Microscale Thermophoresis as a Tool to Study Protein Interactions and Their Implication in Human Diseases
Abstract
1. Introduction
1.1. Implication of Proteins in Human Diseases
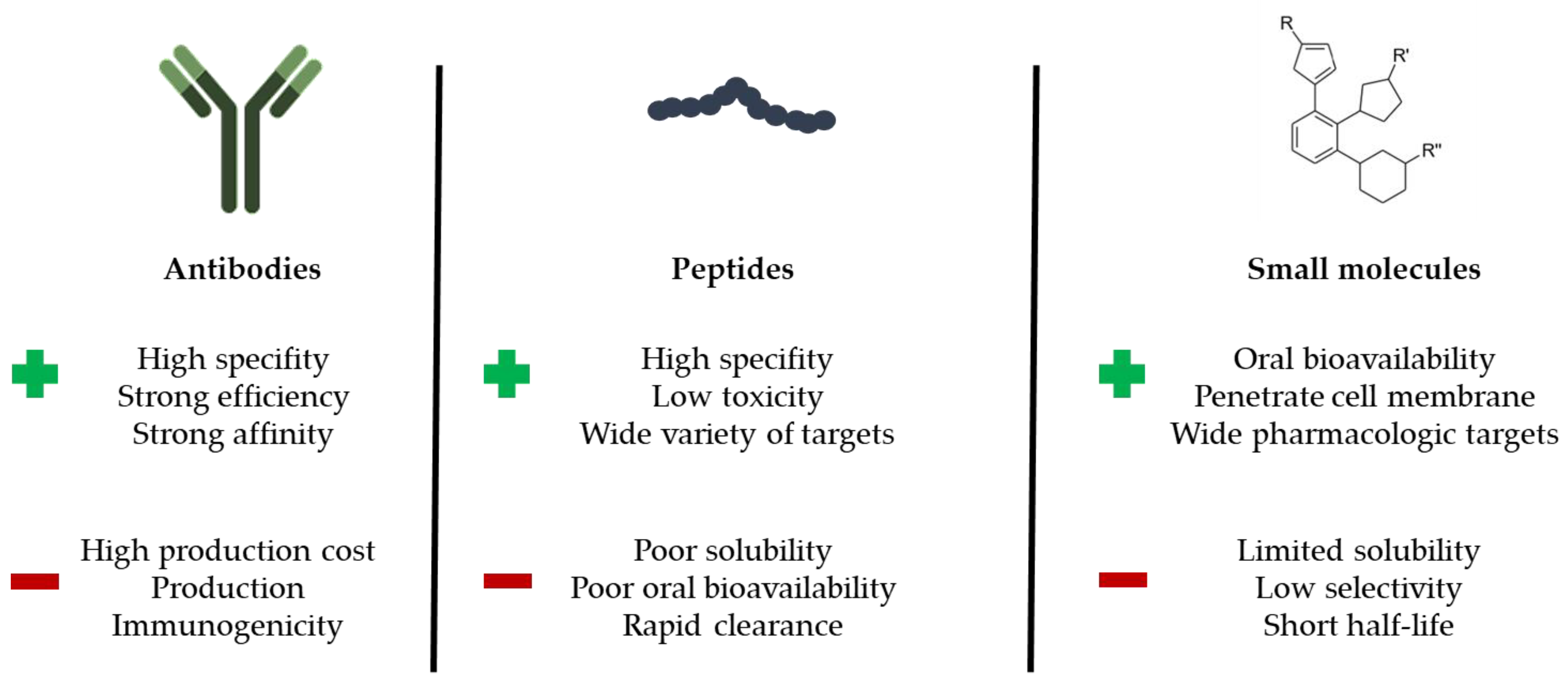
1.2. Overview of Protein Types and Their Relevance
1.3. Technical Approaches Used to Study PPIs
| Methods | Description | Ref. |
|---|---|---|
| Co-immunoprecipitation | Gold standard with endogenous proteins | [21] |
| Affinity electrophoresis | For binding constants | [22] |
| Phage display | HTS | [23] |
| Proximity ligation assay (PLA) | Immuno-histochemical method | [24] |
| Tandem affinity purification (TAP) | High-throughput identification | [25] |
| Surface plasmon resonance (SPR) | Label-free/immobilization required | [26] |
| Dynamic light scattering (DLS) | Screening/No immobilization or labeling | [27] |
| Bio-layer interferometry (BLI) | HTS/Label-free | [28] |
| Isothermal titration calorimetry (ITC) | Quantitative/Thermodynamics/No label or immobilization | [29] |
| Microscale thermophoresis (MST) | HTS/No immobilization/Can work in complex medium | [30] |
1.4. Chemical Types of PPI Modulators
1.5. Quantifying Binding Interactions
1.6. Microscale Thermophoresis
2. Case Studies
2.1. MST Applied to PROTAC Molecules
2.1.1. PROTAC-Induced Degradation of CREPT
2.1.2. PROTAC-Induced Degradation of Brd4
2.2. MST Applied to the PD-1/PD-L1 Immune Checkpoint
2.3. MST in the Context of Gene Therapy
2.3.1. CD19 CAR-T Cell Therapy
2.3.2. CRISPR-Cas9-Based Gene Editing
2.4. MST Applied to Coronavirus Infections
2.4.1. Interactions between CoV Non-Structural Proteins
2.4.2. CoV Protein Nsp9 Binding to Single-Stranded DNA (ssDNA)
2.4.3. Binding to CoV Glycoprotein S
2.5. MST Applied to Other Viruses
2.5.1. Hepatitis C
2.5.2. Influenza A
2.5.3. HIV-1
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nooren, I.M.A.; Thornton, J.M. Diversity of Protein-Protein Interactions. EMBO J. 2003, 22, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Ghadie, M.A.; Xia, Y. Are transient protein-protein interactions more dispensable? PLoS Comput. Biol. 2022, 18, e1010013. [Google Scholar] [CrossRef] [PubMed]
- Ngounou Wetie, A.G.; Sokolowska, I.; Woods, A.G.; Roy, U.; Loo, J.A.; Darie, C.C. Investigation of Stable and Transient Protein-Protein Interactions: Past, Present, and Future. Proteomics 2013, 13, 538–557. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Thornton, J.M. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, J.; Ivaska, J.; Corthals, G.L. Identification of Protein Interactions Involved in Cellular Signaling. Mol. Cell. Proteom. 2013, 12, 1752–1763. [Google Scholar] [CrossRef]
- Nimmagadda, A.; Shi, Y.; Cai, J. γ-AApeptides as a New Strategy for Therapeutic Development. Curr. Med. Chem. 2019, 26, 2313–2329. [Google Scholar] [CrossRef]
- Steinbrecher, T. Towards Accurate Free Energy Calculations in Ligand Protein-Binding Studies. Curr. Med. Chem. 2010, 17, 767–785. [Google Scholar] [CrossRef]
- Bhandari, G.P.; Angdembe, M.R.; Dhimal, M.; Neupane, S.; Bhusal, C. State of non-communicable diseases in Nepal. BMC Public Health 2014, 14, 23. [Google Scholar] [CrossRef]
- Haselkorn, R.; Rothman-Denes, L.B. Protein Synthesis. Annu. Rev. Biochem. 1973, 42, 397–438. [Google Scholar] [CrossRef]
- Sun, P.D.; Foster, C.E.; Boyington, J.C. Overview of Protein Structural and Functional Folds. Curr. Protoc. Protein Sci. 2004, 35, 17.1.1–17.1.189. [Google Scholar] [CrossRef]
- Dannies, P.S. Protein Hormone Storage in Secretory Granules: Mechanisms for Concentration and Sorting1. Endocr. Rev. 1999, 20, 3–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, S.G.; Leclerc, R.F.; Seo, S.-J.; Malone, C. Synthesis and transport of storage proteins by testes inHeliothis virescens. Arch. Insect Biochem. Physiol. 1990, 14, 151–170. [Google Scholar] [CrossRef]
- Obinata, T.; Maruyama, K.; Sugita, H.; Kohama, K.; Ebashi, S. Dynamic aspects of structural proteins in vertebrate skeletal muscle. Muscle Nerve 1981, 4, 456–488. [Google Scholar] [CrossRef]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N.S. Protein-Protein Interaction Detection: Methods and Analysis. Int. J. Proteom. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Analyzing Protein Structure and Function. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Ozbabacan, S.E.; Engin, H.B.; Gursoy, A.; Keskin, O. Transient protein-protein interactions. Protein Eng. Des. Sel. 2011, 24, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Goodrich, J. Protein-protein interaction assays: Eliminating false positive interactions. Nat. Methods 2006, 3, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Williams, J. Origin and evolution of high throughput screening. Br. J. Pharmacol. 2007, 152, 53–61. [Google Scholar] [CrossRef]
- Wells, J.A.; McClendon, C.L. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature 2007, 450, 1001–1009. [Google Scholar] [CrossRef]
- Fischer, G.; Rossmann, M.; Hyvönen, M. Alternative modulation of protein–protein interactions by small molecules. Curr. Opin. Biotechnol. 2015, 35, 78–85. [Google Scholar] [CrossRef]
- Phizicky, E.M.; Fields, S. Protein-protein interactions: Methods for detection and analysis. Microbiol. Rev. 1995, 59, 94–123. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Toraño, J.S.; Pukin, A.; Fu, O.; Boons, G.J.; De Jong, G.J.; Pieters, R.J. Affinity capillary electrophoresis for the assessment of binding affinity of carbohydrate-based cholera toxin inhibitors. Electrophoresis 2017, 39, 344–347. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, O.; Gullberg, M.; Jarvius, M.; Ridderstråle, K.; Leuchowius, K.-J.; Jarvius, J.; Wester, K.; Hydbring, P.; Bahram, F.; Larsson, L.-G.; et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 2006, 3, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Krogan, N.J.; Cagney, G.; Yu, H.; Zhong, G.; Guo, X.; Ignatchenko, A.; Li, J.; Pu, S.; Datta, N.; Tikuisis, A.P.; et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 2006, 440, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, B.; Nylander, C.; Lundström, I. Biosensing with surface plasmon resonance–How it all started. Biosens. Bioelectron. 1995, 10, 1–9. [Google Scholar] [CrossRef]
- Hanlon, A.D.; Larkin, M.I.; Reddick, R.M. Free-Solution, Label-Free Protein-Protein Interactions Characterized by Dynamic Light Scattering. Biophys. J. 2010, 98, 297–304. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Higher-throughput, label-free, real-time molecular interaction analysis. Anal. Biochem. 2007, 361, 1–6. [Google Scholar] [CrossRef]
- Pierce, M.M.; Raman, C.S.; Nall, B.T. Isothermal Titration Calorimetry of Protein–Protein Interactions. Methods 1999, 19, 213–221. [Google Scholar] [CrossRef]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef]
- Moreira, I.; Fernandes, P.; Ramos, M.J. Hot spots-A review of the protein-protein interface determinant amino-acid residues. Proteins: Struct. Funct. Bioinform. 2007, 68, 803–812. [Google Scholar] [CrossRef]
- Hudson, P.J.; Souriau, C. Engineered antibodies. Nat. Med. 2003, 9, 129–134. [Google Scholar] [CrossRef]
- Sperandio, O.; Reynès, C.H.; Camproux, A.-C.; Villoutreix, B. Rationalizing the chemical space of protein–protein interaction inhibitors. Drug Discov. Today 2010, 15, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Azzarito, V.; Long, K.; Murphy, N.S.; Wilson, A.J. Inhibition of α-helix-mediated protein–protein interactions using designed molecules. Nat. Chem. 2013, 5, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhou, Q.; He, J.; Jiang, Z.; Peng, C.; Tong, R.; Shi, J. Recent advances in the development of protein–protein interactions modulators: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2020, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Nero, T.L.; Morton, C.J.; Holien, J.K.; Wielens, J.; Parker, M.W. Oncogenic protein interfaces: Small molecules, big challenges. Nat. Cancer 2014, 14, 248–262. [Google Scholar] [CrossRef]
- Scott, D.E.; Bayly, A.; Abell, C.; Skidmore, J. Small molecules, big targets: Drug discovery faces the protein–protein interaction challenge. Nat. Rev. Drug Discov. 2016, 15, 533–550. [Google Scholar] [CrossRef]
- Churcher, I. Protac-Induced Protein Degradation in Drug Discovery: Breaking the Rules or Just Making New Ones? J. Med. Chem. 2017, 61, 444–452. [Google Scholar] [CrossRef]
- Pollard, T.D. A Guide to Simple and Informative Binding Assays. Mol. Biol. Cell 2010, 21, 4061–4067. [Google Scholar] [CrossRef]
- Rodbard, D.; Feldman, H. [1] Theory of protein-ligand interaction. Methods Enzymol. 1975, 36, 3–16. [Google Scholar] [CrossRef]
- Jarmoskaite, I.; Alsadhan, I.; Vaidyanathan, P.P.; Herschlag, D. How to measure and evaluate binding affinities. eLife 2020, 9, e57264. [Google Scholar] [CrossRef]
- Duhr, S.; Braun, D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. USA 2006, 103, 19678–19682. [Google Scholar] [CrossRef]
- Ludwig, C. Diffusion zwischen ungleich erwärmten Orten gleich zusammengesetzter Lösungen. Naturwiss. 1856, 20, 539. [Google Scholar]
- Seidel, S.A.I.; Dijkman, P.M.; Lea, W.A.; van den Bogaart, G.; Jerabek-Willemsen, M.; Lazic, A.; Joseph, J.S.; Srinivasan, P.; Baaske, P.; Simeonov, A.; et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 2013, 59, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Tso, S.-C.; Chen, Q.; Vishnivetskiy, S.A.; Gurevich, V.V.; Iverson, T.; Brautigam, C.A. Using two-site binding models to analyze microscale thermophoresis data. Anal. Biochem. 2017, 540–541, 64–75. [Google Scholar] [CrossRef] [PubMed]
- López-Méndez, B.; Uebel, S.; Lundgren, L.P.; Sedivy, A. Microscale Thermophoresis and additional effects measured in NanoTemper Monolith instruments. Eur. Biophys. J. 2021, 50, 653–660. [Google Scholar] [CrossRef]
- Nowak, P.M.; Woźniakiewicz, M. The Acid-Base/Deprotonation Equilibrium Can Be Studied with a MicroScale Thermophoresis (MST). Molecules 2022, 27, 685. [Google Scholar] [CrossRef]
- Neklesa, T.K.; Winkler, J.D.; Crews, C.M. Targeted protein degradation by PROTACs. Pharmacol. Ther. 2017, 174, 138–144. [Google Scholar] [CrossRef]
- Cecchini, C.; Pannilunghi, S.; Tardy, S.; Scapozza, L. From Conception to Development: Investigating PROTACs Features for Improved Cell Permeability and Successful Protein Degradation. Front. Chem. 2021, 9, 672267. [Google Scholar] [CrossRef]
- Ma, D.; Zou, Y.; Chu, Y.; Liu, Z.; Liu, G.; Chu, J.; Li, M.; Wang, J.; Sun, S.-Y.; Chang, Z. A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer. Theranostics 2020, 10, 3708–3721. [Google Scholar] [CrossRef]
- Sun, X.; Rao, Y. PROTACs as Potential Therapeutic Agents for Cancer Drug Resistance. Biochemistry 2019, 59, 240–249. [Google Scholar] [CrossRef]
- Proof-of-Concept with PROTACs in Prostate Cancer. Cancer Discov. 2020, 10, 1084. [CrossRef] [PubMed]
- Jin, J.; Wu, Y.; Chen, J.; Shen, Y.; Zhang, L.; Zhang, H.; Chen, L.; Yuan, H.; Chen, H.; Zhang, W.; et al. The peptide PROTAC modality: A novel strategy for targeted protein ubiquitination. Theranostics 2020, 10, 10141–10153. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Guan, Y.; Qin, W.; Zhai, X.; Yu, B.; Liu, H. Targeting Brd4 for cancer therapy: Inhibitors and degraders. MedChemComm 2018, 9, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Zengerle, M.; Chan, K.H.; Ciulli, A. Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem. Biol. 2015, 10, 1770–1777. [Google Scholar] [CrossRef]
- Bartoschik, T.; Zoephel, A.; Rumpel, K.; Ciulli, A.; Heffern, C. MST and TRIC Technology to Reliably Study PROTAC Binary and Ternary Binding in Drug Development. Methods Mol. Biol. 2021, 2365, 115–133. [Google Scholar] [CrossRef]
- Lee, L.; Gupta, M.; Sahasranaman, S. Immune Checkpoint inhibitors: An introduction to the next-generation cancer immunotherapy. J. Clin. Pharmacol. 2015, 56, 157–169. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Sheridan, C. Cautious optimism surrounds early clinical data for PD-1 blocker. Nat. Biotechnol. 2012, 30, 729–730. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Sunshine, J.; Taube, J.M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Linhares, A.; Battin, C.; Jutz, S.; Leitner, J.; Hafner, C.; Tobias, J.; Wiedermann, U.; Kundi, M.; Zlabinger, G.J.; Grabmeier-Pfistershammer, K.; et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci. Rep. 2019, 9, 11472. [Google Scholar] [CrossRef] [PubMed]
- Shankar, B.; Naidoo, J. PD-1 and PD-L1 inhibitor toxicities in non-small cell lung cancer. J. Thorac. Dis. 2018, 10, S4034–S4037. [Google Scholar] [CrossRef] [PubMed]
- Musielak, B.; Kocik, J.; Skalniak, L.; Magiera-Mularz, K.; Sala, D.; Czub, M.; Stec, M.; Siedlar, M.; Holak, T.A.; Plewka, J. CA-170—A Potent Small-Molecule PD-L1 Inhibitor or Not? Molecules 2019, 24, 2804. [Google Scholar] [CrossRef]
- Skalniak, L.; Zak, K.M.; Guzik, K.; Magiera, K.; Musielak, B.; Pachota, M.; Szelazek, B.; Kocik, J.; Grudnik, P.; Tomala, M.; et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget 2017, 8, 72167–72181. [Google Scholar] [CrossRef]
- Park, J.-J.; Thi, E.P.; Carpio, V.H.; Bi, Y.; Cole, A.G.; Dorsey, B.D.; Fan, K.; Harasym, T.; Iott, C.L.; Kadhim, S.; et al. Checkpoint inhibition through small molecule-induced internalization of programmed death-ligand 1. Nat. Commun. 2021, 12, 1222. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.-C.; He, Q.-J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Lin, X.; Lu, X.; Luo, G.; Xiang, H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2019, 186, 111876. [Google Scholar] [CrossRef]
- Li, K.; Tian, H. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J. Drug Target. 2018, 27, 244–256. [Google Scholar] [CrossRef]
- Ganesan, A.; Ahmed, M.; Okoye, I.; Arutyunova, E.; Babu, D.; Turnbull, W.L.; Kundu, J.K.; Shields, J.; Agopsowicz, K.C.; Xu, L.; et al. Comprehensive in vitro characterization of PD-L1 small molecule inhibitors. Sci. Rep. 2019, 9, 12392. [Google Scholar] [CrossRef]
- Leleu-Chavain, N.; Regnault, R.; Ahouari, H.; Le Biannic, R.; Kouach, M.; Klupsch, F.; Magnez, R.; Vezin, H.; Thuru, X.; Bailly, C.; et al. Antioxidant Properties and Aldehyde Reactivity of PD-L1 Targeted Aryl-Pyrazolone Anticancer Agents. Molecules 2022, 27, 3316. [Google Scholar] [CrossRef] [PubMed]
- Le Biannic, R.; Magnez, R.; Klupsch, F.; Leleu-Chavain, N.; Thiroux, B.; Tardy, M.; El Bouazzati, H.; Dezitter, X.; Renault, N.; Vergoten, G.; et al. Pyrazolones as inhibitors of immune checkpoint blocking the PD-1/PD-L1 interaction. Eur. J. Med. Chem. 2022, 236, 114343. [Google Scholar] [CrossRef] [PubMed]
- Koblish, H.K.; Wu, L.; Wang, L.-C.S.; Liu, P.C.; Wynn, R.; Rios-Doria, J.; Spitz, S.; Liu, H.; Volgina, A.; Zolotarjova, N.; et al. Characterization of INCB086550: A Potent and Novel Small-Molecule PD-L1 Inhibitor. Cancer Discov. 2022, 12, 1482–1499. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2014, 6, 224ra25. [Google Scholar] [CrossRef]
- Song, D.-G.; Ye, Q.; Poussin, M.; Liu, L.; Figini, M.D.J.P., Jr. A fully human chimeric antigen receptor with potent activity against cancer cells but reduced risk for off-tumor toxicity. Oncotarget 2015, 6, 21533–21546. [Google Scholar] [CrossRef]
- Maus, M.V.; Haas, A.R.; Beatty, G.L.; Albelda, S.M.; Levine, B.L.; Liu, X.; Zhao, Y.; Kalos, M.; June, C.H. T Cells Expressing Chimeric Antigen Receptors Can Cause Anaphylaxis in Humans. Cancer Immunol. Res. 2013, 1, 26–31. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Wang, X.; Wu, H.; Zhang, J.; Yang, J.; Zhang, F.; Liu, L.; Long, J.; Lu, P.; et al. Treatment with Humanized Selective CD19CAR-T Cells Shows Efficacy in Highly Treated B-ALL Patients Who Have Relapsed after Receiving Murine-Based CD19CAR-T Therapies. Clin. Cancer Res. 2019, 25, 5595–5607. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Hirano, H.; Gootenberg, J.; Horii, T.; Abudayyeh, O.O.; Kimura, M.; Hsu, P.; Nakane, T.; Ishitani, R.; Hatada, I.; Zhang, F.; et al. Structure and Engineering of Francisella novicida Cas9. Cell 2016, 164, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Price, A.A.; Sampson, T.R.; Ratner, H.K.; Grakoui, A.; Weiss, D.S. Cas9-mediated targeting of viral RNA in eukaryotic cells. Proc. Natl. Acad. Sci. USA 2015, 112, 6164–6169. [Google Scholar] [CrossRef] [PubMed]
- Khavrutskii, L.; Yeh, J.; Timofeeva, O.; Tarasov, S.G.; Pritt, S.; Stefanisko, K.; Tarasova, N. Protein Purification-free Method of Binding Affinity Determination by Microscale Thermophoresis. J. Vis. Exp. 2013, 2013, e50541. [Google Scholar] [CrossRef] [PubMed]
- Magnez, R.; Thiroux, B.; Taront, S.; Segaoula, Z.; Quesnel, B.; Thuru, X. PD-1/PD-L1 binding studies using microscale thermophoresis. Sci. Rep. 2017, 7, 17623. [Google Scholar] [CrossRef]
- Acharya, S.; Mishra, A.; Paul, D.; Ansari, A.H.; Azhar, M.; Kumar, M.; Rauthan, R.; Sharma, N.; Aich, M.; Sinha, D.; et al. Francisella novicida Cas9 interrogates genomic DNA with very high specificity and can be used for mammalian genome editing. Proc. Natl. Acad. Sci. USA 2019, 116, 20959–20968. [Google Scholar] [CrossRef]
- Hilgenfeld, R.; Peiris, M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antivir. Res. 2013, 100, 286–295. [Google Scholar] [CrossRef]
- Fehr, A.R.; Channappanavar, R.; Perlman, S. Middle East Respiratory Syndrome: Emergence of a Pathogenic Human Coronavirus. Annu. Rev. Med. 2017, 68, 387–399. [Google Scholar] [CrossRef]
- Shahhamzehei, N.; Abdelfatah, S.; Efferth, T. In Silico and In Vitro Identification of Pan-Coronaviral Main Protease Inhibitors from a Large Natural Product Library. Pharmaceuticals 2022, 15, 308. [Google Scholar] [CrossRef]
- Ivanov, K.A.; Hertzig, T.; Rozanov, M.; Bayer, S.; Thiel, V.; Gorbalenya, A.E.; Ziebuhr, J. Major genetic marker of nidoviruses encodes a replicative endoribonuclease. Proc. Natl. Acad. Sci. USA 2004, 101, 12694–12699. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Yan, L.; Ming, Z.; Jia, Z.; Lou, Z.; Rao, Z. Structural and Biochemical Characterization of Endoribonuclease Nsp15 Encoded by Middle East Respiratory Syndrome Coronavirus. J. Virol. 2018, 92, e00893-18. [Google Scholar] [CrossRef]
- Araújo, J.D.O.; Pinheiro, S.; Zamora, W.J.; Alves, C.N.; Lameira, J.; Lima, A.H. Structural, energetic and lipophilic analysis of SARS-CoV-2 non-structural protein 9 (NSP9). Sci. Rep. 2021, 11, 23003. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Deng, F.; Shi, K.; Ye, G.; Wang, G.; Fang, L.; Xiao, S.; Fu, Z.; Peng, G. Dimerization of Coronavirus nsp9 with Diverse Modes Enhances Its Nucleic Acid Binding Affinity. J. Virol. 2018, 92, e00692-18. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Walls, A.C.; Tortorici, M.A.; Bosch, B.-J.; Frenz, B.; Rottier, P.J.M.; DiMaio, F.; Rey, F.A.; Veesler, D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature 2016, 531, 114–117. [Google Scholar] [CrossRef]
- Wendt, A.; Bourlière, M. An update on the treatment of genotype-1 chronic hepatitis C infection: Lessons from recent clinical trials. Ther. Adv. Infect. Dis. 2013, 1, 191–208. [Google Scholar] [CrossRef]
- Belda, O.; Targett-Adams, P. Small molecule inhibitors of the hepatitis C virus-encoded NS5A protein. Virus Res. 2012, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ascher, D.; Wielens, J.; Nero, T.; Doughty, L.; Morton, C.; Parker, M.W. Potent hepatitis C inhibitors bind directly to NS5A and reduce its affinity for RNA. Sci. Rep. 2014, 4, 4765. [Google Scholar] [CrossRef]
- Hwang, J.; Huang, L.; Cordek, D.G.; Vaughan, R.; Reynolds, S.L.; Kihara, G.; Raney, K.D.; Kao, C.C.; Cameron, C.E. Hepatitis C Virus Nonstructural Protein 5A: Biochemical Characterization of a Novel Structural Class of RNA-Binding Proteins. J. Virol. 2010, 84, 12480–12491. [Google Scholar] [CrossRef]
- Lim, P.J.; Chatterji, U.; Cordek, D.; Sharma, S.; Garcia-Rivera, J.A.; Cameron, C.E.; Lin, K.; Targett-Adams, P.; Gallay, P.A. Correlation between NS5A Dimerization and Hepatitis C Virus Replication. J. Biol. Chem. 2012, 287, 30861–30873. [Google Scholar] [CrossRef]
- Sauter, N.K.; Bednarski, M.D.; Wurzburg, B.A.; Hanson, J.E.; Whitesides, G.M.; Skehel, J.J.; Wiley, D.C. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: A 500-MHz proton nuclear magnetic resonance study. Biochemistry 1989, 28, 8388–8396. [Google Scholar] [CrossRef] [PubMed]
- Lauster, D.; Klenk, S.; Ludwig, K.; Nojoumi, S.; Behren, S.; Adam, L.; Stadtmüller, M.; Saenger, S.; Zimmler, S.; Hönzke, K.; et al. Phage capsid nanoparticles with defined ligand arrangement block influenza virus entry. Nat. Nanotechnol. 2020, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Uchil, P.D.; Quinlan, B.D.; Chan, W.-T.; Luna, J.M.; Mothes, W. TRIM E3 Ligases Interfere with Early and Late Stages of the Retroviral Life Cycle. PLoS Pathog. 2008, 4, e16. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Luban, J. TRIM5α selectively binds a restriction-sensitive retroviral capsid. Retrovirology 2005, 2, 40. [Google Scholar] [CrossRef]
- Walport, L.J.; Low, J.K.K.; Matthews, J.M.; Mackay, J.P. The characterization of protein interactions —What, how and how much? Chem. Soc. Rev. 2021, 50, 12292–12307. [Google Scholar] [CrossRef]
- Stein, J.A.C.; Ianeselli, A.; Braun, D. Kinetic Microscale Thermophoresis for Simultaneous Measurement of Binding Affinity and Kinetics. Angew. Chem. Int. Ed. 2021, 60, 13988–13995. [Google Scholar] [CrossRef]
- Holdgate, G.; Embrey, K.; Milbradt, A.; Davies, G. Biophysical methods in early drug discovery. Admet Dmpk 2019, 7, 222–241. [Google Scholar] [CrossRef]
- Niether, D.; Wiegand, S. Thermophoresis of biological and biocompatible compounds in aqueous solution. J. Physics Condens. Matter 2019, 31, 503003. [Google Scholar] [CrossRef]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular Interaction Studies Using Microscale Thermophoresis. Assay Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef]
- Asmari, M.; Ratih, R.; Alhazmi, H.A.; El Deeb, S. Thermophoresis for characterizing biomolecular interaction. Methods 2018, 146, 107–119. [Google Scholar] [CrossRef]
- Plach, M.; Schubert, T. Biophysical Characterization of Aptamer-Target Interactions. Aptamers Biotechnol. 2019, 2019, 174. [Google Scholar] [CrossRef]
- Al-Jubair, T.; Steffen, J.H.; Missel, J.W.; Kitchen, P.; Salman, M.M.; Bill, R.M.; Gourdon, P.; Törnroth-Horsefield, S. Characterization of human aquaporin protein-protein interactions using microscale thermophoresis (MST). STAR Protoc. 2022, 3, 101316. [Google Scholar] [CrossRef]
- Sparks, R.P.; Lawless, W.; Arango, A.S.; Tajkhorshid, E.; Fratti, R.A. Use of Microscale Thermophoresis to Measure Protein-Lipid Interactions. J. Vis. Exp. 2022, 2022, e60607. [Google Scholar] [CrossRef] [PubMed]
- Plach, M.; Grasser, K.; Schubert, T. MicroScale Thermophoresis as a Tool to Study Protein-peptide Interactions in the Context of Large Eukaryotic Protein Complexes. Bio-Protocol 2017, 7, e2632. [Google Scholar] [CrossRef] [PubMed]

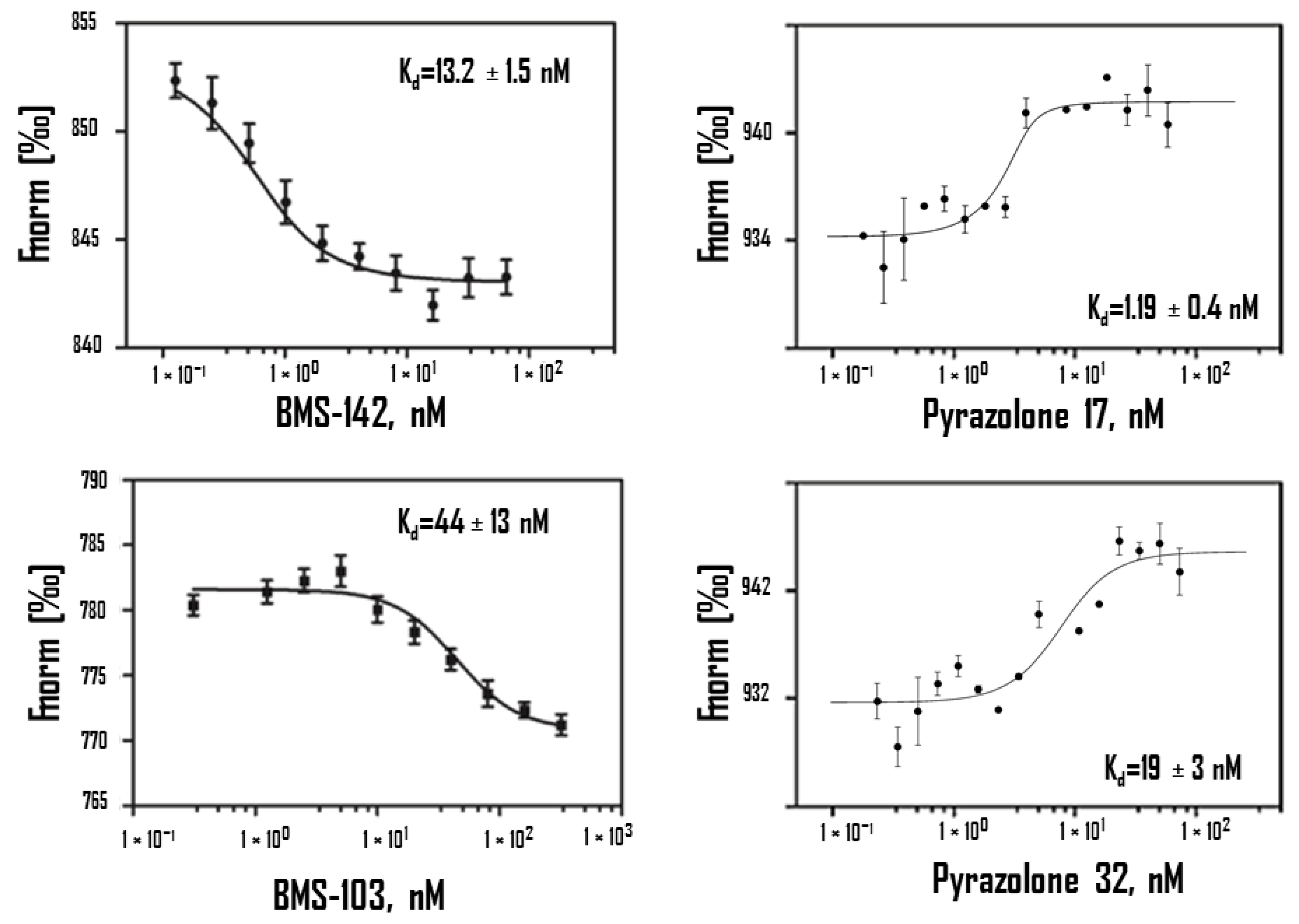
| Compounds | MST Kd (nM) | SPR Kd (nM) | ITC Kd (nM) |
|---|---|---|---|
| BMSpep-57 * | 19 ± 2 | 20 ± 2 | / |
| BMS-103 * | 44 ± 13 | 16 ± 2 | / |
| BMS-142 * | 13.2 ± 1.5 | 12 ± 2 | / |
| Pyrazolone 11 ** | 83 ± 12 | / | 120 |
| Pyrazolone 17 ** | 1.19 ± 0.4 | / | / |
| Pyrazolone 32 ** | 19 ± 3 | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnez, R.; Bailly, C.; Thuru, X. Microscale Thermophoresis as a Tool to Study Protein Interactions and Their Implication in Human Diseases. Int. J. Mol. Sci. 2022, 23, 7672. https://doi.org/10.3390/ijms23147672
Magnez R, Bailly C, Thuru X. Microscale Thermophoresis as a Tool to Study Protein Interactions and Their Implication in Human Diseases. International Journal of Molecular Sciences. 2022; 23(14):7672. https://doi.org/10.3390/ijms23147672
Chicago/Turabian StyleMagnez, Romain, Christian Bailly, and Xavier Thuru. 2022. "Microscale Thermophoresis as a Tool to Study Protein Interactions and Their Implication in Human Diseases" International Journal of Molecular Sciences 23, no. 14: 7672. https://doi.org/10.3390/ijms23147672
APA StyleMagnez, R., Bailly, C., & Thuru, X. (2022). Microscale Thermophoresis as a Tool to Study Protein Interactions and Their Implication in Human Diseases. International Journal of Molecular Sciences, 23(14), 7672. https://doi.org/10.3390/ijms23147672







