Nutrient Supply Is Essential for Shifting Tree Peony Reflowering Ahead in Autumn and Sugar Signaling Is Involved
Abstract
:1. Introduction
2. Results
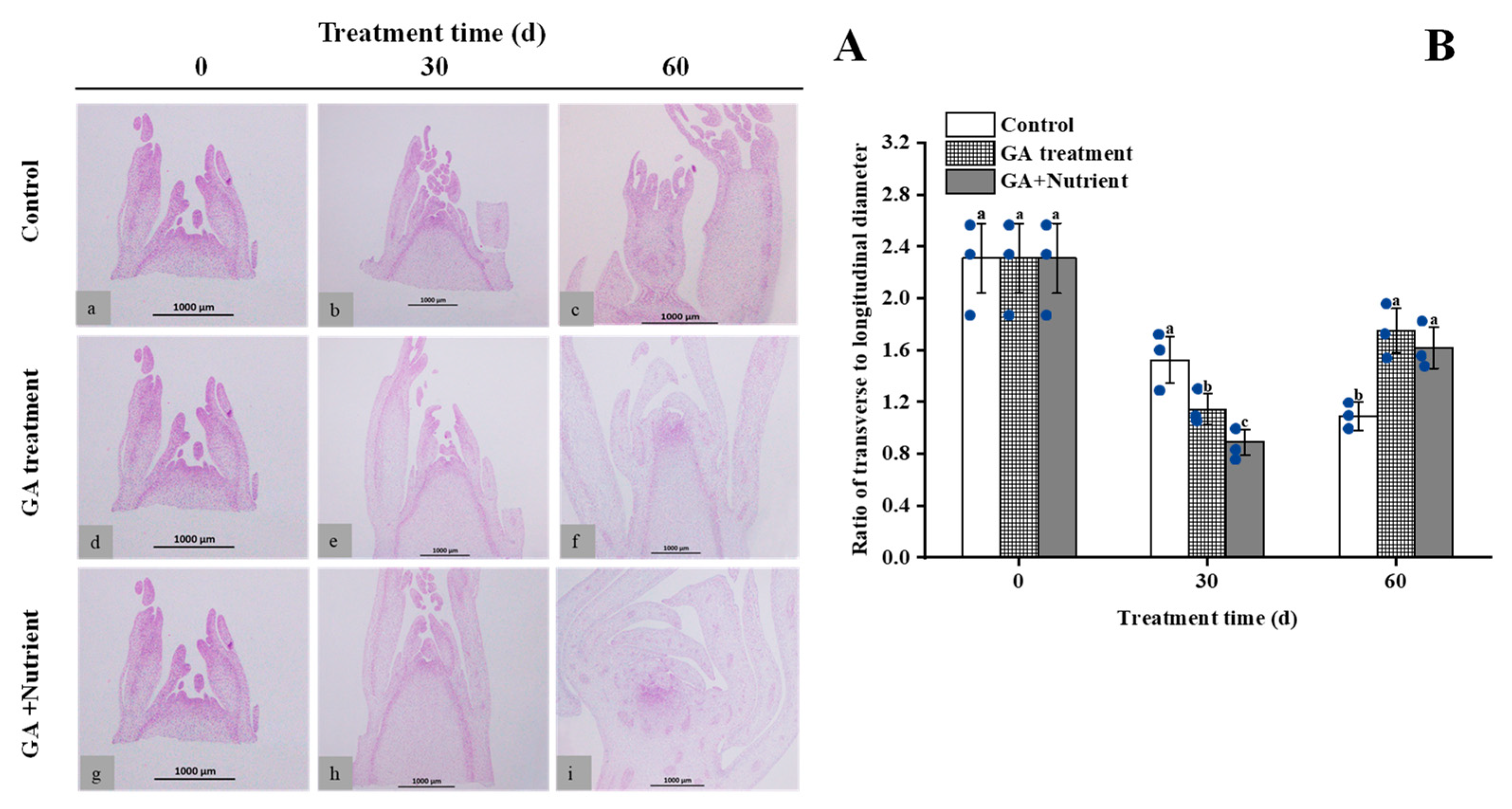
2.1. Effects of Different Treatments on the Morphological Changes in Tree Peony Flower Buds during Growth and Development
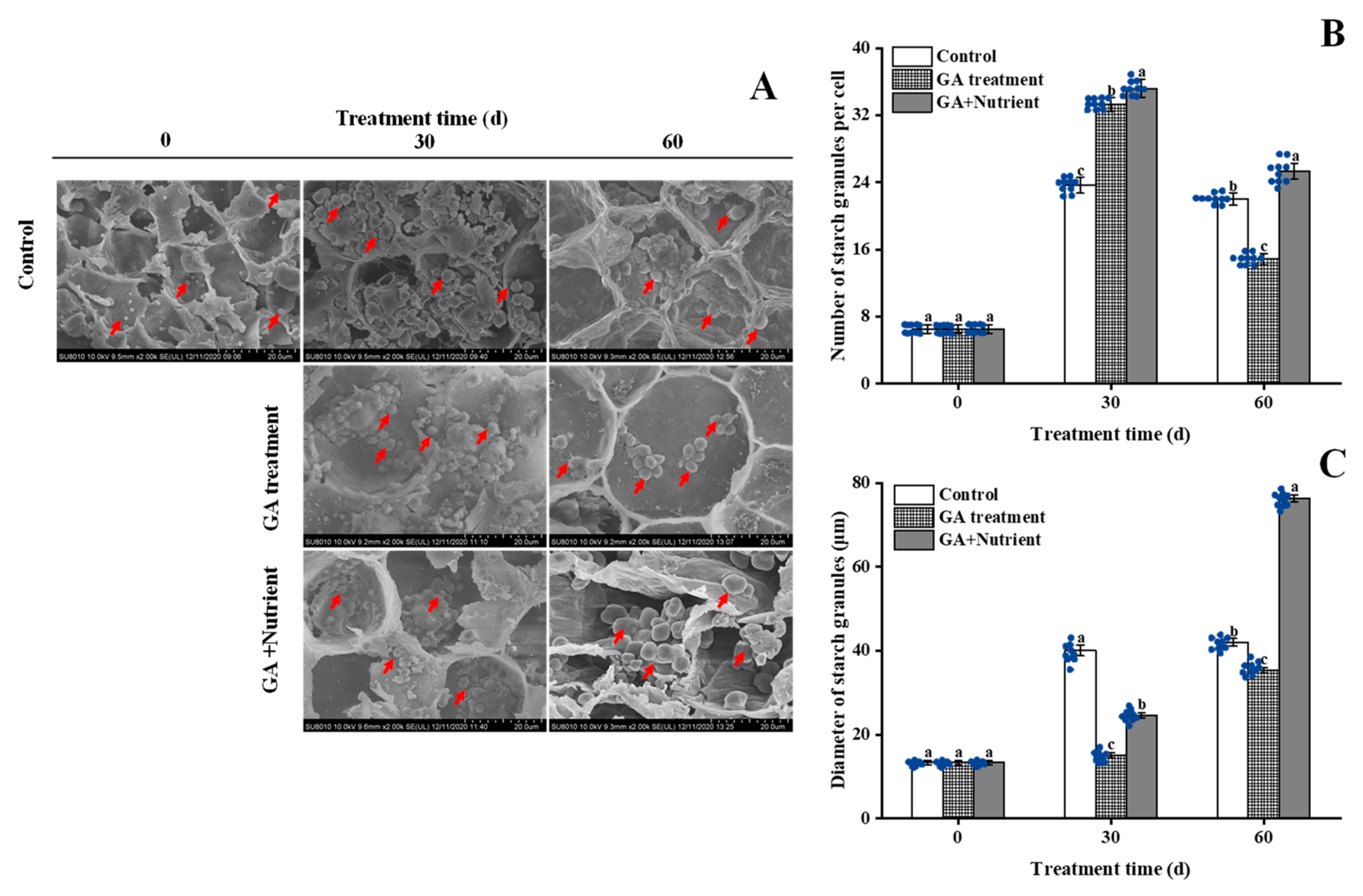
2.2. Effects of Different Treatments on the Starch Metabolism in Tree Peony Buds
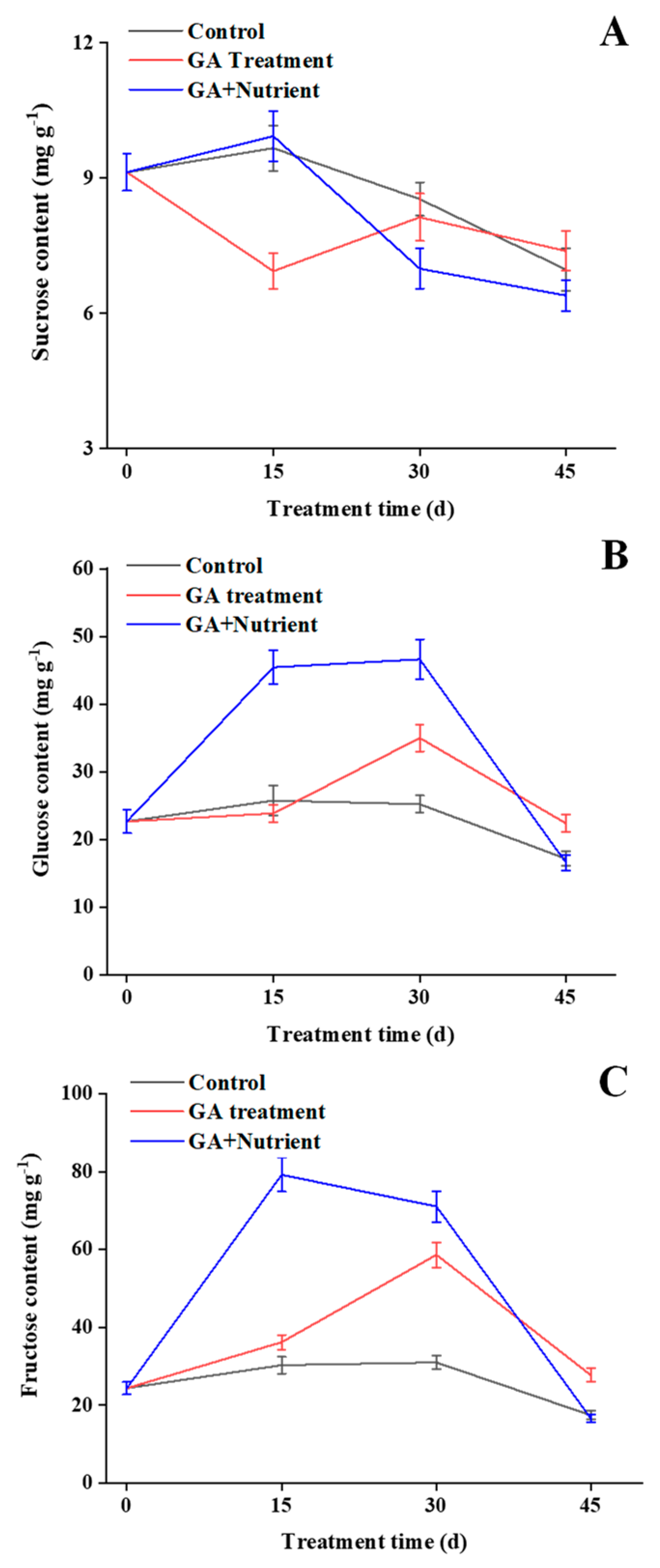
2.3. Effects of Different Treatments on Soluble Sugar Content in Tree Peony Buds
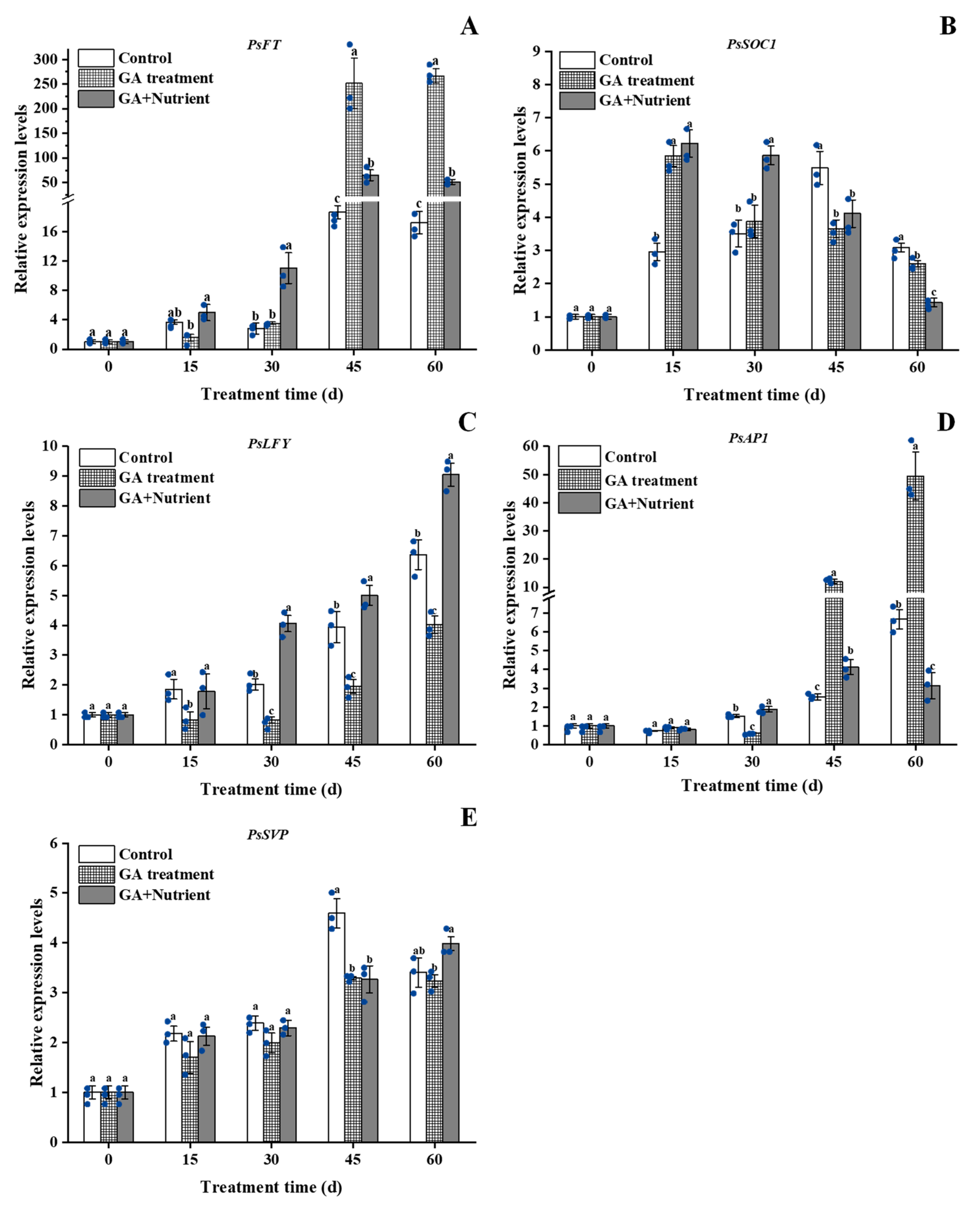
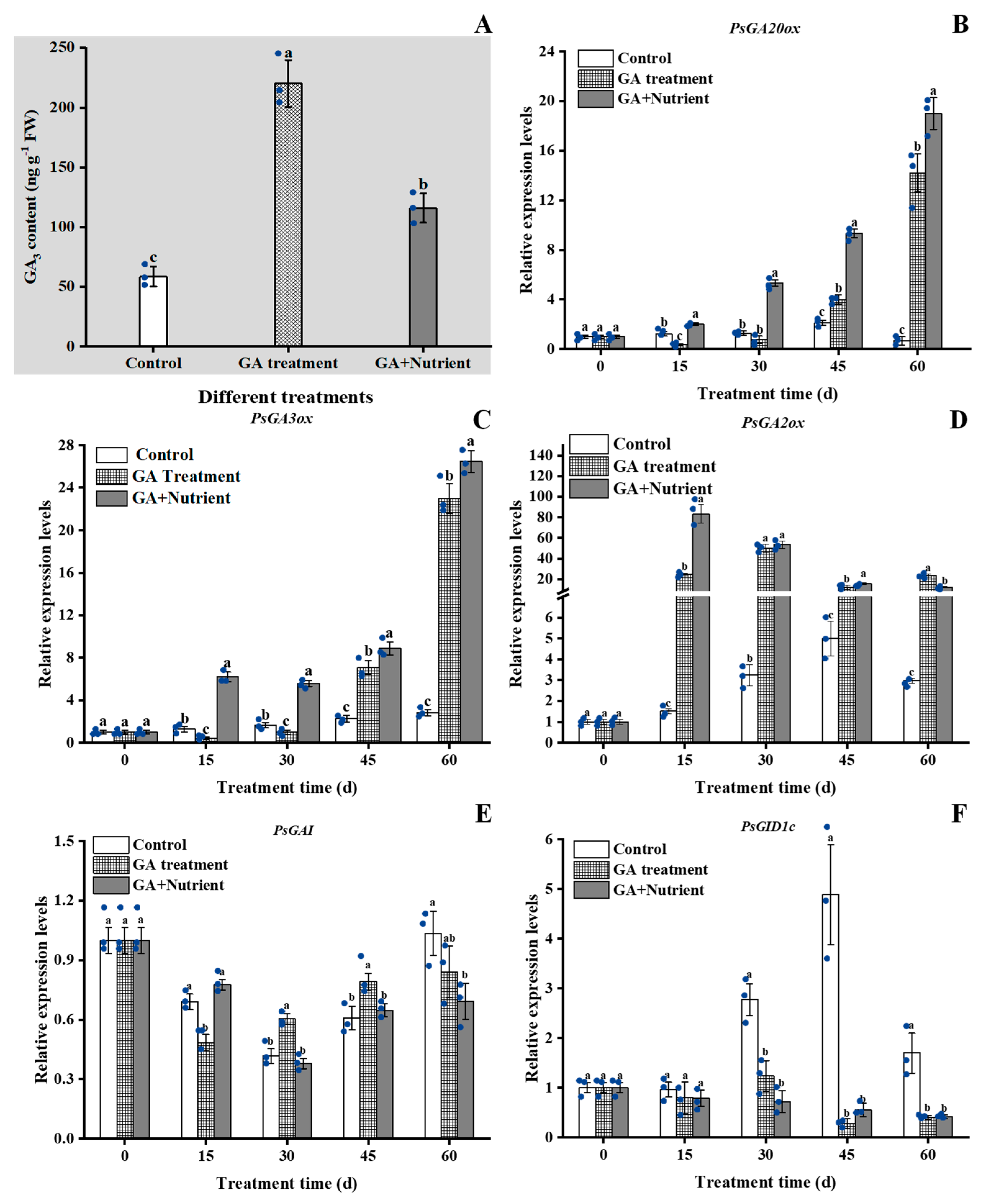
2.4. Effects of Different Treatments on the Expression of Related Genes in Tree Peony Flower Buds during Growth and Development
2.4.1. Floral Pathway-Related Genes
2.4.2. GA Signal Pathway-Related Genes
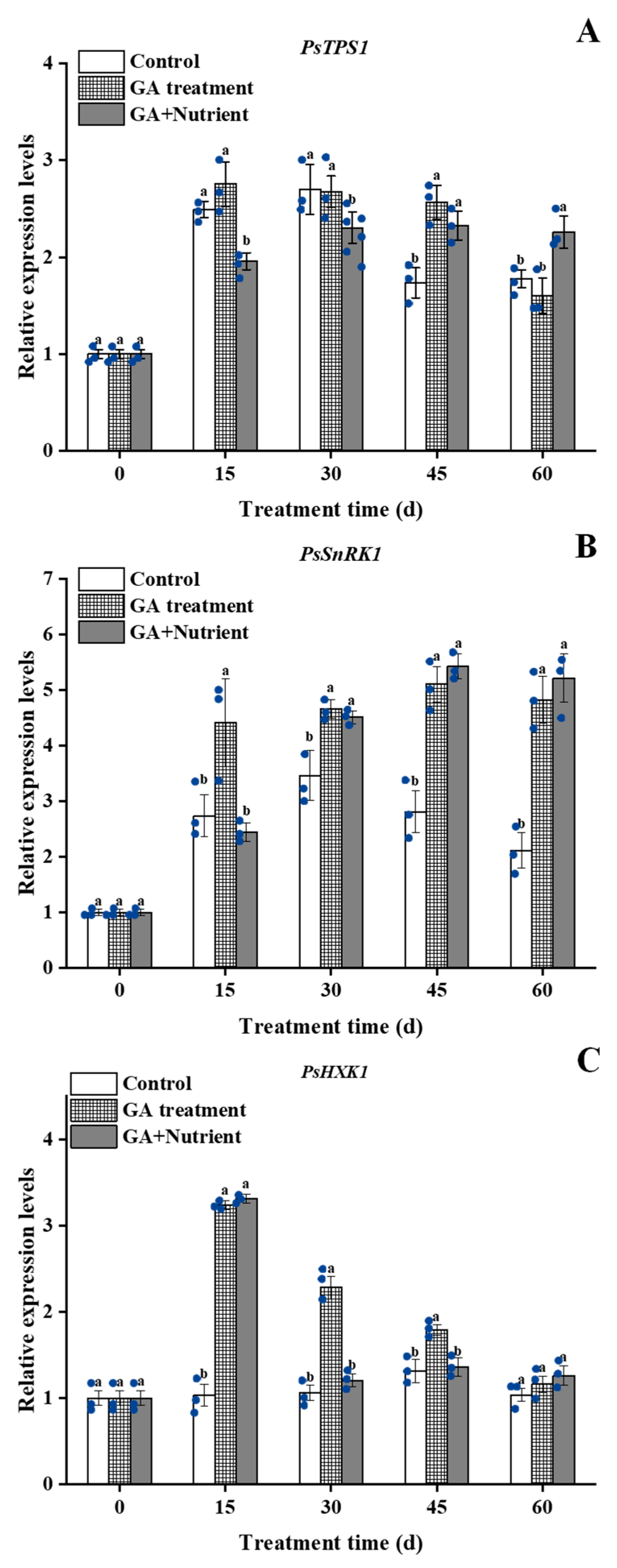
2.4.3. Sugar Signal Pathway-Related Genes
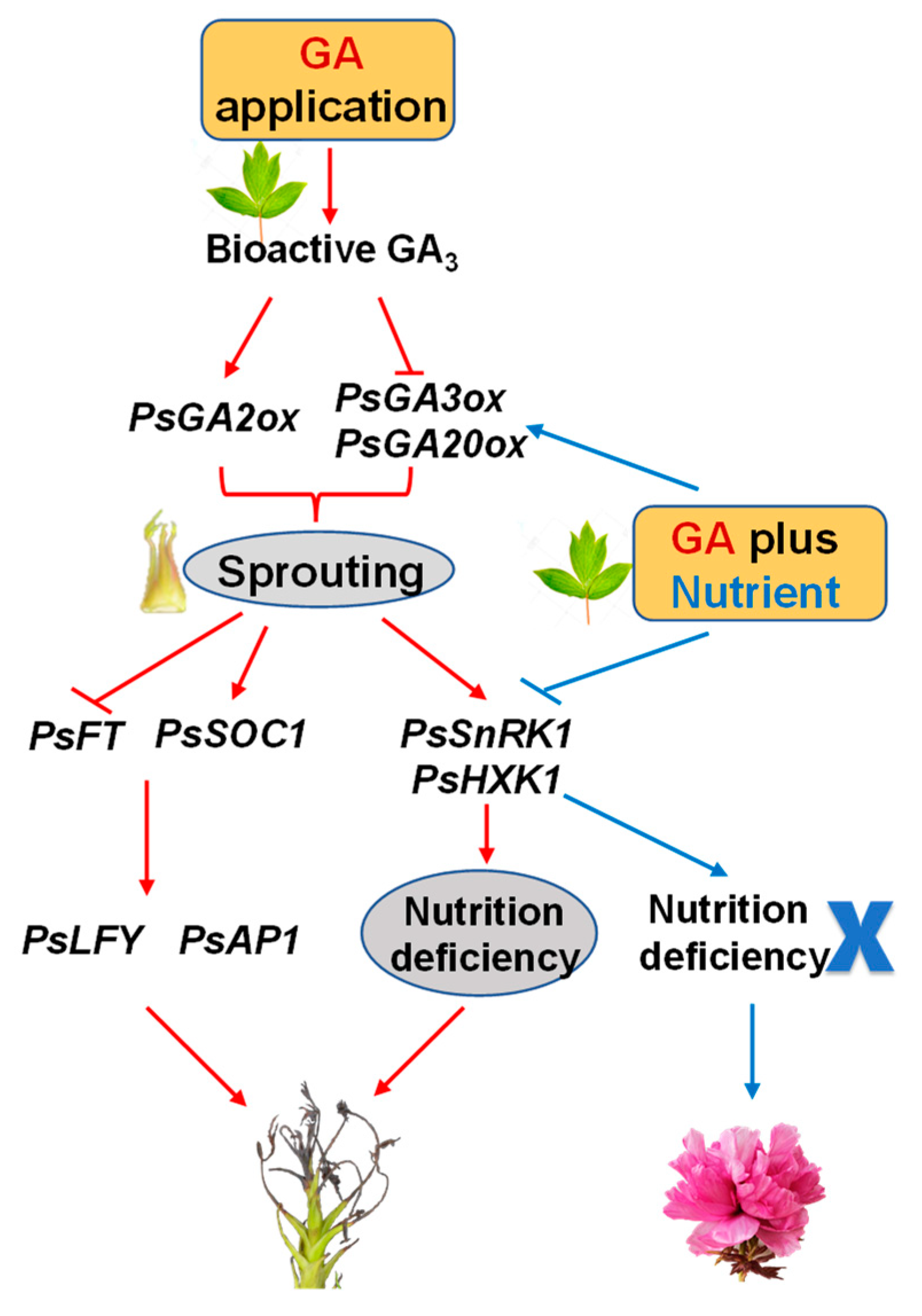
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Paraffin Section and Scanning Electron Microscope Observations
4.3. Soluble Sugar and GA3 Content Measurement
4.4. Total RNA Extraction and cDNA Synthesis
4.5. RT-qPCR
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.X. Development status and suggestions of peony industry in Heze city. Agric. Eng. 2021, 11, 141–144. [Google Scholar]
- Wang, X.P.; Wang, S.L.; Xue, Y.Q.; Ren, X.X.; Xue, J.Q.; Zhang, X.X. Defoliation, not gibberellin, induces tree peony autumn reflowering regulated by carbon allocation and metabolism in buds and leaves. Plant Physiol. Biochem. 2020, 151, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Shi, Y.T.; Xue, Y.Q.; Wang, X.P.; Huang, Z.; Xue, J.Q.; Zhang, X.X. Non-structural carbohydrates coordinate tree peony flowering both as energy substrates and as sugar signaling triggers, with the bracts playing an essential role. Plant Physiol. Biochem. 2021, 159, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Q.; Li, T.T.; Wang, S.L.; Xue, Y.Q.; Hu, F.R.; Zhang, X.X. Elucidation of the mechanism of reflowering in tree peony (Paeonia suffruticosa) ‘Qiu Fa No.1’ by defoliation and gibberellic acid application. Plant Physiol. Biochem. 2018, 132, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Komeda, Y. Genetic regulation of time to flower in Arabidopsis thaliana. Annu. Rev. Plant Biol. 2004, 55, 521–535. [Google Scholar] [CrossRef] [Green Version]
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550–550.e2. [Google Scholar] [CrossRef] [Green Version]
- Wahl, V.; Ponnu, J.; Schlereth, A.; Arrivault, S.; Langenecker, T.; Franke, A.; Feil, R.; Lunn, J.E.; Stitt, M.; Schmid, M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 2013, 339, 704–707. [Google Scholar] [CrossRef]
- Wang, J.W. Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 2014, 65, 4723–4730. [Google Scholar] [CrossRef] [Green Version]
- Fukazawa, J.; Ohashi, Y.; Takahashi, R.; Nakai, K.; Takahashi, Y. DELLA degradation by gibberellin promotes flowering via GAF1-TPR-dependent repression of floral repressors in Arabidopsis. Plant Cell 2021, 33, 2258–2272. [Google Scholar] [CrossRef]
- Xu, G.K.; Tao, Z.; He, Y.H. Embryonic reactivation of FLOWRING LOCUS C by ABACISIC ACID-INSENSITIVE 3 establishes the vernalization rrequirement in each Arabidopsis generation. Plant Cell 2022, 34, 2205–2221. [Google Scholar] [CrossRef]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.L.; Gao, J.; Xue, J.Q.; Xue, Y.Q.; Li, D.D.; Guan, Y.Y.; Zhang, X.X. De novo sequencing of tree peony (Paeonia suffruticosa) transcriptome to identify critical genes involved in flowering and floral organ development. BMC Genom. 2019, 20, 572. [Google Scholar] [CrossRef] [PubMed]
- Mandel, M.A.; Gustafson-Brown, C.; Savidge, B.; Yanofsky, M.F. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 1992, 360, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef]
- Wang, S.L.; Xue, J.Q.; Ahmadi, N.; Holloway, P.; Zhu, F.Y.; Ren, X.X.; Zhang, X.X. Molecular characterization and expression patterns of PsSVP genes reveal distinct roles in flowering bud abortion and flowering in tree peony (Paeonia Suffruticosa). Can. J. Plant Sci. 2014, 94, 1181–1193. [Google Scholar] [CrossRef] [Green Version]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.I.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Murase, K.; Hirano, Y.; Sun, T.P.; Hakoshima, T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 2008, 456, 459–463. [Google Scholar] [CrossRef]
- Santner, A.; Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 2009, 459, 1071–1078. [Google Scholar] [CrossRef]
- Du, L.S.; Qi, S.Y.; Ma, J.J.; Xing, L.B.; Fan, S.; Zhang, S.W.; Li, Y.M.; Shen, Y.W.; Zhang, D.; Han, M.Y. Identification of TPS family members in apple (Malus X Domestica Borkh.) and the effect of sucrose sprays on TPS expression and floral induction. Plant Physiol. Biochem. 2007, 120, 10–23. [Google Scholar] [CrossRef]
- Sanagi, M.; Shoki, A.; Akio, K.; Yu, L.; Yasutake, S.; Shogo, I.; Mitsutomo, A.; Nobutaka, M.; Masaru, O.T.; Takatoshi, K.; et al. Low nitrogen conditions accelerate flowering by modulating the phosphorylation state of FLOWEING BHLH4 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2022942118. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Peng, F.T.; Zhang, L.; Shi, X.Z.; Wang, Z.Y. Cloning and characterization of a SnRK1-encodinig gene form Malus hupehensis Rehd. and heterologous expression in tomato. Mol. Biol. Rep. 2010, 37, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Baena-Gonzalez, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Claeyssen, E.; Rivoal, J. Isozymes of plant hexokinase: Occurrence, properties and functions. Phytochemistry 2007, 68, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Tang, Y.Z.; Jiang, A.H. Effects of PPP333 and GA3 on the mechanism of flower bud induction in apple tree. Acta Hortic. Sin. 2001, 28, 339–341. [Google Scholar]
- An, L.J.; Jin, L.; Yang, C.Q.; Li, T.H. Effect and functional mechanism of the action of exogenous gibberellin on flowering peach. Sci. Agric. Sin. 2009, 42, 1324–1332. [Google Scholar]
- Li, W.Q.; Yong, Y.B.; Zhang Yue Lyu, Y.M. Transcriptional regulatory network of GA floral induction pathway in LA Hybrid Lily. Int. J. Mol. Sci. 2019, 20, 2694. [Google Scholar] [CrossRef] [Green Version]
- Li, M.F.; Yang, Y.; Wang, H.; Liu, J.S.; Jin, W.M. Effects of gibberellin on growth and development of Rose ‘Carola’. Acta Hortic. Sin. 2019, 46, 749–760. [Google Scholar]
- Guan, Y.R.; Xue, J.Q.; Xue, Y.Q.; Yang, R.W.; Wang, S.L.; Zhang, X.X. Effect of exogenous GA3 on flowering quality, endogenous hormones, and hormone- and flowering-associated gene expression in forcing-cultured tree peony (Paeonia suffruticosa). J. Integr. Agric. 2019, 18, 1295–1311. [Google Scholar] [CrossRef]
- Hisamatsu, T.; King, R.W. The nature of floral signals in Arabidopsis. II. Roles for FLOWERING LOCUS T (FT) and gibberellin. J. Exp. Bot. 2008, 59, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, S.; Böhlenius, H.; Moritz, T.; Nilsson, O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 2006, 18, 2172–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, N.; Winter, C.M.; Wu, M.F.; Kanno, Y.; Yamaguchi, A.; Seo, M.; Wagner, D. Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science 2014, 344, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, F.; Kurz, B.; Melzer, S.; Bernier, G.; Jacqmard, A. Cytokinin and gibberellin activate SaMADS A, a gene apparently involved in regulation of the floral transition in Sinapis alba. Plant J. 2000, 24, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, D.; Fan, S.; Du, L.; Shen, Y.; Xing, L.; Li, Y.; Ma, J.; Han, M. Effect of exogenous GA3 and its inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2016, 107, 178–186. [Google Scholar] [CrossRef]
- Dong, F.; Fan, S.; Ma, X.L.; Meng, Y.; Zuo, X.Y.; Liu, X.J.; Li, K.; Liu, Z.; Han, M.Y.; Zhang, D. Genome-wide indentification and expression analysis of GA2ox, GA3ox, and GA20ox in apple. Acta Hortic. Sin. 2018, 45, 613–626. [Google Scholar]
- Gao, Y.; Chen, J.M.; Zhao, Y.; Li, T.T.; Wang, M.L. Molecular cloning and expression analysis of a RGA-like gene responsive to plant hormones in Brassica napus. Mol. Biol. Rep. 2012, 39, 1957–1962. [Google Scholar] [CrossRef]
- Fukazawa, J.; Teramura, H.; Murakoshi, S.; Nasuno, K.; Nishida, N.; Ito, T.; Yoshida, M.; Kamiya, Y.; Yamaguchi, S.; Takahashi, Y. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 2014, 26, 2920–2938. [Google Scholar] [CrossRef] [Green Version]
- Ramon, M.; Dang, T.V.T.; Broeckx, T.; Hulsmans, S.; Crepin, N.; Sheen, J.; Rolland, F. Default activation and nuclear translocation of the plant cellular energy sensor SnRK1 regulate metabolic stress responses and development. Plant Cell 2019, 31, 1614–1632. [Google Scholar] [CrossRef]
- Fichtner, F.; Olas, J.J.; Feil, R.; Watanabe, M.; Krause, U.; Hoefgen, R.; Stitt, M.; Lunn, J.E. Functional features of TREHALOSE-6-PHOSPHATE SYNTHASE1, an essential enzyme in Arabidopsis. Plant Cell 2020, 32, 1949–1972. [Google Scholar] [CrossRef] [Green Version]
- Ponnu, J.; Schlereth, A.; Zacharaki, V.; Działo, M.A.; Abel, C.; Feil, R.; Schmid, M.; Wahl, V. The trehalose 6-phosphate pathway impacts vegetative phase change in Arabidopsis thaliana. Plant J. 2020, 104, 768–780. [Google Scholar] [CrossRef]
- Baena-Gonzalez, E.; Lunn, J.E. SnRK1 and trehalose 6-phosphate—Two ancient pathways converge to regulate plant metabolism and growth. Curr. Opin. Plant Biol. 2020, 55, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Yu, W.; Luo, J.J.; Wang, W.R.; Zhang, S.H.; Xiao, S.Y.; Peng, F.T. Peach PpSnRK1α gene regulates the growth and development processes of plants. Plant Physiol. J. 2018, 54, 1553–1560. [Google Scholar]
- Granot, D. Role of tomato hexose kinases. Funct. Plant Biol. 2007, 34, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Cheng, S.; Wang, Z.; Li, S.; Jin, X.; Lan, L.; Yang, B.; Yu, K.; Ni, X.; Li, N.; et al. Draft genome of the famous ornamental plant Paeonia suffruticosa. Ecol. Evol. 2020, 10, 4518–4530. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Xue, Y.; Prijic, Z.; Wang, S.; Xue, J.; Zhang, X. DNA Demethylation Induces Tree Peony Flowering with a Low Deformity Rate Compared to Gibberellin by Inducing PsFT Expression under Forcing Culture Conditions. Int. J. Mol. Sci. 2022, 23, 6632. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Gai, S.P.; Liu, C.Y.; Mu, P.; Zheng, G.S. Selection of control genes in real-time qPCR analyss during bud dormancy release in tree peony (Paeonia suffruticosa). Mol. Plant Breed. 2011, 9, 1052–1056. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Xue, J.; Ren, X.; Li, C.; Sun, K.; Cui, L.; Lyu, Y.; Zhang, X. Nutrient Supply Is Essential for Shifting Tree Peony Reflowering Ahead in Autumn and Sugar Signaling Is Involved. Int. J. Mol. Sci. 2022, 23, 7703. https://doi.org/10.3390/ijms23147703
Xue Y, Xue J, Ren X, Li C, Sun K, Cui L, Lyu Y, Zhang X. Nutrient Supply Is Essential for Shifting Tree Peony Reflowering Ahead in Autumn and Sugar Signaling Is Involved. International Journal of Molecular Sciences. 2022; 23(14):7703. https://doi.org/10.3390/ijms23147703
Chicago/Turabian StyleXue, Yuqian, Jingqi Xue, Xiuxia Ren, Changyue Li, Kairong Sun, Litao Cui, Yingmin Lyu, and Xiuxin Zhang. 2022. "Nutrient Supply Is Essential for Shifting Tree Peony Reflowering Ahead in Autumn and Sugar Signaling Is Involved" International Journal of Molecular Sciences 23, no. 14: 7703. https://doi.org/10.3390/ijms23147703
APA StyleXue, Y., Xue, J., Ren, X., Li, C., Sun, K., Cui, L., Lyu, Y., & Zhang, X. (2022). Nutrient Supply Is Essential for Shifting Tree Peony Reflowering Ahead in Autumn and Sugar Signaling Is Involved. International Journal of Molecular Sciences, 23(14), 7703. https://doi.org/10.3390/ijms23147703







