Effects of Combined Allogenic Adipose Stem Cells and Hyperbaric Oxygenation Treatment on Pathogenesis of Osteoarthritis in Knee Joint Induced by Monoiodoacetate
Abstract
:1. Introduction
2. Results
2.1. Severity of Arthritis–Measured by Diameter of Knee
2.2. Radiography of OA
2.3. Effect of ADMSCs and HBO on the Repair of Cartilage Defects
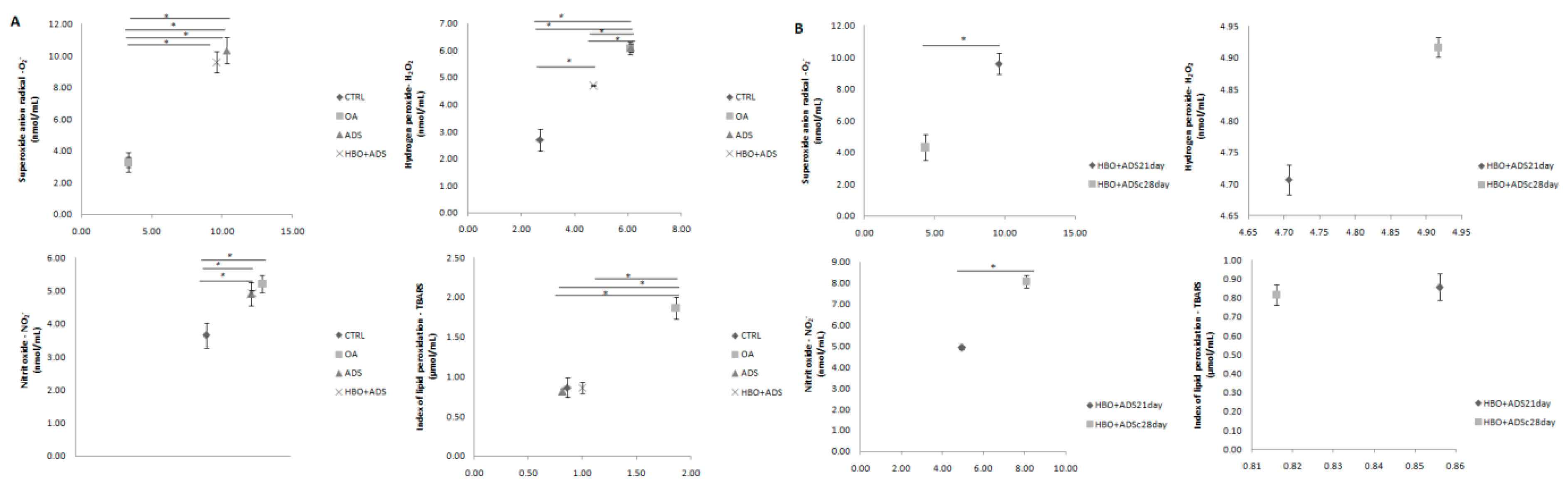
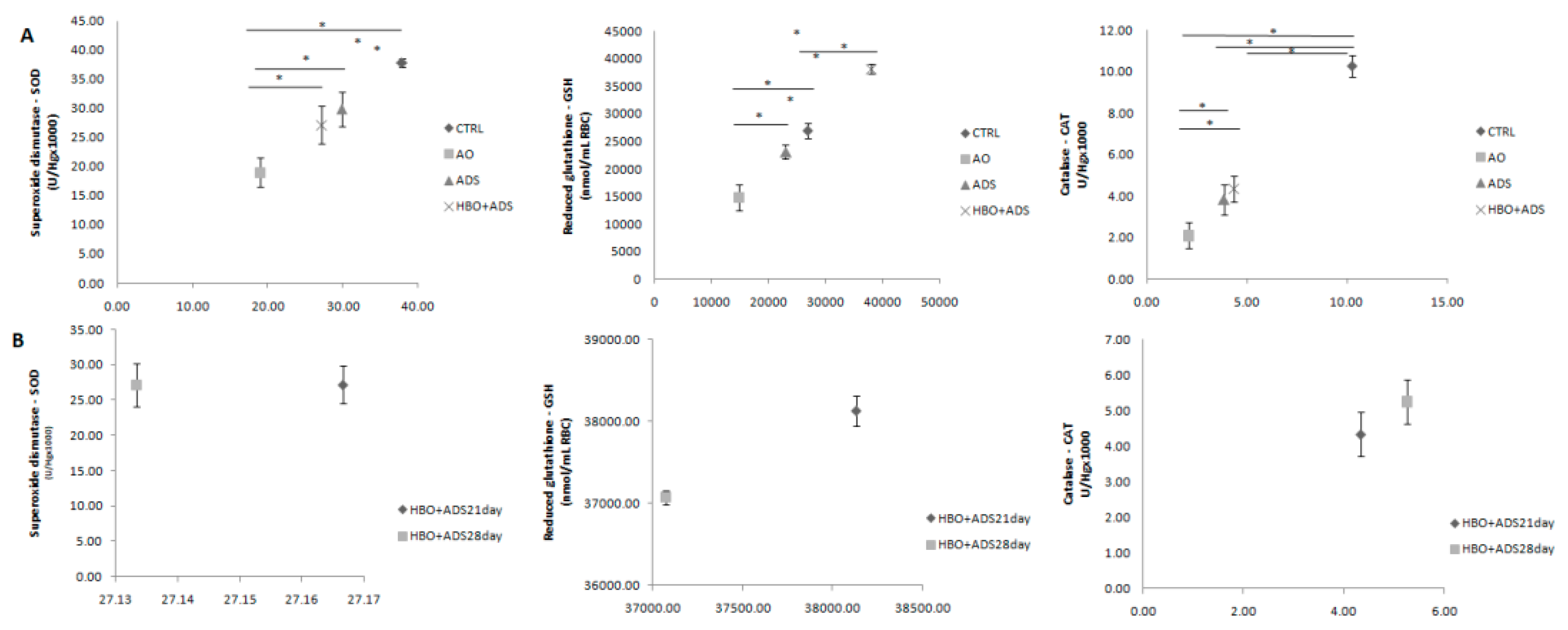
2.4. Parameters of Oxidative Stress
2.4.1. Prooxidative Markers
2.4.2. Antioxidative Enzymes
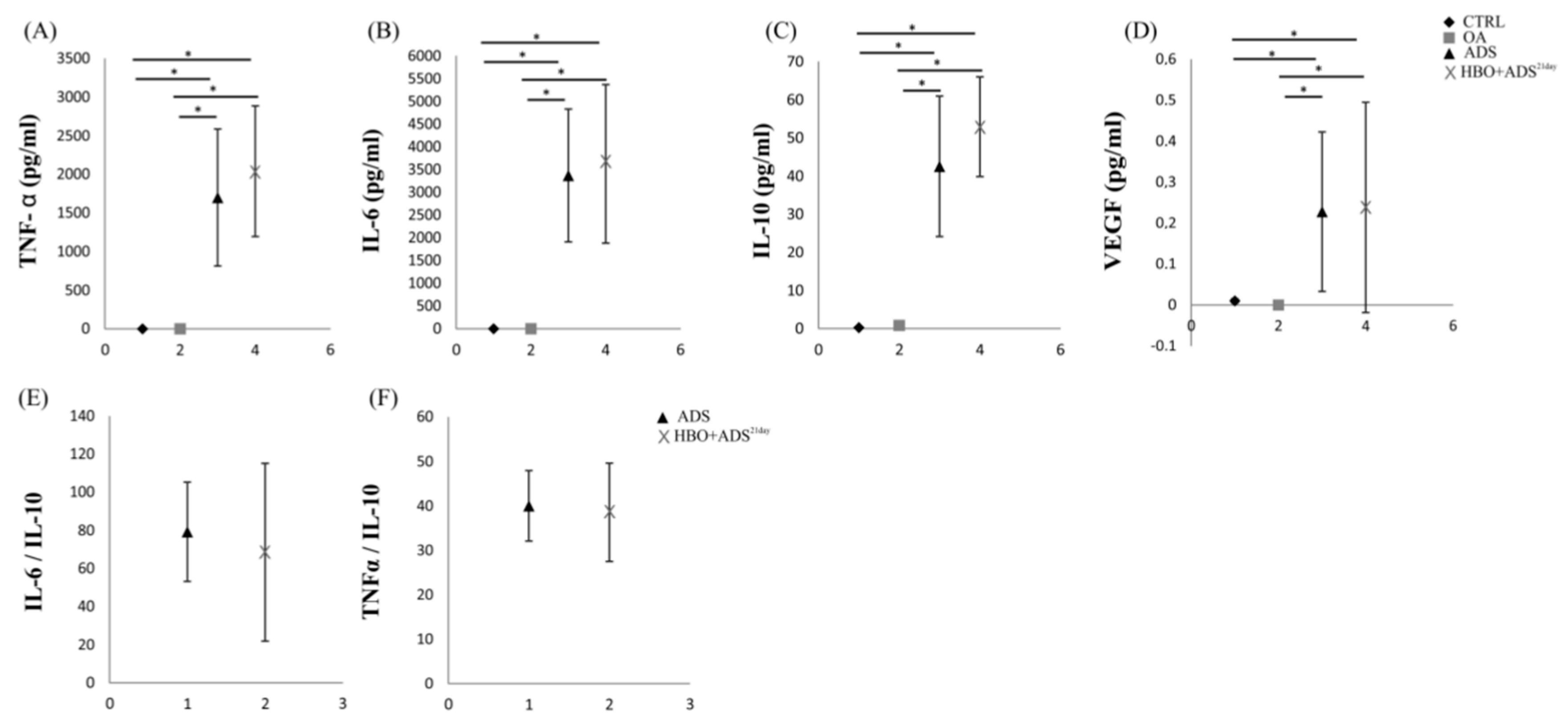
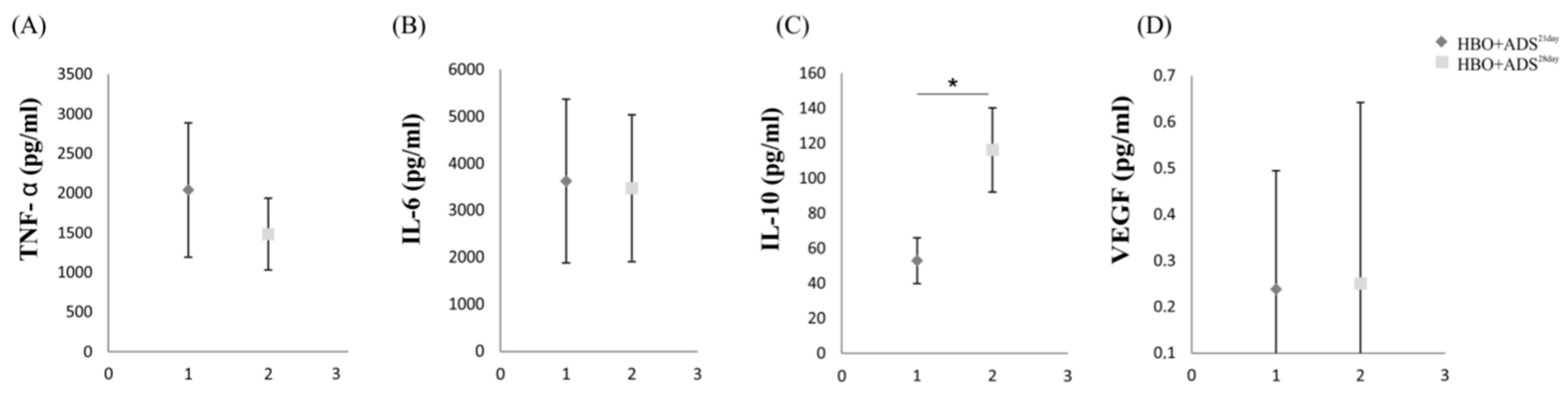
2.5. ELISA of Cytokines
3. Discussion
4. Materials and Methods
4.1. Induction of the Osteoarthritis Model
4.2. Cell Culture
4.3. Experimental Animals and Animal Care
- Healthy control rats (CTRL)
- Rats with induced OA without therapeutic intervention, untreated (OA)
- Rats with induced OA subjected to ADMSCs treatment (ADS)
- Rats with induced OA subjected to single ADMSCs injection and HBO treatment for 14 days (HBO+ADS21day)
4.4. ADMSCs Treatment Protocol
4.5. HBO Treatment
4.6. Radiographic Analysis of OA
4.7. Knee Diameter
4.8. Histological Preparation
4.9. Markers of Oxidative Stress and Inflammation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clouet, J.; Vinatier, C.; Merceron, C.; Pot-vaucel, M.; Maugars, Y.; Weiss, P.; Grimandi, G.; Guicheux, J. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discov. 2009, 14, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Jeffrey, B.D.; Charles, B.E.; Timothy, E.M.; Leslie, R.H.; Kate, L.L. Objectively measured physical activity and symptoms change in knee osteoarthritis. Am. J. Med. 2016, 129, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peat, G.; McCarney, R.; Croft, P. Knee pain and osteoarthritis in older adults: A review of community burden and current use of primary health care. Ann. Rheum. Dis. 2001, 60, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D. Practical considerations for the pharmacologic management of osteoarthritis. Am. J. Manag. Care. 2009, 15, 236–243. [Google Scholar]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Mehrabani, D.; Mehrabani, G.; Zare, S.; Manafi, A. Adipose-derived stem cells (ADSC) and aesthetic surgery: A mini review. World J. Plast Surg. 2013, 2, 65–70. [Google Scholar]
- Desando, G.; Cavallo, C.; Sartoni, F.; Martini, L.; Parrilli, A.; Veronesi, F.; Fini, M.; Giardino, R.; Facchini, A.; Grigolo, B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res. Ther. 2013, 15, R22. [Google Scholar] [CrossRef] [Green Version]
- Schelbergen, R.F.; van Dalen, S.; terHuurne, M.; Roth, J.; Vogl, T.; Noel, D.; Jorgensen, C.; van den Berg, W.B.; van de Loo, F.A.; Blom, A.B.; et al. Treatment efficacy of adipose—derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthr. Cartil. 2014, 22, 1158–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toghraie, F.; Razmkhah, M.; Gholipour, M.A.; Faghih, Z.; Chenari, N.; Nezhad, S.; Nazhvani, D.S.; Ghaderi, A. Scaffold-free adiposederived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch. Iran. Med. 2012, 15, 495–499. [Google Scholar] [PubMed]
- Zhou, J.; Wang, Y.; Liu, Y.; Zeng, H.; Xu, H.; Lian, F. Adipose derived mesenchymal stem cells alleviated osteoarthritis and chondrocyte apoptosis through autophagy inducing. J. Cell Biochem. 2018, 120, 2198–2212. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Jung, Y.; Kim, S.H. Insight on stem cell preconditioning and instructive biomaterials to enhance cell adhesion, retention and engraftmentfor tissue repair. Biomaterials 2016, 90, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondía, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef] [Green Version]
- Joshi, J.N.; Rodríguez, L.; Reverté-Vinaixa, M.M.; Navarro, A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop. J. Sports Med. 2017, 5, 2325967116689386. [Google Scholar]
- Wang, D.W.; Fermor, B.; Gimble, J.M.; Awad, H.A.; Guilak, F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J. Cell Physiol. 2005, 204, 184–191. [Google Scholar] [CrossRef]
- Wilson, H.D.; Toepfer, V.E.; Senapati, A.K.; Wilson, J.R.; Fuchs, P.N. Hyperbaric oxygen treatment is comparable to acetylsalicylic acid treatment in an animal model of arthritis. J. Pain 2007, 8, 924–930. [Google Scholar] [CrossRef]
- Lindell, K.W. Hyperbaric Oxygen Therapy Indications, 13th ed.; The Hyperbaric Oxygen Therapy Committee Report; Best Publishing Company: North Palm Beach, FL, USA, 2014. [Google Scholar]
- Yuan, L.J.; Ueng, S.W.; Lin, S.S.; Yeh, W.L.; Yang, C.Y.; Lin, P.Y. Attenuation of apoptosis and enhancement of proteoglycan synthesis in rabbit cartilage defects by hyperbaric oxygen treatment are related to the suppression of nitric oxide production. J. Orthop. Res. 2004, 22, 1126–1134. [Google Scholar] [CrossRef]
- Ueng, S.W.N.; Yuan, L.-J.; Lin, S.-S.; Niu, C.-C.; Chan, Y.-S.; Wang, I.-C.; Yang, C.-Y.; Chen, W.-J. Hyperbaric oxygen treatment prevents nitric oxide-induced apoptosis in articular cartilage injury via enhancement of the expression of heat shock protein. J. Orthop. Res. 2013, 3, 376–384. [Google Scholar] [CrossRef]
- Nagatomo, F.; Gu, N.; Fujino, H.; Okiura, T.; Morimatsu, F.; Takeda, I.; Ishihara, A. Effects of exposure to hyperbaric oxygen on oxidative stress in rats with type II collagen-induced arthritis. Clin. Exp. Med. 2010, 1, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yılmaz, O.; Bilge, A.; Erken, H.Y.; Kuru, T. The effects of systemic ozone application and hyperbaric oxygen therapy on knee osteoarthritis: An experimental study in rats. Int. Orthop. 2021, 45, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Bosco, G.; Ferroni, L.; Quartesan, S.; Rizzato, A.; Tatullo, M.; Zavan, B. Hyperbaric Oxygen Therapy Improves the Osteogenic and Vasculogenic Properties of Mesenchymal Stem Cells in the Presence of Inflammation In Vitro. Int. J. Mol. Sci. 2020, 20, 1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.celixir.com/stem-cell-treatment-group-to-combine-treatment-with-hyperbaric-oxygen-therapy-hbot/ (accessed on 2 June 2022).
- Si, Y.L.; Zhao, Y.L.; Hao, H.J.; Fu, X.B.; Han, W.D. MSCs: Biological characteristics, clinical applications and their outstanding concerns. Ageing Res. Rev. 2011, 10, 93–103. [Google Scholar] [CrossRef]
- Mei, L.; Shen, B.; Ling, P.; Liu, S.; Xue, J.; Liu, F.; Shao, H.; Chen, J.; Ma, A.; Liu, X. Culture-expanded allogenic adipose tissuederived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS ONE 2017, 12, e0176107. [Google Scholar] [CrossRef]
- Harnanik, T.; Soeroso, J.; Suryokusumo, M.G.; Juliandhy, T. Effects of Hyperbaric Oxygen on T helper 17/regulatory T Polarization in Antigen and Collagen-induced Arthritis: Hypoxia-inducible Factor-1α as a Target. Oman Med. J. 2020, 35, e90. [Google Scholar] [CrossRef]
- Wu, L.; Cai, X.; Zhang, S.; Karperien, M.; Lin, Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: Perspectives from stem cell biology and molecular medicine. J. Cellular Physiol. 2013, 228, 938–944. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, G.; Yu, X.; Xu, W. A novel approach to estimate ROS origination by hyperbaric oxygen exposure, targeted probes and specific inhibitors. Cell Physiol. Biochem. 2018, 47, 1800–1808. [Google Scholar] [CrossRef]
- Ivana, J.; Mihael, M.; Martina, M.; Zrinka, M.; Sanela, U.; Dijana, K.; Aleksandar, K. Mechanisms of HBO-Induced Vascular Functional Changes in Diabetic Animal Models. Hyperbaric Oxygen Treatment in Research and Clinical Practice; Drenjancevic, I., Ed.; IntechOpen: London, UK, 2018; pp. 87–108. [Google Scholar]
- Sureda, A.; Batle, J.M.; Martorell, M.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A. Antioxidant response of chronic wounds to hyperbaric oxygen therapy. PLoS ONE 2016, 11, e0163371. [Google Scholar] [CrossRef] [Green Version]
- Nyoman, S.; Hendry, I. Blood glucose and lipid profile in patients with diabetic foot ulcer that underwent hyperbaric oxygen therapy. Bali Med. J. 2017, 6, 405–408. [Google Scholar]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS ONE 2018, 13, e0196625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, S.J.P.; Kotikalapudi, N.; Venkatesan, V. A novel therapeutic combination of mesenchymal stem cells and stigmasterol to attenuate osteoarthritis in rodent model system-a proof of concept study. Stem Cell Investig. 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yehuda, S.; Rath-Wolfson, L.; Del Valle, L.; Ochaion, A.; Cohen, S.; Patoka, R.; Zozulya, G.; Barer, F.; Atar, E.; Piña-Oviedo, S.; et al. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 2009, 60, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Shen, B.; Xue, J.; Liu, S.; Ma, A.; Liu, F.; Shao, H.; Chen, J.; Chen, Q.; Liu, F.; et al. Adipose tissue-derived stem cells in combination with xanthan gum attenuate osteoarthritis progression in an experimental rat model. Biochem. Biophys. Res. Commun. 2017, 494, 285–291. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, N.; Laverty, S.; Kraus, V.B.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartilage. 2010, 18 (Suppl. 3), S113–S116. [Google Scholar] [CrossRef] [Green Version]
- Auclair, C.; Voisin, E. Nitrobluetetrazolium reduction. In Handbook of Methods for Oxygen Radical Research; Greenvvald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B. MImproved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Beutler, E. Superoxide dismutase. Red cell metabolism. In A Manual of Biochemical Methods; Beutler, I.N.E., Ed.; Grune& Stratton: Philadelphia, PA, USA, 1984; pp. 83–85. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juskovic, A.; Nikolic, M.; Ljujic, B.; Matic, A.; Zivkovic, V.; Vucicevic, K.; Milosavljevic, Z.; Vojinovic, R.; Jovicic, N.; Zivanovic, S.; et al. Effects of Combined Allogenic Adipose Stem Cells and Hyperbaric Oxygenation Treatment on Pathogenesis of Osteoarthritis in Knee Joint Induced by Monoiodoacetate. Int. J. Mol. Sci. 2022, 23, 7695. https://doi.org/10.3390/ijms23147695
Juskovic A, Nikolic M, Ljujic B, Matic A, Zivkovic V, Vucicevic K, Milosavljevic Z, Vojinovic R, Jovicic N, Zivanovic S, et al. Effects of Combined Allogenic Adipose Stem Cells and Hyperbaric Oxygenation Treatment on Pathogenesis of Osteoarthritis in Knee Joint Induced by Monoiodoacetate. International Journal of Molecular Sciences. 2022; 23(14):7695. https://doi.org/10.3390/ijms23147695
Chicago/Turabian StyleJuskovic, Aleksandar, Marina Nikolic, Biljana Ljujic, Aleksandar Matic, Vladimir Zivkovic, Ksenija Vucicevic, Zoran Milosavljevic, Radisa Vojinovic, Nemanja Jovicic, Suzana Zivanovic, and et al. 2022. "Effects of Combined Allogenic Adipose Stem Cells and Hyperbaric Oxygenation Treatment on Pathogenesis of Osteoarthritis in Knee Joint Induced by Monoiodoacetate" International Journal of Molecular Sciences 23, no. 14: 7695. https://doi.org/10.3390/ijms23147695
APA StyleJuskovic, A., Nikolic, M., Ljujic, B., Matic, A., Zivkovic, V., Vucicevic, K., Milosavljevic, Z., Vojinovic, R., Jovicic, N., Zivanovic, S., Milivojevic, N., Jakovljevic, V., Bolevich, S., & Miletic Kovacevic, M. (2022). Effects of Combined Allogenic Adipose Stem Cells and Hyperbaric Oxygenation Treatment on Pathogenesis of Osteoarthritis in Knee Joint Induced by Monoiodoacetate. International Journal of Molecular Sciences, 23(14), 7695. https://doi.org/10.3390/ijms23147695






