Exploring the Links between Obesity and Psoriasis: A Comprehensive Review
Abstract
:1. Introduction
2. Materials and Methods
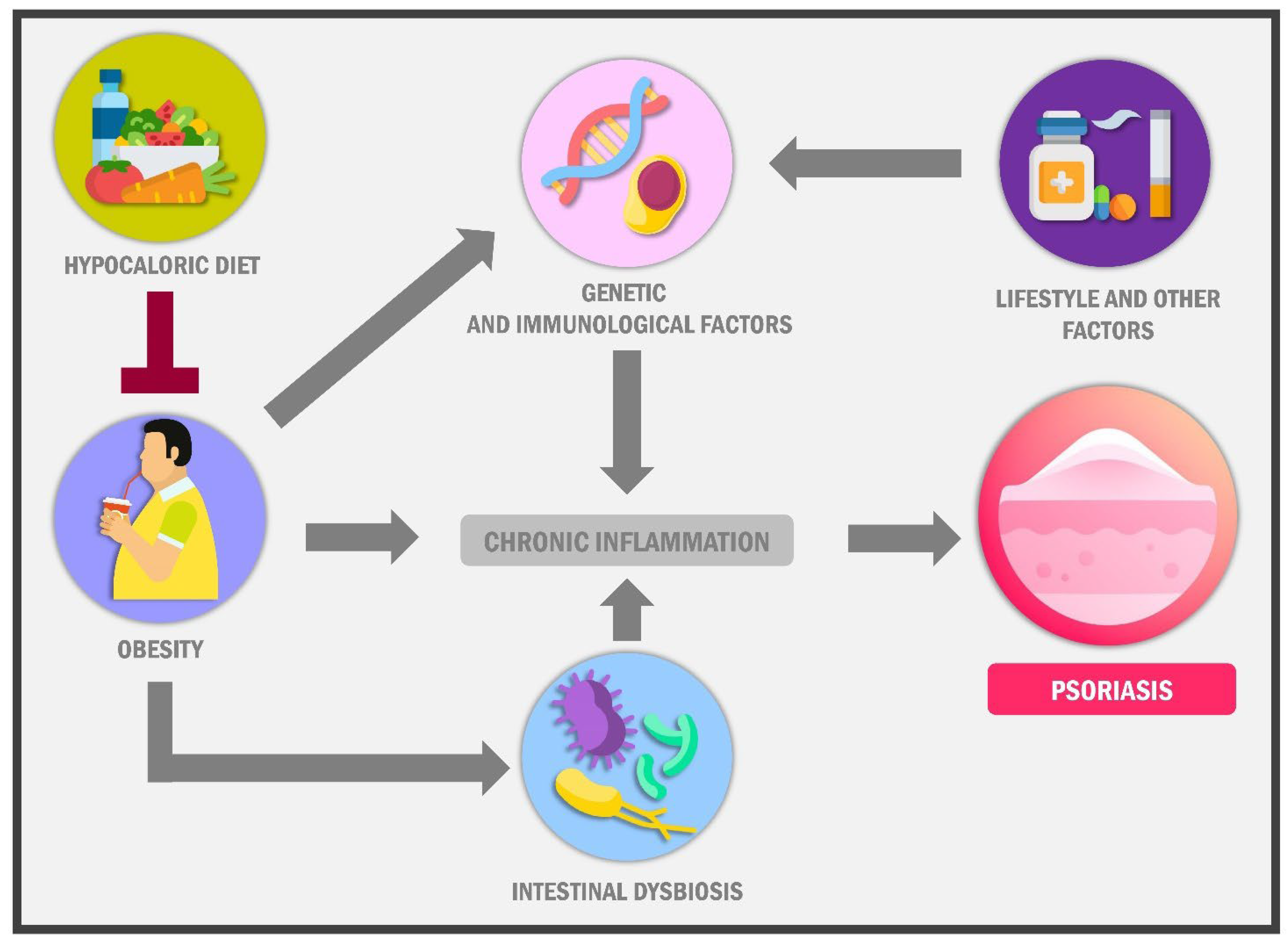
3. The Link between Psoriasis and Obesity
3.1. Is There Epidemiological Evidence That the Increase in the Prevalence of Obesity Coincides with the Epidemiological Behaviour of Psoriasis over the Last 50 Years?
3.2. Relationship between Anthropometric Indices and Psoriasis
3.3. Food and Psoriasis: Is There a Link?
3.4. Role of the Gut and Skin Microbiota
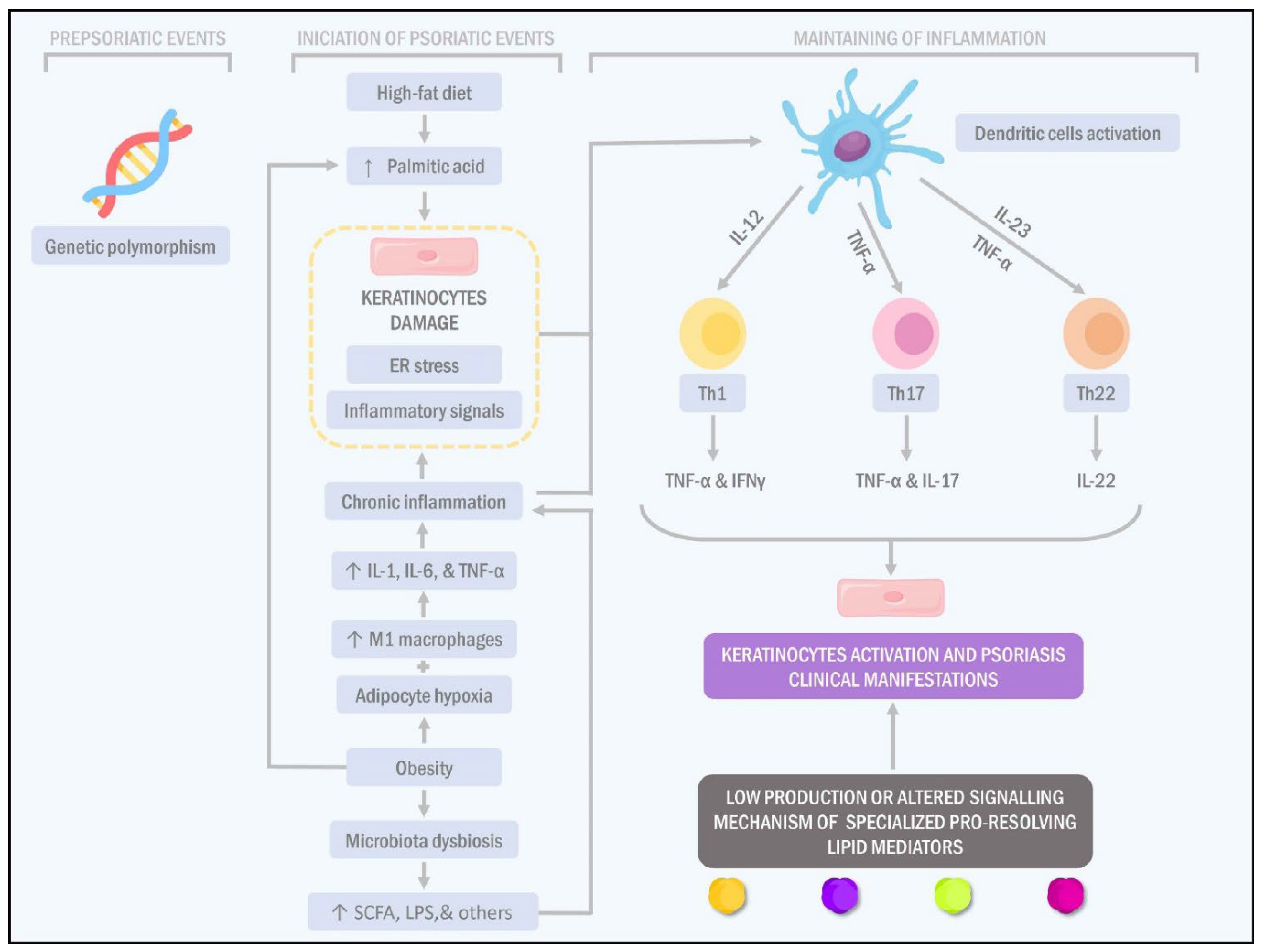
3.5. A High-Fat Diet and Palmitic Acid Consumption Break the Gut Epithelium Integrity and Initiate Pro-Inflammatory Cytokine Production
3.6. Obesity and Endoplasmic Reticulum Stress in Adipocytes Increases Pro-Inflammatory Cytokine Production in Adipose Tissue
3.7. Palmitic Acid Directly Affects the Activation of Antigen-Presenting Cells and Keratinocytes
3.8. Typical High-Fat Diet (Palmitic Acid) of the Westernised Countries Produces Smooth Endoplasmic Reticulum Stress in Multiple Tissues, including Keratinocytes
3.9. Alteration in the Inflammation Resolution in Psoriasis
3.10. Is Maresin-1 the Missing Link in the Resolution of Psoriasis Inflammation?
3.11. Immunomolecular and Genetic Factors: The Missing Link between Psoriasis and Obesity?
3.12. Role of Adipokines in Obesity and Psoriasis
3.13. Is Psoriasis Clinic Related to Obesity?
3.14. Psoriasis Treatment Considerations in Obese Patients
3.15. New Hope on the Horizon: Microbiota Manipulation and Polyphenols in the Obesity-Psoriasis Intervention
3.16. Antiobesity Therapy: Evidence for the Role of Bariatric Surgery and Incretin Analogues against Psoriasis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, P.; Skov, L. Psoriasis and Obesity. Dermatology 2016, 232, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Cheng, Y.; Chang, D. The Important Role of Leptin in Modulating the Risk of Dermatological Diseases. Front. Immunol. 2021, 11, 593564. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef] [PubMed]
- Bonanad, C.; González-Parra, E.; Rivera, R.; Carrascosa, J.M.; Daudén, E.; Olveira, A.; Botella-Estrada, R. Clinical, Diagnostic, and Therapeutic Implications in Psoriasis Associated With Cardiovascular Disease. Actas Dermosifiliogr. 2017, 108, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Timmins, K.A.; Leech, R.D.; Batt, M.E.; Edwards, K.L. Running and Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2017, 45, 1447–1457. [Google Scholar] [CrossRef]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Cortese, S.; Tessari, L. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: Update 2016. Curr. Psychiatry Rep. 2017, 19, 4. [Google Scholar] [CrossRef] [Green Version]
- Kelishadi, R.; Roufarshbaf, M.; Soheili, S.; Payghambarzadeh, F.; Masjedi, M. Association of Childhood Obesity and the Immune System: A Systematic Review of Reviews. Child. Obes. 2017, 13, 332–346. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Carretero, J.; Ena, J.; Arévalo, J.C.; Seguí, J.M.; Carrasco, F.J.; Gómez, R.; Pérez, M.I.; Delgado, J.; Pérez, P. Obesity is a chronic disease. Positioning statement of the Diabetes, Obesity and Nutrition Workgroup of the Spanish Society of Internal Medicine (SEMI) for an approach centred on individuals with obesity. Rev. Clínica Esp. 2021, 221, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- CDC CDC Overweight & Obesity. Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/obesity/index.html (accessed on 27 October 2021).
- Orloff, J.; Kaminetsky, J.; Aziz, M. Psoriasis and Obesity: A Review of the Current Literature. SKIN J. Cutan. Med. 2018, 2, 364–379. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- An, R.; Ji, M.; Zhang, S. Global warming and obesity: A systematic review. Obes. Rev. 2018, 19, 150–163. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Nga, V.T.; Dung, V.N.T.; Chu, D.-T.; Tien, N.L.B.; Van Thanh, V.; Ngoc, V.T.N.; Hoan, L.N.; Phuong, N.T.; Pham, V.-H.; Tao, Y.; et al. School education and childhood obesity: A systemic review. Diabetes Metab. Syndr. 2019, 13, 2495–2501. [Google Scholar] [CrossRef]
- Koch, C.A.; Sharda, P.; Patel, J.; Gubbi, S.; Bansal, R.; Bartel, M.J. Climate Change and Obesity. Horm. Metab. Res. 2021, 53, 575–587. [Google Scholar] [CrossRef]
- Dhurandhar, E.J. The food-insecurity obesity paradox: A resource scarcity hypothesis. Physiol. Behav. 2016, 162, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Figueira, T.C.F.; Corrente, J.E.; Miot, L.D.B.; Papini, S.J.; Miot, H.A. Dietary patterns of patients with psoriasis at a public healthcare institution in Brazil. An. Bras. Dermatol. 2020, 95, 452–458. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.-M.; Fan, X.-Y.; Jin, Y.-L.; Li, X.; Wu, S.-R.; Ge, W.-W.; Lv, C.-H.; Wang, Y.-K.; Chen, J.-G. Gut-Brain-Skin Axis in Psoriasis: A Review. Derm. Ther. 2021, 11, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Liakou, A.; Zouboulis, C. Links and risks associated with psoriasis and metabolic syndrome. Psoriasis Targets Ther. 2015, 5, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langan, S.M.; Seminara, N.M.; Shin, D.B.; Troxel, A.B.; Kimmel, S.E.; Mehta, N.N.; Margolis, D.J.; Gelfand, J.M. Prevalence of Metabolic Syndrome in Patients with Psoriasis: A Population-Based Study in the United Kingdom. J. Investig. Dermatol. 2012, 132, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisondi, P.; Fostini, A.C.; Fossà, I.; Girolomoni, G.; Targher, G. Psoriasis and the metabolic syndrome. Clin. Dermatol. 2018, 36, 21–28. [Google Scholar] [CrossRef]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Myers, B.; Brownstone, N.; Reddy, V.; Chan, S.; Thibodeaux, Q.; Truong, A.; Bhutani, T.; Chang, H.-W.; Liao, W. The gut microbiome in psoriasis and psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101494. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Simon, J.C.; Saalbach, A. Psoriasis: Obesity and Fatty Acids. Front. Immunol. 2019, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-N.; Han, K.; Park, Y.-G.; Lee, J.H. Metabolic syndrome is associated with an increased risk of psoriasis: A nationwide population-based study. Metabolism 2019, 99, 19–24. [Google Scholar] [CrossRef]
- Kyriakou, A.; Patsatsi, A.; Sotiriadis, D.; Goulis, D.G. Effects of treatment for psoriasis on circulating levels of leptin, adiponectin and resistin: A systematic review and meta-analysis. Br. J. Derm. 2018, 179, 273–281. [Google Scholar] [CrossRef]
- Ogawa, K.; Stuart, P.E.; Tsoi, L.C.; Suzuki, K.; Nair, R.P.; Mochizuki, H.; Elder, J.T.; Okada, Y. A Transethnic Mendelian Randomization Study Identifies Causality of Obesity on Risk of Psoriasis. J. Investig. Dermatol. 2019, 139, 1397–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdugo, A. Prevalencia de sobrepeso y obesidad en adultos de la ciudad de Cuenca-Ecuador, 2014. Rev. Fac. Cienc. Méd. Univ. Cuenca 2018, 36, 54–60. [Google Scholar]
- Hirt, P.A.; Castillo, D.E.; Yosipovitch, G.; Keri, J.E. Skin changes in the obese patient. J. Am. Acad. Dermatol. 2019, 81, 1037–1057. [Google Scholar] [CrossRef]
- Paroutoglou, K.; Papadavid, E.; Christodoulatos, G.S.; Dalamaga, M. Deciphering the Association Between Psoriasis and Obesity: Current Evidence and Treatment Considerations. Curr. Obes. Rep. 2020, 9, 165–178. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, S.; Wu, R.; Su, X.; Peng, D.; Zhao, M.; Su, Y. New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 2019, 18, 171. [Google Scholar] [CrossRef] [Green Version]
- Stjernholm, T.; Ommen, P.; Langkilde, A.; Johansen, C.; Iversen, L.; Rosada, C.; Stenderup, K. Leptin deficiency in mice counteracts imiquimod (IMQ)-induced psoriasis-like skin inflammation while leptin stimulation induces inflammation in human keratinocytes. Exp. Derm. 2017, 26, 338–345. [Google Scholar] [CrossRef]
- Snekvik, I.; Smith, C.H.; Nilsen, T.I.L.; Langan, S.M.; Modalsli, E.H.; Romundstad, P.R.; Saunes, M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017, 137, 2484–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Han, J.; Li, T.; Qureshi, A.A. Obesity, waist circumference, weight change and the risk of psoriasis in US women: Risk of psoriasis in US women. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1293–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.-H.; Chi, C.-C.; Yeh, M.-L.; Wang, S.-H.; Tsai, Y.-S.; Hsu, M.-Y. Lifestyle changes for treating psoriasis. Cochrane Database Syst. Rev. 2019, 7, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.B.; Lebwohl, M.G. Psoriasis: Which therapy for which patient. J. Am. Acad. Dermatol. 2019, 80, 27–40. [Google Scholar] [CrossRef]
- Dopytalska, K.; Baranowska-Bik, A.; Roszkiewicz, M.; Bik, W.; Walecka, I. The role of leptin in selected skin diseases. Lipids Health Dis. 2020, 19, 215. [Google Scholar] [CrossRef]
- Tollefson, M.M.; Crowson, C.S.; McEvoy, M.T.; Maradit Kremers, H. Incidence of psoriasis in children: A population-based study. J. Am. Acad. Dermatol. 2010, 62, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, G.; Rastrelli, L.; Galdo, G.; Molettieri, P.; Rotondi Aufiero, F.; Cereda, E. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: A proof-of-concept, single-arm, open-label clinical trial. Nutrition 2020, 74, 110757. [Google Scholar] [CrossRef]
- Llamas-Velasco, M.; Ovejero-Merino, E.; Salgado-Boquete, L. Obesidad: Factor de riesgo para psoriasis y COVID-19. Actas Dermo-Sifiliográficas 2020, 112, 489–494. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Vanderpuye-Orgle, J.; Zhao, Y.; Lu, J.; Shrestha, A.; Sexton, A.; Seabury, S.; Lebwohl, M. Evaluating the economic burden of psoriasis in the United States. J. Am. Acad. Derm. 2015, 72, 961–967.e5. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Icen, M.; Crowson, C.S.; McEvoy, M.T.; Dann, F.J.; Gabriel, S.E.; Maradit Kremers, H. Trends in incidence of adult-onset psoriasis over three decades: A population-based study. J. Am. Acad. Dermatol. 2009, 60, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevalence of Psoriasis in Italy 2010–2017. Statista. Available online: https://www.statista.com/statistics/944680/prevalence-of-psoriasis-in-italy/ (accessed on 22 October 2021).
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Huang, H.; Deng, X.; Gao, D.; Zhang, Z. Modifiable lifestyle and environmental factors associated with onset of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational studies. J. Am. Acad. Derm. 2021, 84, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Green, L.J.; Lebwohl, M.G.; Wu, J.J.; Blauvelt, A.; Jacobson, A.A. Comparable efficacy and safety of brodalumab in obese and nonobese patients with psoriasis: Analysis of two randomized controlled trials. Br. J. Derm. 2020, 182, 880–888. [Google Scholar] [CrossRef]
- Norden, A.; Rekhtman, S.; Strunk, A.; Garg, A. Risk of psoriasis according to body mass index: A retrospective cohort analysis. J. Am. Acad. Dermatol. 2021, 86, 1020–1026. [Google Scholar] [CrossRef]
- Karmacharya, P.; Ogdie, A.; Eder, L. Psoriatic arthritis and the association with cardiometabolic disease: A narrative review. Adv. Musculoskelet. Dis. 2021, 13, 1759720X21998279. [Google Scholar] [CrossRef]
- Bavoso, N.C.; Pinto, J.M.; Soares, M.M.S.; dos Santos Diniz, M.; Teixeira Júnior, A.L. Psoriasis in obesity: Comparison of serum levels of leptin and adiponectin in obese subjects—Cases and controls. An. Bras. Dermatol. 2019, 94, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, J.; Zhu, W.; Kuang, Y.; Liu, T.; Zhang, W.; Chen, X.; Peng, C. Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front. Microbiol. 2020, 11, 589726. [Google Scholar] [CrossRef]
- Setty, A.R. Obesity, Waist Circumference, Weight Change, and the Risk of Psoriasis in Women: Nurses’ Health Study II. Arch. Intern. Med. 2007, 167, 1670–1675. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Derm. 2019, 181, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.; Frings, V.G.; Geier, A.; Goebeler, M.; Kerstan, A. Non-alcoholic fatty liver disease and psoriasis—Is there a shared proinflammatory network? J. Dtsch. Derm. Ges. 2021, 19, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.R.H.; Sonkoly, E.; Eberhardson, M.; Ståhle, M. Inflammatory bowel disease and psoriasis: Modernizing the multidisciplinary approach. J. Intern. Med. 2021, 290, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Todberg, T.; Egeberg, A.; Zachariae, C.; Sørensen, N.; Pedersen, O.; Skov, L. Patients with psoriasis have a dysbiotic taxonomic and functional gut microbiota. Br. J. Derm. 2022, bjd.21245. [Google Scholar] [CrossRef]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome-Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Supp. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef]
- Lv, Y.; Qin, X.; Jia, H.; Chen, S.; Sun, W.; Wang, X. The association between gut microbiota composition and BMI in Chinese male college students, as analysed by next-generation sequencing. Br. J. Nutr. 2019, 122, 986–995. [Google Scholar] [CrossRef]
- Barengolts, E.; Green, S.J.; Chlipala, G.E.; Layden, B.T.; Eisenberg, Y.; Priyadarshini, M.; Dugas, L.R. Predictors of Obesity among Gut Microbiota Biomarkers in African American Men with and without Diabetes. Microorganisms 2019, 7, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut. Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamanu, E.; Rai, S.N. The Link between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis. Diseases 2021, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, H.J.; Tseng, C.; Lai, Z.; Shieh, J.; Wu, C. Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp. Derm. 2018, 27, 1336–1343. [Google Scholar] [CrossRef]
- Ghezzal, S.; Postal, B.G.; Quevrain, E.; Brot, L.; Seksik, P.; Leturque, A.; Thenet, S.; Carrière, V. Palmitic acid damages gut epithelium integrity and initiates inflammatory cytokine production. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158530. [Google Scholar] [CrossRef]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.-E.; Vaillant, J.-C.; Oppert, J.-M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef]
- Tsai, Y.-W.; Lu, C.-H.; Chang, R.C.-A.; Hsu, Y.-P.; Ho, L.-T.; Shih, K.-C. Palmitoleic acid ameliorates palmitic acid-induced proinflammation in J774A.1 macrophages via TLR4-dependent and TNF-α-independent signallings. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102270. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.; Zhang, Q.; Permatasari, F.; Xu, Y.; Wu, D.; Yin, Z.; Luo, D. Palmitic Acid Induces Production of Proinflammatory Cytokines Interleukin-6, Interleukin-1β, and Tumor Necrosis Factor-α via a NF-κB-Dependent Mechanism in HaCaT Keratinocytes. Mediat. Inflamm. 2013, 2013, 530429. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Campos, H.; McGarvey, S.; Wu, Z.; Goldberg, R.; Baylin, A. Adipose tissue palmitoleic acid and obesity in humans: Does it behave as a lipokine? 123. Am. J. Clin. Nutr. 2011, 93, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermúdez, V.; Durán, P.; Rojas, E.; Díaz, M.P.; Rivas, J.; Nava, M.; Chacín, M.; Cabrera de Bravo, M.; Carrasquero, R.; Ponce, C.C.; et al. The Sick Adipose Tissue: New Insights Into Defective Signaling and Crosstalk With the Myocardium. Front Endocrinol. 2021, 12, 735070. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, M.M.; Mizusawa, M.; Hayashi, S.; Arai, W.; Sakata, S.; Yonekura, S. Effects of fatty acids on inducing endoplasmic reticulum stress in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 8643–8654. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dror, K.; Birk, R. Oleic acid ameliorates palmitic acid-induced ER stress and inflammation markers in naive and cerulein-treated exocrine pancreas cells. Biosci. Rep. 2019, 39, BSR20190054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Rozpędek, W.; Pytel, D.; Mucha, B.; Leszczyńska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Suzuki, T.; Gao, J.; Ishigaki, Y.; Kondo, K.; Sawada, S.; Izumi, T.; Uno, K.; Kaneko, K.; Tsukita, S.; Takahashi, K.; et al. ER Stress Protein CHOP Mediates Insulin Resistance by Modulating Adipose Tissue Macrophage Polarity. Cell Rep. 2017, 18, 2045–2057. [Google Scholar] [CrossRef] [Green Version]
- Maya-Monteiro, C.M.; Bozza, P.T. Leptin and mTOR: Partners in metabolism and inflammation. Cell Cycle 2008, 7, 1713–1717. [Google Scholar] [CrossRef] [Green Version]
- Cooper, P.O.; Haas, M.R.; Noonepalle, S.K.R.; Shook, B.A. Dermal Drivers of Injury-Induced Inflammation: Contribution of Adipocytes and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 1933. [Google Scholar] [CrossRef]
- Rivera-Gonzalez, G.; Shook, B.; Horsley, V. Adipocytes in Skin Health and Disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015271. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Dehairs, J.; L’homme, L.; Herinckx, G.; Esser, N.; Jansen, O.; Habraken, Y.; Lassence, C.; Swinnen, J.V.; Rider, M.H.; et al. Saturated fatty acids induce NLRP3 activation in human macrophages through K+ efflux resulting from phospholipid saturation and Na, K-ATPase disruption. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Everts, B. Fa(c)t checking: How fatty acids shape metabolism and function of macrophages and dendritic cells. Eur. J. Immunol. 2021, 51, 1628–1640. [Google Scholar] [CrossRef]
- Stelzner, K.; Herbert, D.; Popkova, Y.; Lorz, A.; Schiller, J.; Gericke, M.; Klöting, N.; Blüher, M.; Franz, S.; Simon, J.C.; et al. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur. J. Immunol. 2016, 46, 2043–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, J. It is all about fluidity: Fatty acids and macrophage phagocytosis. Eur. J. Pharm. 2016, 785, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Luo, J.; Xiao, B.; Tang, H.; Song, F.; Ding, X.; Yang, G. Endoplasmic reticulum stress links psoriasis vulgaris with keratinocyte inflammation. Postepy Derm. Alergol. 2020, 37, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Kanemaru, K.; Matsuyuki, A.; Nakamura, Y.; Fukami, K. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hyperplasia and interleukin-17 and interleukin-22 production in mice. Exp. Derm. 2015, 24, 436–442. [Google Scholar] [CrossRef]
- Vasseur, P.; Serres, L.; Jégou, J.-F.; Pohin, M.; Delwail, A.; Petit-Paris, I.; Levillain, P.; Favot, L.; Samson, M.; Yssel, H.; et al. High-Fat Diet-Induced IL-17A Exacerbates Psoriasiform Dermatitis in a Mouse Model of Steatohepatitis. Am. J. Pathol. 2016, 186, 2292–2301. [Google Scholar] [CrossRef]
- Klapan, K.; Simon, D.; Karaulov, A.; Gomzikova, M.; Rizvanov, A.; Yousefi, S.; Simon, H.-U. Autophagy and Skin Diseases. Front. Pharmacol. 2022, 13, 380–389. [Google Scholar] [CrossRef]
- Chávez-Castillo, M.; Ortega, Á.; Cudris-Torres, L.; Duran, P.; Rojas, M.; Manzano, A.; Garrido, B.; Salazar, J.; Silva, A.; Rojas-Gomez, D.M.; et al. Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy? Int. J. Mol. Sci. 2021, 22, 10370. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Domenichiello, A.F.; Dey, A.K.; Yuan, Z.-X.; Goyal, A.; Rose, S.M.; Playford, M.P.; Ramsden, C.E.; Mehta, N.N. Bioactive Lipid Mediator Profiles in Human Psoriasis Skin and Blood. J. Investig. Derm. 2018, 138, 1518–1528. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.H. Prostaglandin E2 Induces Skin Aging via E-Prostanoid 1 in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5555. [Google Scholar] [CrossRef] [Green Version]
- Saito-Sasaki, N.; Sawada, Y.; Nakamura, M. Maresin-1 and Inflammatory Disease. Int. J. Mol. Sci. 2022, 23, 1367. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Wang, W.; Xu, R.-L.; He, P.; Chen, R. MAR1 suppresses inflammatory response in LPS-induced RAW 264.7 macrophages and human primary peripheral blood mononuclear cells via the SIRT1/PGC-1α/PPAR-γ pathway. J. Inflamm. 2021, 18, 8. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [Green Version]
- Sasaki-Saito, N.; Sawada, Y.; Nakamura, M. 954 Maresin-1 inhibits IL-23 receptors via down-regulation of RORt expression and internalization in an imiquimod-induced psoriasis model mouse. J. Investig. Derm. 2018, 138, S162. [Google Scholar] [CrossRef]
- Abdulnour, R.-E.E.; Dalli, J.; Colby, J.K.; Krishnamoorthy, N.; Timmons, J.Y.; Tan, S.H.; Colas, R.A.; Petasis, N.A.; Serhan, C.N.; Levy, B.D. Maresin 1 biosynthesis during platelet–neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA 2014, 111, 16526–16531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito-Sasaki, N.; Sawada, Y.; Mashima, E.; Yamaguchi, T.; Ohmori, S.; Yoshioka, H.; Haruyama, S.; Okada, E.; Nakamura, M. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci. Rep. 2018, 8, 5522. [Google Scholar] [CrossRef] [PubMed]
- Fania, L.; Morelli, M.; Scarponi, C.; Mercurio, L.; Scopelliti, F.; Cattani, C.; Scaglione, G.L.; Tonanzi, T.; Pilla, M.A.; Pagnanelli, G.; et al. Paradoxical psoriasis induced by TNF-α blockade shows immunological features typical of the early phase of psoriasis development. J. Pathol. Clin. Res. 2020, 6, 55–68. [Google Scholar] [CrossRef]
- Yunanto, K.A.; Waspodo, N.N.; Tabri, F.; Ilyas, F. Obesity as a comorbid factor in a boy with psoriasis vulgaris. Enfermería Clínica 2020, 30, 259–262. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Hugh, J.M.; Weinberg, J.M. Update on the pathophysiology of psoriasis. Cutis 2018, 102, 6–12. [Google Scholar]
- Grän, F.; Kerstan, A.; Serfling, E.; Goebeler, M.; Muhammad, K. Current Developments in the Immunology of Psoriasis. Yale J. Biol. Med. 2020, 93, 97–110. [Google Scholar]
- Hwang, J.; Yoo, J.A.; Yoon, H.; Han, T.; Yoon, J.; An, S.; Cho, J.Y.; Lee, J. The Role of Leptin in the Association between Obesity and Psoriasis. Biomol. Ther. 2021, 29, 11–21. [Google Scholar] [CrossRef]
- Thomas, J.; Küpper, M.; Batra, R.; Jargosch, M.; Atenhan, A.; Baghin, V.; Krause, L.; Lauffer, F.; Biedermann, T.; Theis, F.J.; et al. Is the humoral immunity dispensable for the pathogenesis of psoriasis? J. Eur. Acad. Derm. Venereol. 2019, 33, 115–122. [Google Scholar] [CrossRef]
- Kyriakou, A.; Patsatsi, A.; Sotiriadis, D.; Goulis, D.G. Serum Leptin, Resistin, and Adiponectin Concentrations in Psoriasis: A Meta-Analysis of Observational Studies. Dermatology 2017, 233, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.; Takeshita, J.; Mehta, N.N.; Kimmel, S.E.; Ogdie, A.; Margolis, D.J.; Shin, D.B.; Attor, R.; Troxel, A.B.; Gelfand, J.M. Psoriasis Severity and the Prevalence of Major Medical Comorbidity: A Population-Based Study. JAMA Derm. 2013, 149, 1173–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Syrimi, Z.; Hughes, D.M.; Zhao, S.S. Comorbidities in psoriatic arthritis: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hile, G.; Kahlenberg, J.M.; Gudjonsson, J.E. Recent genetic advances in innate immunity of psoriatic arthritis. Clin. Immunol. 2020, 214, 108405. [Google Scholar] [CrossRef]
- Ben Abdallah, H.; Johansen, C.; Iversen, L. Key Signaling Pathways in Psoriasis: Recent Insights from Antipsoriatic Therapeutics. Psoriasis Targets Ther. 2021, 11, 83–97. [Google Scholar] [CrossRef]
- Storer, M.A.; Danesh, M.J.; Sandhu, M.E.; Pascoe, V.; Kimball, A.B. An assessment of the relative impact of hidradenitis suppurativa, psoriasis, and obesity on quality of life. Int. J. Womens Dermatol. 2018, 4, 198–202. [Google Scholar] [CrossRef]
- Salihbegovic, E.M.; Kurtalic, S.; Omerkic, E. Comorbidity in Men with Psoriasis. Med. Arch. 2021, 75, 31–34. [Google Scholar] [CrossRef]
- Higgins, E. Psoriasis. Medicine 2021, 49, 361–369. [Google Scholar] [CrossRef]
- Shi, L.-Q.; Lian, N.; Sun, J.-T.; Liu, L.-H.; Chen, M. Association between the systemic treatment of psoriasis and cardiovascular risk. Chin. Med. J. 2021, 134, 518–520. [Google Scholar] [CrossRef]
- Jacobi, A.; Langenbruch, A.; Purwins, S.; Augustin, M.; Radtke, M.A. Prevalence of Obesity in Patients with Psoriasis: Results of the National Study PsoHealth3. Dermatology 2015, 231, 231–238. [Google Scholar] [CrossRef]
- Shah, S.; Nikam, B.; Kale, M.; Jamale, V.; Chavan, D. Safety and efficacy profile of oral cyclosporine vs oral methotrexate vs oral acitretin in palmoplantar psoriasis: A hospital based prospective investigator blind randomized controlled comparative study. Dermatol. Ther. 2021, 34, e14650. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef]
- Mylonas, A.; Conrad, C. Psoriasis: Classical vs. Paradoxical. The Yin-Yang of TNF and Type I Interferon. Front. Immunol. 2018, 9, 2746. [Google Scholar] [CrossRef]
- Le, S.T.; Toussi, A.; Maverakis, N.; Marusina, A.I.; Barton, V.R.; Merleev, A.A.; Luxardi, G.; Raychaudhuri, S.P.; Maverakis, E. The cutaneous and intestinal microbiome in psoriatic disease. Clin. Immunol. 2020, 218, 108537. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, Y.; Liao, Y.; Shi, Y.; Zhang, L. Emerging Roles of Adipose Tissue in the Pathogenesis of Psoriasis and Atopic Dermatitis in Obesity. JID Innov. 2022, 2, 100064. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Fung, M.A.; Chen, H.-L. Role of skin and gut microbiota in the pathogenesis of psoriasis, an inflammatory skin disease. Med. Microecol. 2020, 4, 100016. [Google Scholar] [CrossRef]
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis—Probiotics as new tools for skin disorder management? Exp. Derm. 2019, 28, 1210–1218. [Google Scholar] [CrossRef] [Green Version]
- Thye, A.Y.-K.; Bah, Y.-R.; Law, J.W.-F.; Tan, L.T.-H.; He, Y.-W.; Wong, S.-H.; Thurairajasingam, S.; Chan, K.-G.; Lee, L.-H.; Letchumanan, V. Gut–Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, C.-S.; Chao, Y.-H.; Lin, C.-C.; Tsai, H.-Y.; Li, Y.-R.; Chen, Y.-Z.; Tsai, W.-H.; Chen, Y.-K. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J. Food Drug Anal. 2017, 25, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Thio, H.B. The Microbiome in Psoriasis and Psoriatic Arthritis: The Skin Perspective. J. Rheumatol. 2018, 94, 30–31. [Google Scholar] [CrossRef]
- Vijayashankar, M.; Raghunath, N. Pustular psoriasis responding to Probiotics—A new insight. Our Derm. Online 2012, 3, 326–329. [Google Scholar] [CrossRef]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Bifidobacterium infantis 35,624 modulates host inflammatory processes beyond the gut. Gut. Microbes. 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Zeng, T.; Deng, Y.; Yang, W.; Xiong, J. [Treatment of psoriasis vulgaris using Bacteroides fragilis BF839: A single-arm, open preliminary clinical study]. Sheng Wu Gong Cheng Xue Bao 2021, 37, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Carrara, M.; Kelly, M.T.; Roso, F.; Larroque, M.; Margout, D. Potential of Olive Oil Mill Wastewater as a Source of Polyphenols for the Treatment of Skin Disorders: A Review. J. Agric. Food Chem. 2021, 69, 7268–7284. [Google Scholar] [CrossRef] [PubMed]
- Medina, M. Tratamiento Alternativo al Uso Crónico de Esteroides e Inmunosupresores en Psoriasis Mediante Polifenoles Aislados del Aceite Del Oliva. Ph.D. Thesis, Universidad del Zulia, Maracaibo, Venezuela.
- Medina, M.; Cano, R.; Contreras, I.; Prado, A.; Cano, C. Estudio comparativo de la eficiencia terapéutica entre imiquimod crema al 5% y una loción basada en polifenoles en el tratamiento de la queratosis actínica. Arch. Venez. Farmacol. Ter. 2015, 34, 16–20. [Google Scholar]
- Yan, D.; Blauvelt, A.; Dey, A.K.; Golpanian, R.S.; Hwang, S.T.; Mehta, N.N.; Myers, B.; Shi, Z.; Yosipovitch, G.; Bell, S.; et al. New Frontiers in Psoriatic Disease Research, Part II: Comorbidities and Targeted Therapies. J. Investig. Dermatol. 2021, 141, 2328–2337. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Pirro, F.; Caldarola, G.; Chiricozzi, A.; Burlando, M.; Mariani, M.; Parodi, A.; Peris, K.; De Simone, C. Impact of Body Mass Index on the Efficacy of Biological Therapies in Patients with Psoriasis: A Real-World Study. Clin. Drug Investig. 2021, 41, 917–925. [Google Scholar] [CrossRef]
- Su, X.; Zhang, G.; Cheng, Y.; Wang, B. Leptin in skin disease modulation. Clin. Chim. Acta 2021, 516, 8–14. [Google Scholar] [CrossRef]
- Madden, S.K.; Flanagan, K.L.; Jones, G. How lifestyle factors and their associated pathogenetic mechanisms impact psoriasis. Clin. Nutr. 2020, 39, 1026–1040. [Google Scholar] [CrossRef]
- Villarreal-Calderón, J.R.; Cuéllar, R.X.; Ramos-González, M.R.; Rubio-Infante, N.; Castillo, E.C.; Elizondo-Montemayor, L.; García-Rivas, G. Interplay between the Adaptive Immune System and Insulin Resistance in Weight Loss Induced by Bariatric Surgery. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maglio, C.; Peltonen, M.; Rudin, A.; Carlsson, L.M.S. Bariatric Surgery and the Incidence of Psoriasis and Psoriatic Arthritis in the Swedish Obese Subjects Study: Effect of Bariatric Surgery on Psoriasis. Obesity 2017, 25, 2068–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeberg, A.; Sørensen, J.A.; Gislason, G.H.; Knop, F.K.; Skov, L. Incidence and Prognosis of Psoriasis and Psoriatic Arthritis in Patients Undergoing Bariatric Surgery. JAMA Surg. 2017, 152, 344. [Google Scholar] [CrossRef] [PubMed]
- Farias, M.M.; Achurra, P.; Boza, C.; Vega, A.; de la Cruz, C. Psoriasis following bariatric surgery: Clinical evolution and impact on quality of life on 10 patients. Obes. Surg. 2012, 22, 877–880. [Google Scholar] [CrossRef]
- Alruwaili, H.; Dehestani, B.; le Roux, C.W. Clinical Impact of Liraglutide as a Treatment of Obesity. CPAA 2021, 13, 53–60. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Zobel, E.H.; Ripa, R.S.; von Scholten, B.J.; Rotbain Curovic, V.; Kjaer, A.; Hansen, T.W.; Rossing, P.; Størling, J. Effect of liraglutide on expression of inflammatory genes in type 2 diabetes. Sci. Rep. 2021, 11, 18522. [Google Scholar] [CrossRef]
- Chang, G.; Chen, B.; Zhang, L. Efficacy of GLP-1rA, liraglutide, in plaque psoriasis treatment with type 2 diabetes: A systematic review and meta-analysis of prospective cohort and before-after studies. J. Dermatol. Treat. 2022, 33, 1299–1305. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Chen, P.; Yu, Y.; Chen, S.; Chen, X.; Shao, Z. Treatment with liraglutide, a glucagon-like peptide-1 analogue, improves effectively the skin lesions of psoriasis patients with type 2 diabetes: A prospective cohort study. Diabetes Res. Clin. Pract. 2019, 150, 167–173. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.; Yu, Y.; Ye, H.; He, X.; Chen, S.; Chen, X.; Shao, Z.; Chen, P. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: A randomized-controlled trial. J. Dermatol. Treat. 2022, 33, 1428–1434. [Google Scholar] [CrossRef]
- Costanzo, G.; Curatolo, S.; Busà, B.; Belfiore, A.; Gullo, D. Two birds one stone: Semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
| Author (REF). | Methodology | Relevant Results |
|---|---|---|
| Norden et al. [58] | A prospective cohort analysis which evaluated the incidence of psoriasis according to BMI, in a sample of more than 1.5 million patients in the United States, over a period between 1 January 2008, through 9 September 2019 | The crude incidence of psoriasis per 10,000 person-years was 9.5% (95% CI, 9.1–10.0) in normal weight patients, 11.9 (95% CI, 11.4–12.4) in overweight, 14.2 (95% CI, 13.6–14.9) in obese class 1 patients, and 17.4 (95% CI, 16.6–18.2) among obese class 2/3 patients. |
| Setty et al. [62] | A prospective longitudinal 14 years study assessed the relationship between BMI, WC, HC, and psoriasis incidence in 78,626 nurses aged 25 to 42 years in the USA with a biannual follow-up. | A total of 892 new cases of psoriasis were collected; the incidence rate was 82 per 100,000 person-years. The multivariate RRs for psoriasis were 1.40 (95% CI, 1.13–1.73) with BMI 25.0–29.9; 1.48 (95% CI, 1.15–1.91) BMI 30.0–34.9; and 2.69 (95% CI, 2.12–3.40) for BMI 35.0 or greater (p < 0.001). |
| Castaldo et al. [48] | Open-label, single-arm, clinical trial, in 37 adult patients, overweight or obese, with stable chronic plaque psoriasis, without previous treatment. Patients underwent a WL program, through a protein-sparing VLCKD for four weeks and subsequently a Mediterranean-type, hypocaloric diet for six weeks. | The diet produced a reduction in the mean body weight of 12% (−10.6 kg), as well as a significant reduction in the mean PASI score of −10.6 (95% CI, −12.8 to −8.4; p < 0.001). 97% (n = 36) and 64.9% (n = 24) of patients recorded a PASI score response ≥50% and ≥75%, respectively. In addition, a reduction in the DLQI score of −13.4 points was observed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, G.; Duran, P.; Vera, I.; Bermúdez, V. Exploring the Links between Obesity and Psoriasis: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 7499. https://doi.org/10.3390/ijms23147499
Barros G, Duran P, Vera I, Bermúdez V. Exploring the Links between Obesity and Psoriasis: A Comprehensive Review. International Journal of Molecular Sciences. 2022; 23(14):7499. https://doi.org/10.3390/ijms23147499
Chicago/Turabian StyleBarros, Gabriela, Pablo Duran, Ivana Vera, and Valmore Bermúdez. 2022. "Exploring the Links between Obesity and Psoriasis: A Comprehensive Review" International Journal of Molecular Sciences 23, no. 14: 7499. https://doi.org/10.3390/ijms23147499
APA StyleBarros, G., Duran, P., Vera, I., & Bermúdez, V. (2022). Exploring the Links between Obesity and Psoriasis: A Comprehensive Review. International Journal of Molecular Sciences, 23(14), 7499. https://doi.org/10.3390/ijms23147499






