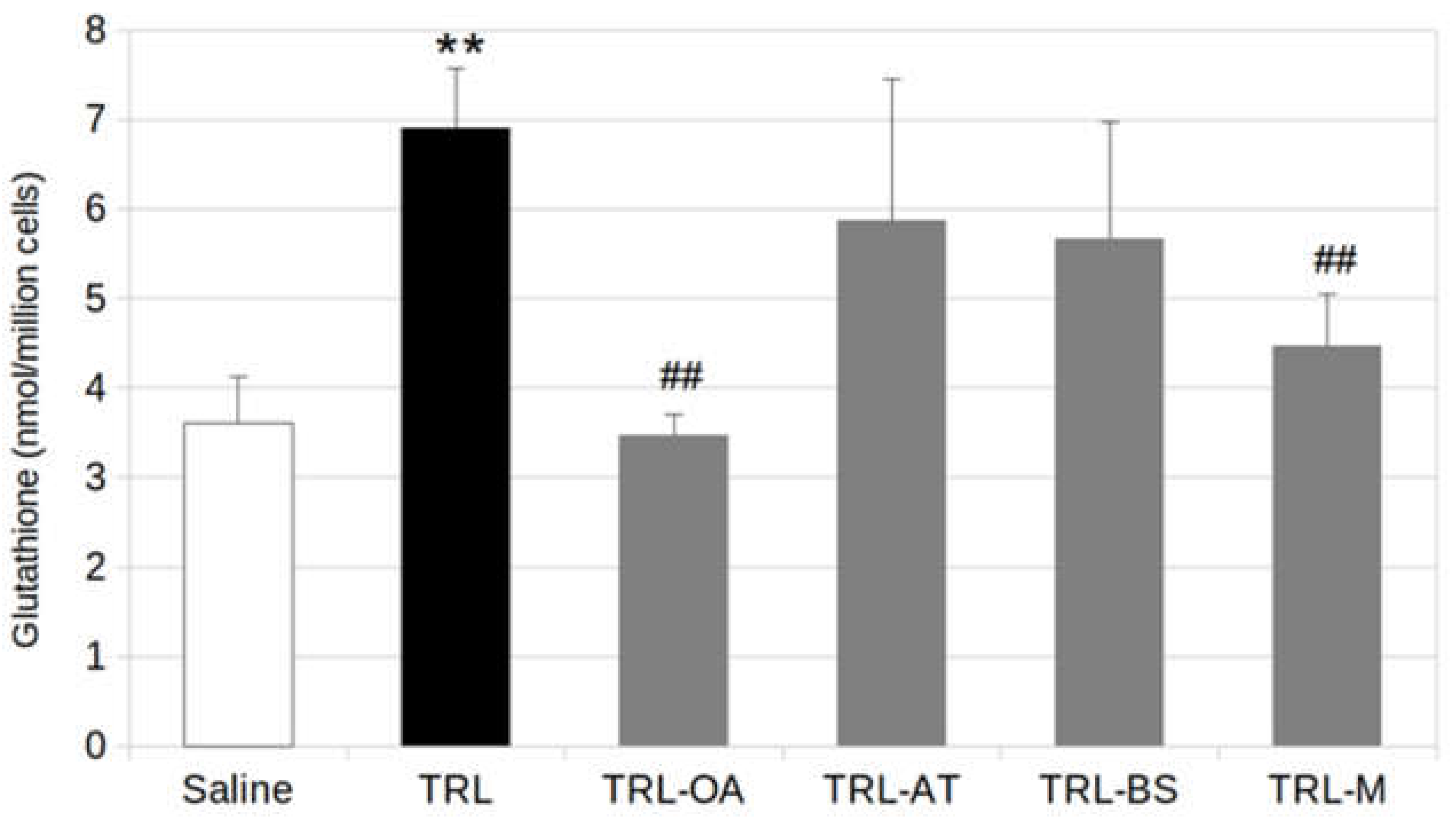
2.3. Glutathione Production in BV2 Cells
3. Discussion
4. Materials and Methods
4.1. Oleanolic Acid Isolation and Characterization
4.2. Preparation of Lab-Made TRL
4.3. Microglia Cell Model
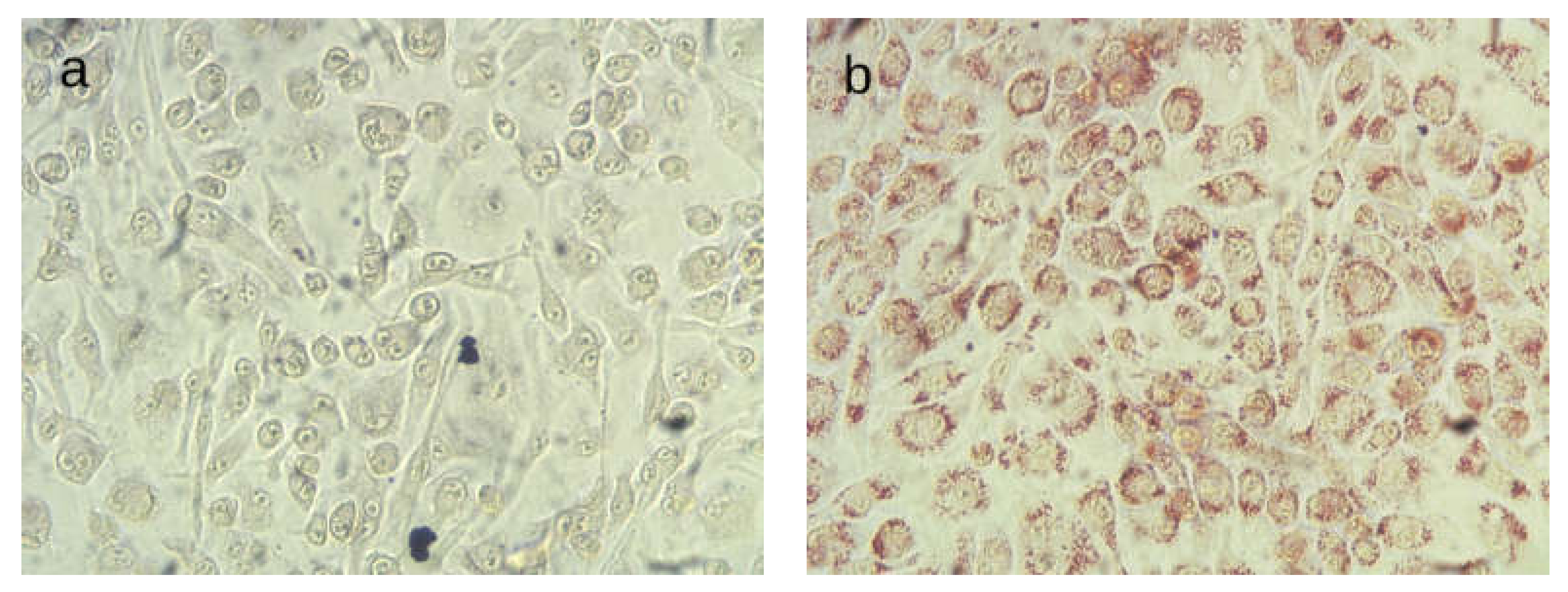
4.4. Oil Red O Staining
4.5. Inflammatory Cytokine Production
4.6. Nitric Oxide Assay
4.7. Glutathione Assay
4.8. Gene Expression
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Bulck, M.; Sierra-Magro, A.; Alarcon-Gil, J.; Perez-Castillo, A.; Morales-Garcia, J. Novel Approaches for the Treatment of Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 719. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Helkala, E.L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissien, A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ 2001, 322, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Skoog, I.; Kalaria, R.N.; Breteler, M.M. Vascular factors and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1999, 13 (Suppl. S3), S106–S114. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.; Hofman, A.; Breteler, M. Dietary fat intake and the risk of incident dementia in the Rotterdam study. Ann. Neurol. 1997, 42, 776–782. [Google Scholar] [CrossRef]
- Grant, W.B. Dietary links to Alzheimer’s disease: 1999 update. J. Alzheimers Dis. 1999, 1, 197–201. [Google Scholar] [CrossRef]
- Galloway, S.; Jian, L.; Johnsen, R.; Chew, S.; Mamo, J.C.L. β-amyloid or its precursor protein is found in epithelial cells of the small intestine and is stimulated by high-fat feeding. J. Nutr. Biochem. 2007, 18, 279–284. [Google Scholar] [CrossRef]
- Roher, A.E.; Esh, C.L.; Kokjohn, T.A.; Castaño, E.M.; Van Vickle, G.D.; Kalback, W.M.; Patton, R.L.; Luehrs, D.C.; Daugs, I.D.; Kuo, Y.-M.; et al. Amyloid β peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 2009, 5, 18–29. [Google Scholar] [CrossRef]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.; Wellington, C.; Johnsen, R.; Mamo, J.C. Three-dimensional colocalization analysis of plasma-derived apolipoprotein B with amyloid plaques in APP/PS1 transgenic mice. Histochem. Cell Biol. 2009, 131, 661–666. [Google Scholar] [CrossRef]
- Dyall, S.C. Amyloid-Beta Peptide, Oxidative Stress and Inflammation in Alzheimer’s Disease: Potential Neuroprotective Effects of Omega-3 Polyunsaturated Fatty Acids. Int. J. Alzheimers Dis. 2010, 2010, 274128. [Google Scholar] [CrossRef]
- Galloway, S.; Takechi, R.; Nesbit, M.; Pallebage-Gamarallage, M.M.; Lam, V.; Mamo, J.C.L. The differential effects of fatty acids on enterocytic abundance of amyloid-β. Lipids Health Dis. 2019, 18, 209. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Veremeyko, T.; Weiner, H.L. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 2013, 61, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Feinstein, D.L.; Shen, Q.; Bhat, A.N. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-β, and activating transcription fact. J. Biol. Chem. 2002, 277, 29584–29592. [Google Scholar] [CrossRef] [PubMed]
- Button, E.B.; Mitchell, A.S.; Domingos, M.M.; Chung, J.H.J.; Bradley, R.M.; Hashemi, A.; Marvyn, P.M.; Patterson, A.C.; Stark, K.D.; Quadrilatero, J.; et al. Microglial cell activation increases saturated and decreases monounsaturated fatty acid content, but both lipid species are proinflammatory. Lipids 2014, 49, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Toscano, R.; Millan-Linares, M.C.; Lemus-Conejo, A.; Claro, C.; Sanchez-Margalet, V.; Montserrat-de la Paz, S. Postprandial triglyceride-rich lipoproteins promote M1/M2 microglia polarization in a fatty-acid-dependent manner. J. Nutr. Biochem. 2020, 75, 108248. [Google Scholar] [CrossRef]
- Castellano, J.M.; Espinosa, J.M.; Perona, J.S. Modulation of Lipid Transport and Adipose Tissue Deposition by Small Lipophilic Compounds. Front. Cell Dev. Biol. 2020, 8, 985. [Google Scholar] [CrossRef]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic Acid Exerts a Neuroprotective Effect Against Microglial Cell Activation by Modulating Cytokine Release and Antioxidant Defense Systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Guinda, Á.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Jeanes, Y.M.; Hall, W.L.; Ellard, S.; Lee, E.; Lodge, J.K. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 2004, 92, 575–579. [Google Scholar] [CrossRef]
- Mortimer, B.C.; Tso, P.; Phan, C.T.; Beveridge, D.J.; Wen, J.; Redgrave, T.G. Features of cholesterol structure that regulate the clearance of chylomicron-like lipid emulsions. J. Lipid Res. 1995, 36, 2038–2053. [Google Scholar] [CrossRef]
- Kasem Kasem, I. Bioavailability of the Unsaponifiable Fraction Components of Olive Oil in Humans. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2019. Available online: https://hdl.handle.net/11441/70732 (accessed on 2 June 2022).
- Batt, K.V.; Avella, M.; Moore, E.H.; Jackson, B.; Suckling, K.E.; Botham, K.M. Differential effects of low-density lipoprotein and chylomicron remnants on lipid accumulation in human macrophages. Exp. Biol. Med. 2004, 229, 528–537. [Google Scholar] [CrossRef]
- Moore, E.H.; Napolitano, M.; Avella, M.; Bejta, F.; Suckling, K.E.; Bravo, E.; Botham, K.M. Protection of chylomicron remnants from oxidation by incorporation of probucol into the particles enhances their uptake by human macrophages and increases lipid accumulation in the cells. Eur. J. Biochem. 2004, 271, 2417–2427. [Google Scholar] [CrossRef]
- Antelo, A.; Perona, J.S. Evaluation of a method of preparation of lipid emulsions as a model for chylomicron-like particles. J. Liposome Res. 2013, 23, 126–133. [Google Scholar] [CrossRef]
- Shemer, A.; Erny, D.; Jung, S.; Prinz, M. Microglia Plasticity during Health and Disease: An Immunological Perspective. Trends Immunol. 2015, 36, 614–624. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Liu, B.; Hong, J.-S. Role of Microglia in Inflammation-Mediated Neurodegenerative Diseases: Mechanisms and Strategies for Therapeutic Intervention. J. Pharm. Exp. 2003, 304, 1–7. [Google Scholar] [CrossRef]
- Refolo, L.M.; Malester, B.; LaFrancois, J.; Bryant-Thomas, T.; Wang, R.; Tint, G.S.; Sambamurti, K.; Duff, K.; Pappolla, M.A. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000, 7, 321–331. [Google Scholar] [CrossRef]
- Thirumangalakudi, L.; Prakasam, A.; Zhang, R.; Bimonte-Nelson, H.; Sambamurti, K.; Kindy, M.S.; Bhat, N.R. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008, 106, 475–485. [Google Scholar] [CrossRef]
- Davidson, T.L.; Monnot, A.; Neal, A.U.; Martin, A.A.; Horton, J.J.; Zheng, W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol. Behav. 2012, 107, 26–33. [Google Scholar] [CrossRef]
- Maesako, M.; Uemura, K.; Iwata, A.; Kubota, M.; Watanabe, K.; Uemura, M.; Noda, Y.; Asada-Utsugi, M.; Kihara, T.; Takahashi, R.; et al. Continuation of exercise is necessary to inhibit high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. PLoS ONE 2013, 8, e72796. [Google Scholar] [CrossRef]
- Eiselein, L.; Wilson, D.W.; Lamé, M.W.; Rutledge, J. C Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2745–H2753. [Google Scholar] [CrossRef]
- Doherty, G.H. Nitric oxide in neurodegeneration: Potential benefits of non-steroidal anti-inflammatories. Neurosci. Bull. 2011, 27, 366–382. [Google Scholar] [CrossRef]
- Chatterjee, S.; Noack, H.; Possel, H.; Keilhoff, G.; Wolf, G. Glutathione levels in primary glial cultures: Monochlorobimane provides evidence of cell type-specific distribution. Glia 1999, 27, 152–161. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Lindenau, J.; Noack, H.; Asayama, K.; Wolf, G. Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia 1998, 24, 252–256. [Google Scholar] [CrossRef]
- Hirrlinger, J.; Gutterer, J.M.; Kussmaul, L.; Hamprecht, B.; Dringen, R. Microglial cells in culture express a prominent glutathione system for the defense against reactive oxygen species. Dev. Neurosci. 2000, 22, 384–392. [Google Scholar] [CrossRef]
- Colton, C.A.; Gilbert, D.L. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987, 223, 284–288. [Google Scholar] [CrossRef]
- Minghetti, L.; Levi, G. Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog. Neurobiol. 1998, 54, 99–125. [Google Scholar] [CrossRef]
- Persson, M.; Sandberg, M.; Hansson, E.; Rönnbäck, L. Microglial glutamate uptake is coupled to glutathione synthesis and glutamate release. Eur. J. Neurosci. 2006, 24, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, F.; Vallat-Decouvelaere, A.V.; Bossuet, C.; Rimaniol, A.C.; Le Grand, R.; Le Pavec, G.; Créminon, C.; Dormont, D.; Gray, F.; Gras, G. Expression of excitatory amino acid transporter-2 (EAAT-2) and glutamine synthetase (GS) in brain macrophages and microglia of SIVmac251-infected macaques. Neuropathol. Appl. Neurobiol. 2002, 28, 410–417. [Google Scholar] [CrossRef]
- Persson, M.; Rönnbäck, L. Microglial self-defence mediated through GLT-1 and glutathione. Amin. Acids 2012, 42, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Graham, V.S.; Lawson, C.; Wheeler-Jones, C.P.D.; Perona, J.S.; Ruiz-Gutierrez, V.; Botham, K.M. Triacylglycerol-rich lipoproteins derived from healthy donors fed different olive oils modulate cytokine secretion and cyclooxygenase-2 expression in macrophages: The potential role of oleanolic acid. Eur. J. Nutr. 2012, 51, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Matumba, M.G.; Ayeleso, A.O.; Nyakudya, T.; Erlwanger, K.; Chegou, N.N.; Mukwevho, E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients 2019, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-F.; Cheng, Y.-D.; Shen, C.-R.; Cherng, J.-Y. A molecular pharmacology study into the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J. Nat. Med. 2010, 64, 330–335. [Google Scholar] [CrossRef]
- Simão da Silva, K.A.B.; Paszcuk, A.F.; Passos, G.F.; Silva, E.S.; Bento, A.F.; Meotti, F.C.; Calixto, J.B. Activation of cannabinoid receptors by the pentacyclic triterpene α,β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain 2011, 152, 1872–1887. [Google Scholar] [CrossRef]
- Cho, N.; Moon, E.H.; Kim, H.W.; Hong, J.; Beutler, J.A.; Sung, S.H. Inhibition of Nitric Oxide Production in BV2 Microglial Cells by Triterpenes from Tetrapanax papyriferus. Molecules 2016, 21, 459. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Perl, K.; Ushakov, K.; Pozniak, Y.; Yizhar-Barnea, O.; Bhonker, Y.; Shivatzki, S.; Geiger, T.; Avraham, K.B.; Shamir, R. Reduced changes in protein compared to mRNA levels across non-proliferating tissues. BMC Genom. 2017, 18, 305. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pereira-Caro, G.; Goya, L.; Bravo, L.B. Biodisponibilidad y metabolismo de los compuestos fenólicos del aceite de oliva virgen. Aliment. Nutr. Salud. 2011, 18, 2–9. [Google Scholar]
- Bentley, C.; Hathaway, N.; Widdows, J.; Bejta, F.; De Pascale, C.; Avella, M.; Wheeler-Jones, C.P.D.; Botham, K.M.; Lawson, C. Influence of chylomicron remnants on human monocyte activation in vitro. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Bravo, E. Lipid metabolism and TNF-α secretion in response to dietary sterols in human monocyte derived macrophages. Eur. J. Clin. Invest. 2005, 35, 482–490. [Google Scholar] [CrossRef]
- De Pascale, C.; Graham, V.; Fowkes, R.C.; Wheeler-Jones, C.P.D.; Botham, K.M. Suppression of nuclear factor-κB activity in macrophages by chylomicron remnants: Modulation by the fatty acid composition of the particles. FEBS J. 2009, 276, 5689–5702. [Google Scholar] [CrossRef]
- Cabello-Moruno, R.; Sinausia, L.; Botham, K.M.; Montero, E.; Avella, M.; Perona, J.S. Postprandial phase time influences the uptake of TAG from postprandial TAG-rich lipoproteins by THP-1 macrophages. Br. J. Nutr. 2014, 112, 1469–1477. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhang, X.; Yan, T.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Schisandra chinensis acts via LRP-1 to reverse microglia activation through suppression of the NF-κB and MAPK signaling. J. Ethnopharmacol. 2020, 25, 112798. [Google Scholar] [CrossRef]
- Wen, X.; Xiao, L.; Zhong, Z.; Wang, L.; Li, Z.; Pan, X.; Liu, Z. Astaxanthin acts via LRP-1 to inhibit inflammation and reverse lipopolysaccharide-induced M1/M2 polarization of microglial cells. Oncotarget 2017, 8, 69370–69385. [Google Scholar] [CrossRef]
- Shi, Y.; Andhey, P.S.; Ising, C.; Wang, K.; Snipes, L.L.; Boyer, K.; Lawson, S.; Yamada, K.; Qin, W.; Manis, M.; et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron 2021, 109, 2413–2426.e7. [Google Scholar] [CrossRef]
- Katsouri, L.; Georgopoulos, S. Lack of LDL receptor enhances amyloid deposition and decreases glial response in an Alzheimer’s disease mouse model. PLoS ONE 2011, 6, e21880. [Google Scholar] [CrossRef] [PubMed]
- Rutkowsky, J.M.; Lee, L.L.; Puchowicz, M.; Golub, M.S.; Befroy, D.E.; Wilson, D.W.; Anderson, S.; Cline, G.; Bini, J.; Borkowski, K.; et al. Reduced cognitive function, increased blood-brain-barrier transport and inflammatory responses, and altered brain metabolites in LDLr -/- and C57B,L/6 mice fed a western diet. PLoS ONE 2018, 13, e0191909. [Google Scholar] [CrossRef]
- Hughes, M.M.; Field, R.H.; Perry, V.H.; Murray, C.L.; Cunningham, C. Microglia in the degenerating brain are capable of phagocytosis of beads and of apoptotic cells, but do not efficiently remove PrPSc, even upon LPS stimulation. Glia 2010, 58, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Kouadir, M.; Yang, L.; Tu, J.; Yin, X.; Zhou, X.; Zhao, D. Comparison of mRNA expression patterns of class B scavenger receptors in BV2 microglia upon exposure to amyloidogenic fragments of β-amyloid and prion proteins. DNA Cell Biol. 2011, 30, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Jiménez-Altayó, F.; Zagmutt, S.; Rodriguez-Rodriguez, R. Molecular Mechanisms Underlying the Effects of Olive Oil Triterpenic Acids in Obesity and Related Diseases. Nutrients 2022, 14, 1606. [Google Scholar] [CrossRef]
- Santos-Lozano, J.M.; Rada, M.; Lapetra, J.; Guinda, Á.; Jiménez-Rodríguez, M.C.; Cayuela, J.A.; Ángel-Lugo, A.; Vilches-Arenas, Á.; Gómez-Martín, A.M.; Ortega-Calvo, M.; et al. Prevention of type 2 diabetes in prediabetic patients by using functional olive oil enriched in oleanolic acid: The PREDIABOLE study, a randomized controlled trial. Diabetes Obes. Metab. 2019, 21, 2526–2534. [Google Scholar] [CrossRef]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol. 2021, 12, 671734. [Google Scholar] [CrossRef]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 115. [Google Scholar] [CrossRef]
- Lee, L.L.; Aung, H.H.; Wilson, D.W.; Anderson, S.E.; Rutledge, J.C.; Rutkowsky, J.M. Triglyceride-rich lipoprotein lipolysis products increase blood-brain barrier transfer coefficient and induce astrocyte lipid droplets and cell stress. Am. J. Physiol. Cell Physiol. 2017, 312, C500–C516. [Google Scholar] [CrossRef] [PubMed]
- Albi, T.; Guinda Garín, M.Á.; Lanzón, A. Procedimiento de obtención y determinación de ácidos terpénicos de la hoja de olivo (Olea europaea). Grasas Aceites 2001, 52, 275–278. [Google Scholar]
- Rada, M.; Castellano, J.M.; Perona, J.S.; Guinda, A. GC-FID determination and pharmacokinetic studies of oleanolic acid in human serum. Biomed. Chromatogr. 2015, 29, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Fanti, P.; Rossi, R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013, 8, 1660–1669. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa, J.M.; Castellano, J.M.; Garcia-Rodriguez, S.; Quintero-Flórez, A.; Carrasquilla, N.; Perona, J.S. Lipophilic Bioactive Compounds Transported in Triglyceride-Rich Lipoproteins Modulate Microglial Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 7706. https://doi.org/10.3390/ijms23147706
Espinosa JM, Castellano JM, Garcia-Rodriguez S, Quintero-Flórez A, Carrasquilla N, Perona JS. Lipophilic Bioactive Compounds Transported in Triglyceride-Rich Lipoproteins Modulate Microglial Inflammatory Response. International Journal of Molecular Sciences. 2022; 23(14):7706. https://doi.org/10.3390/ijms23147706
Chicago/Turabian StyleEspinosa, Juan M., Jose M. Castellano, Silvia Garcia-Rodriguez, Angélica Quintero-Flórez, Natalia Carrasquilla, and Javier S. Perona. 2022. "Lipophilic Bioactive Compounds Transported in Triglyceride-Rich Lipoproteins Modulate Microglial Inflammatory Response" International Journal of Molecular Sciences 23, no. 14: 7706. https://doi.org/10.3390/ijms23147706
APA StyleEspinosa, J. M., Castellano, J. M., Garcia-Rodriguez, S., Quintero-Flórez, A., Carrasquilla, N., & Perona, J. S. (2022). Lipophilic Bioactive Compounds Transported in Triglyceride-Rich Lipoproteins Modulate Microglial Inflammatory Response. International Journal of Molecular Sciences, 23(14), 7706. https://doi.org/10.3390/ijms23147706






