Sperm of Fruit Fly Drosophila melanogaster under Space Flight
Abstract
1. Introduction
2. Results
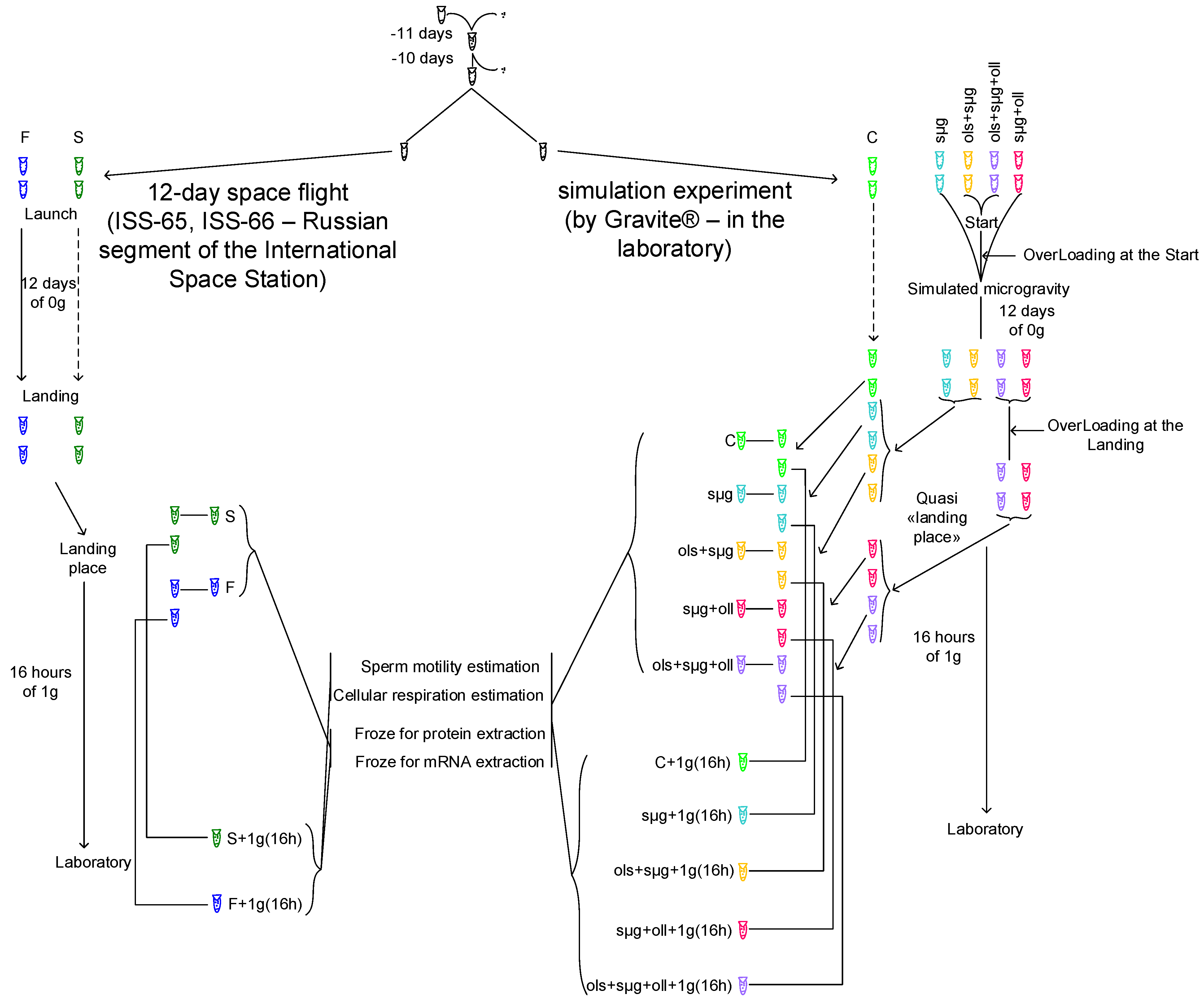
2.1. Experimental Design
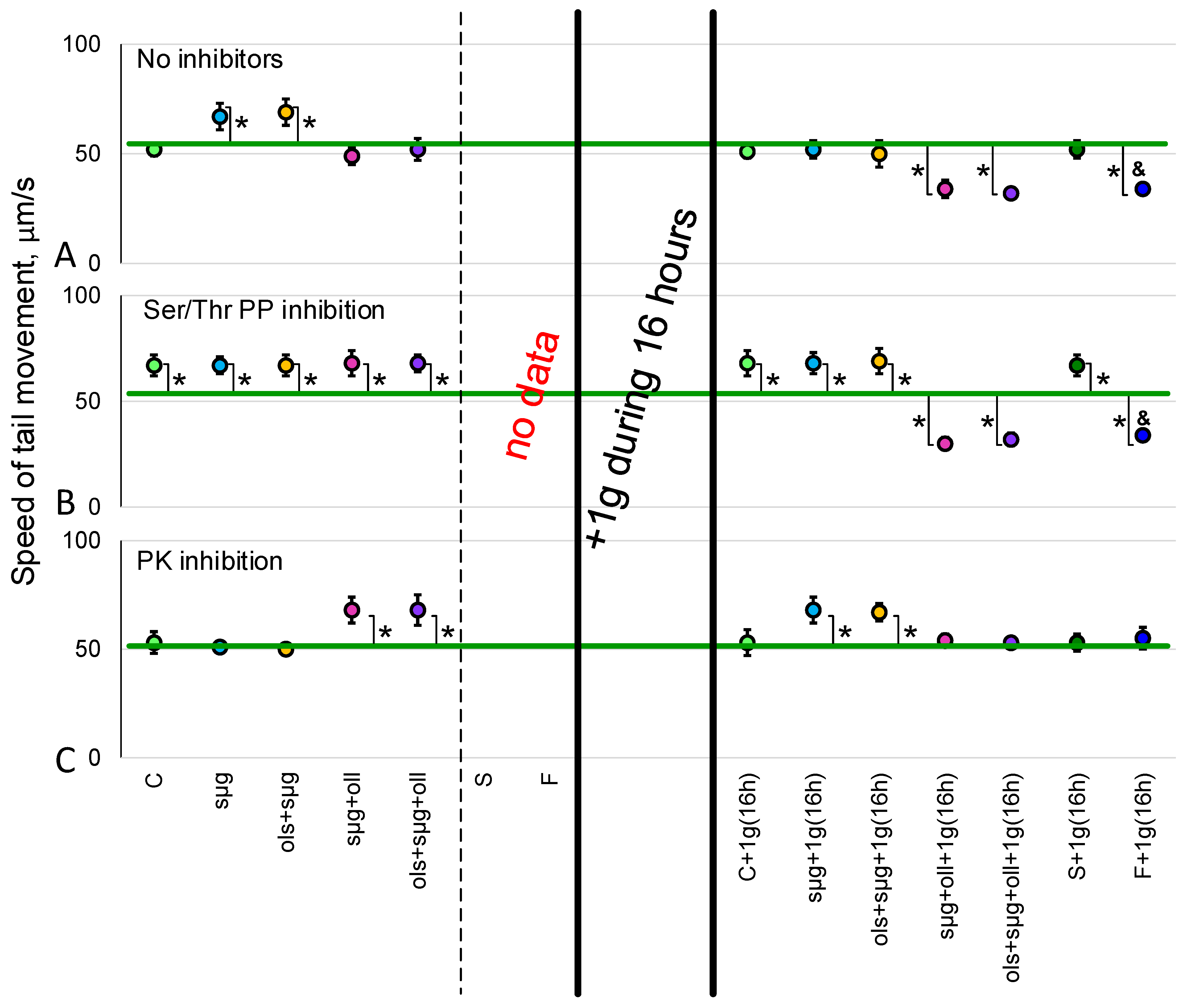
2.2. Sperm Motility
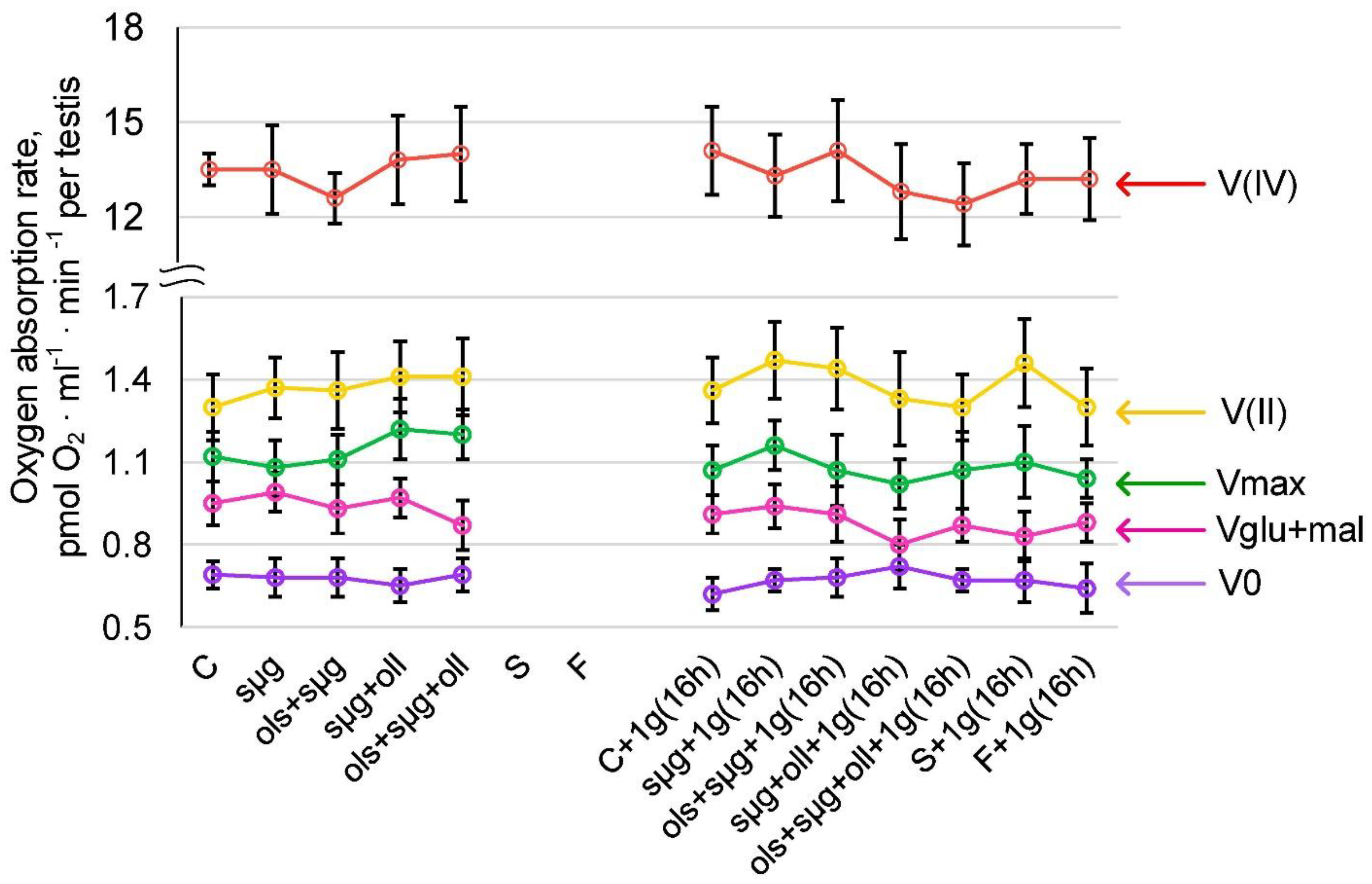
2.3. Cellular Respiration and the Content of Proteins Involved in It
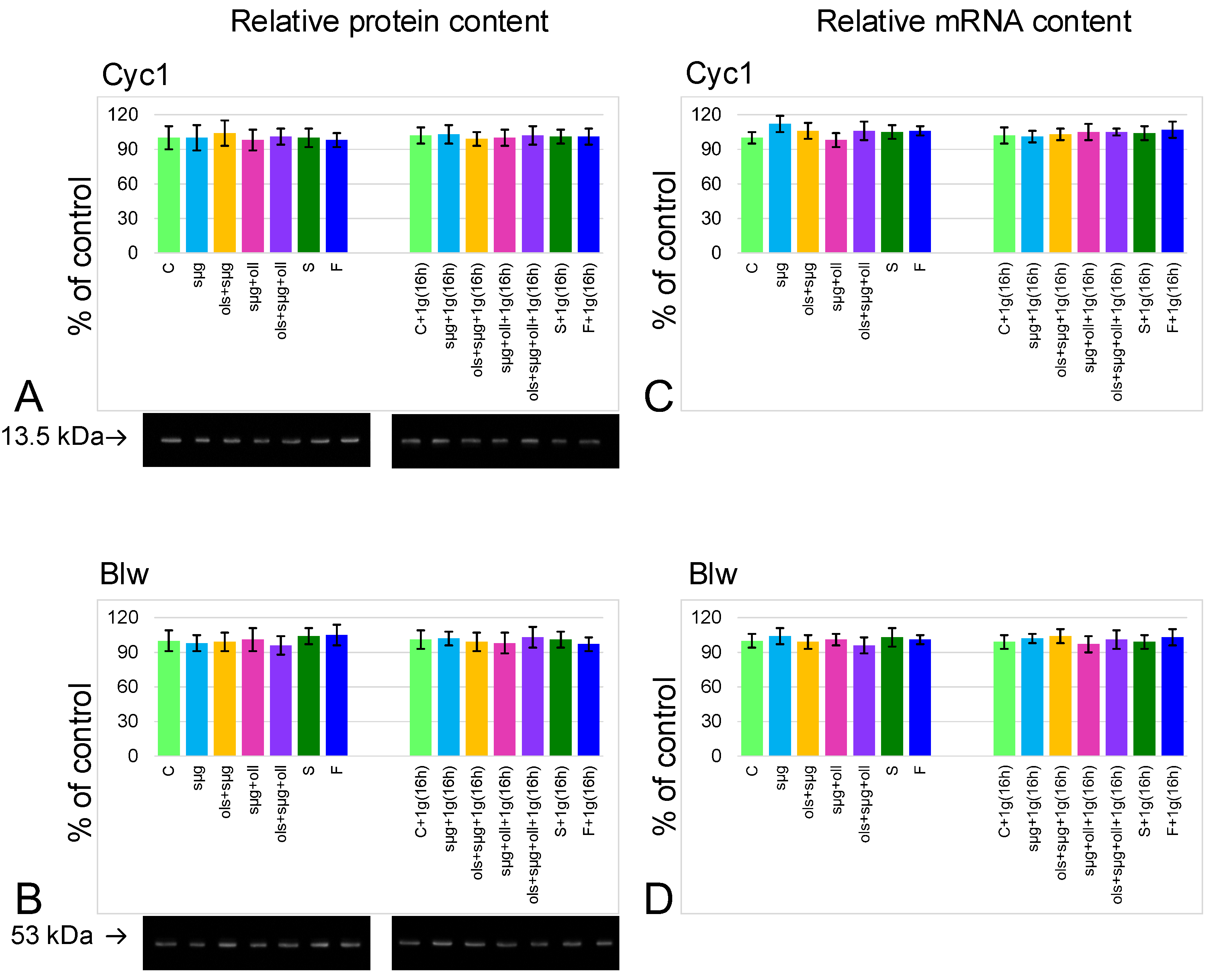
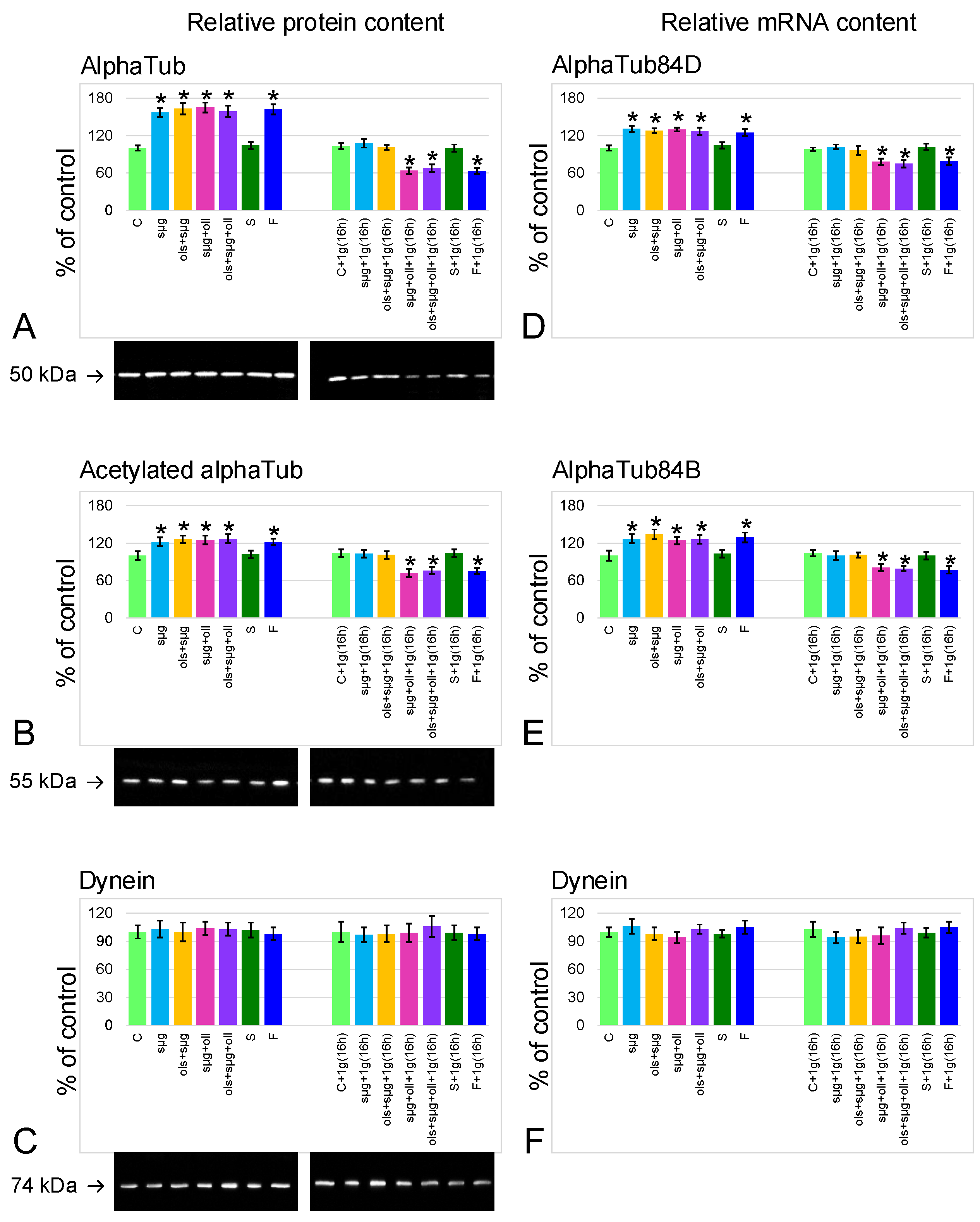
2.4. Relative Content of the Main Cytoskeletal Proteins and mRNA of the Corresponding Genes
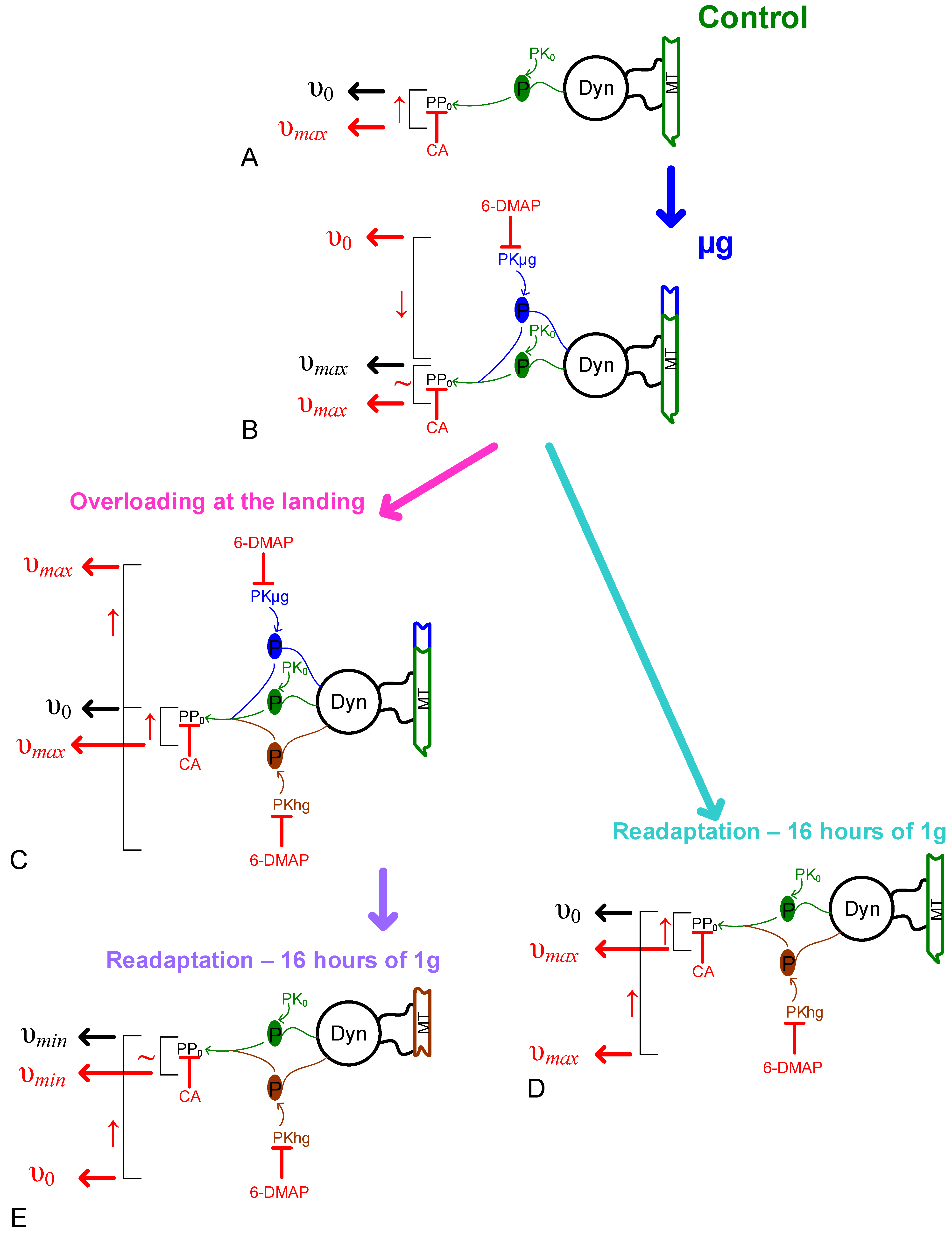
3. Discussion
4. Materials and Methods
4.1. Determination of the Parameters of the Sperm Motility
4.2. Determination of Cellular Respiration Parameters
4.3. Determination of the Relative Content of Proteins
4.4. Determination of Relative mRNA Content
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parfyonov, G.P.; Platonova, R.N.; Tairbekov, M.G.; Zhvalikovskaya, V.P.; Mozgovaya, I.E.; Rostopshina, A.V.; Rozov, A.N. Biological experiments carried out aboard the biological satellite Cosmos-936. Life Sci. Space Res. 1979, 17, 297–299. [Google Scholar] [PubMed]
- Parfenov, G.P. Development of organisms in a state of weightlessness. Kosm. Issled. 1964, 2, 330–335. [Google Scholar]
- Vernos, I.; Gonzalez-Jurado, J.; Calleja, M.; Marco, R. Microgravity effects on the oogenesis and development of embryos of Drosophila melanogaster laid in the Spaceshuttle during the Biorack experiment (ESA). Int. J. Dev. Biol. 1989, 33, 213–226. [Google Scholar] [PubMed]
- Marco, R.; Bengura, A.; Sanchez, J.; de Juan, E. Effects of the space environment on Drosophila melanogaster development. Implications of the IML-2 experiment. J. Biotechnol. 1996, 47, 179–189. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Belyakin, S.N.; Sarantseva, S.V. The development of drosophila melanogaster under different duration space flight and subsequent adaptation to Earth gravity. PLoS ONE 2016, 11, e0166885. [Google Scholar] [CrossRef]
- Kupriyanova, M.S.; Usik, M.A.; Ogneva, I.V. Cytoskeletal proteins content in the oocytes of the third generation of the fruit fly Drosophila melanogaster, obtained after 44.5-day space flight. Aviakosmicheskaia Ekol. Meditsina 2016, 50, 27–34. [Google Scholar]
- Parfenov, G.P. Appearance of dominant lethal mutations in Drosophila during space flight on the satellite. Iskusstv. Sput. Zemli 1961, 10, 69–71. [Google Scholar]
- Uva, B.M.; Masini, M.A.; Sturla, M.; Prato, P.; Passalacqua, M.; Giuliani, M.; Tagliafierro, G.; Strollo, F. Clinorotation-induced weightlessness influences the cytoskeleton of glial cells in culture. Brain Res. 2002, 934, 132–139. [Google Scholar] [CrossRef]
- Plett, P.A.; Abonour, R.; Frankovitz, S.M.; Orschell, C.M. Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Exp. Hematol. 2004, 32, 773–781. [Google Scholar] [CrossRef]
- Crawford-Young, S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2006, 50, 183–191. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Meyers, V.E.; Zayzafoon, M.; Douglas, J.T.; McDonald, J.M. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J. Bone Miner. Res. 2005, 20, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.J.; Liu, W.; Sykes, M.C.; Ward, N.E.; Risin, S.A.; Risin, D.; Jo, H. Identification of mechanosensitive genes in osteoblasts by comparative microarray studies using the rotating wall vessel and the random positioning machine. J. Cell Biochem. 2007, 101, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Sordella, R.; Jiang, W.; Chen, G.C.; Curto, M.; Settleman, J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 2003, 113, 147–158. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Serova, L.V.; Denisova, L.A. The effect of weightlessness on the reproductive function of mammals. Physiologist 1982, 25, S9–S12. [Google Scholar]
- Serova, L.V.; Denisova, L.A.; Baikova, O.V. The effect of microgravity on the reproductive function of male-rats. Physiologist 1989, 32 (Suppl. 1), S29–S30. [Google Scholar]
- Tash, J.S.; Johnson, D.C.; Enders, G.C. Long-term (6-wk) hindlimb suspension inhibits spermatogenesis in adult male rats. J. Appl. Physiol. 1985, 92, 1191–1198. [Google Scholar] [CrossRef]
- Usik, M.A.; Ogneva, I.V. Cytoskeleton Structure in Mouse Sperm and Testes after 30 Days of Hindlimb Unloading and 12 Hours of Recovery. Cell Physiol. Biochem. 2018, 51, 375–392. [Google Scholar] [CrossRef]
- Loktev, S.S.; Ogneva, I.V. DNA methylation of mouse testes, cardiac and lung tissue during long-term microgravity simulation. Sci. Rep. 2019, 9, 7974. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Usik, M.A.; Loktev, S.S.; Zhdankina, Y.U.S.; Biryukov, N.S.; Orlov, O.I.; Sychev, V.N. Testes and duct deferens of mice during space flight: Cytoskeleton structure, sperm-specific proteins and epigenetic events. Sci. Rep. 2019, 9, 9730. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V.; Usik, M.A.; Burtseva, M.V.; Biryukov, N.S.; Zhdankina, Y.S.; Sychev, V.N.; Orlov, O.I. Drosophila melanogaster Sperm under Simulated Microgravity and a Hypomagnetic Field: Motility and Cell Respiration. Int. J. Mol. Sci. 2020, 21, 5985. [Google Scholar] [CrossRef] [PubMed]
- Ogneva, I.V. Mouse and fly sperm motility changes differently under modelling microgravity. Curr. Issues Mol. Biol. 2021, 43, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, J.J.W.A. Some history and use of the Random Positioning Machine, RPM, in gravity related research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Tash, J.S.; Bracho, G.E. Microgravity alters protein phosphorylation changes during initiation of sea urchin sperm motility. FASEB J. 1999, 13, 43–54. [Google Scholar] [CrossRef]
- Tash, J.S.; Kim, S.; Schuber, M.; Seibt, D.; Kinsey, W.H. Fertilization of sea urchin eggs and sperm motility are negatively impacted under low hypergravitational forces significant to space flight. Biol. Reprod. 2001, 65, 1224–1231. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Usik, M.A.; Biryukov, N.S.; Zhadnkina, Y.S. Sperm motility of mice under simulated microgravity and hypergravity. Int. J. Mol. Sci. 2020, 21, 5054. [Google Scholar] [CrossRef]
- Ikeuchi, T.; Sasaki, S.; Umemoto, Y.; Kubota, Y.; Kubota, H.; Kaneko, T.; Kohri, K. Human sperm motility in a microgravity environment. Reprod. Med. Biol. 2005, 4, 161–168. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Mirzoev, T.M.; Biryukov, N.S.; Veselova, O.M.; Larina, I.M. Structure and functional characteristics of rat’s left ventricle cardiomyocytes under antiorthostatic suspension of various duration and subsequent reloading. J. Biomed. Biotechnol. 2012, 2012, 659869. [Google Scholar] [CrossRef]
- Nomura, M.; Inaba, K.; Morisawa, M. Cyclic AMP- and calmodulin-dependent phosphorylation of 21 and 26 kda proteins in axoneme is a prerequisite for SAAF-induced motile activation in ascidian spermatozoa. Dev. Growth Differ. 2000, 42, 129–138. [Google Scholar] [CrossRef]
- Bajpai, M.; Asin, S.; Doncel, G.F. Effect of tyrosine kinase inhibitors on tyrosine phosphorylation and motility parameters in human sperm. Arch. Androl. 2003, 49, 229–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luconi, M.; Porazzi, I.; Ferruzzi, P.; Marchiani, S.; Forti, G.; Baldi, E. Tyrosine phosphorylation of the a kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol. Reprod. 2005, 72, 22–32. [Google Scholar] [CrossRef]
- Aparicio, I.M.; Gil, M.C.; Garcia-Herreros, M.; Peña, F.J.; Garcia-Marin, L.J. Inhibition of phosphatidylinositol 3-kinase modifies boar sperm motion parameters. Reproduction 2005, 129, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.M.; Bragado, M.J.; Gil, M.C.; Garcia-Herreros, M.; Gonzalez-Fernandez, L.; Tapia, J.A.; Garcia-Marin, L.J. Porcine sperm motility is regulated by serine phosphorylation of the glycogen synthase kinase-3alpha. Reproduction 2007, 134, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Battistone, M.A.; Da Ros, V.G.; Salicioni, A.M.; Navarrete, F.A.; Krapf, D.; Visconti, P.E.; Cuasnicú, P.S. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol. Hum. Reprod. 2013, 19, 570–580. [Google Scholar] [CrossRef]
- Dudiki, T.; Kadunganattil, S.; Ferrara, J.K.; Kline, D.W.; Vijayaraghavan, S. Changes in Carboxy Methylation and Tyrosine Phosphorylation of Protein Phosphatase PP2A Are Associated with Epididymal Sperm Maturation and Motility. PLoS ONE 2015, 10, e0141961. [Google Scholar] [CrossRef]
- Ishikawa, H.; Thompson, J.; Yates, J.R., 3rd; Marshall, W.F. Proteomic analysis of mammalian primary cilia. Curr. Biol. 2012, 22, 414–419. [Google Scholar] [CrossRef]
- Follit, J.A.; Tuft, R.A.; Fogarty, K.E.; Pazour, G.J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 2006, 17, 3781–3792. [Google Scholar] [CrossRef]
- Besschetnova, T.Y.; Roy, B.; Shah, J.V. Imaging intraflagellar transport in mammalian primary cilia. Methods Cell Biol. 2009, 93, 331–346. [Google Scholar] [CrossRef]
- Luo, W.; Ruba, A.; Takao, D.; Zweifel, L.P.; Lim, R.Y.H.; Verhey, K.J.; Yang, W. Axonemal Lumen Dominates Cytosolic Protein Diffusion inside the Primary Cilium. Sci. Rep. 2017, 7, 15793. [Google Scholar] [CrossRef]
- Rotem, R.; Paz, G.F.; Homonnai, Z.T.; Kalina, M.; Naor, Z. Protein kinase C is present in human sperm: Possible role in flagellar motility. Proc. Natl. Acad. Sci. USA 1990, 87, 7305–7308. [Google Scholar] [CrossRef] [PubMed]
- Ain, R.; Uma Devi, K.; Shivaji, S.; Seshagiri, P.B. Pentoxifylline-stimulated capacitation and acrosome reaction in hamster spermatozoa: Involvement of intracellular signalling molecules. Mol. Hum. Reprod. 1999, 5, 618–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devi, K.U.; Jha, K.; Shivaji, S. Plasma membrane-associated protein tyrosine phosphatase activity in hamster spermatozoa. Mol. Reprod. Dev. 1999, 53, 42–50. [Google Scholar] [CrossRef]
- González-Fernández, L.; Ortega-Ferrusola, C.; Macias-Garcia, B.; Salido, G.M.; Peña, F.J.; Tapia, J.A. Identification of protein tyrosine phosphatases and dual-specificity phosphatases in mammalian spermatozoa and their role in sperm motility and protein tyrosine phosphorylation. Biol. Reprod. 2009, 80, 1239–1252. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Stephens, D.T.; Trautman, K.; Smith, G.D.; Khatra, B.; da Cruz e Silva, E.F.; Greengard, P. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol. Reprod. 1996, 54, 709–718. [Google Scholar] [CrossRef]
- Allan, V. Protein phosphatase 1 regulates the cytoplasmic dynein-driven formation of endoplasmic reticulum networks in vitro. J. Cell Biol. 1995, 128, 879–891. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Vaksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef]
- Towbin, H.; Staehlin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some application. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 2, 402–408. [Google Scholar] [CrossRef]
| C | sµg | ols+sµg | sµg+oll | ols+sµg+oll | S | F | |
|---|---|---|---|---|---|---|---|
| no inhibitors | 1.54 ± 0.15 | 1.48 ± 0.10 | 1.51 ± 0.12 | 1.46 ± 0.13 | 1.50 ± 0.14 | no data | no data |
| 200 µM Na3VO4 | 0 | 0 | 0 | 0 | 0 | no data | no data |
| 20 nM CA | 1.49 ± 0.16 | 1.49 ± 0.11 | 1.51 ± 0.17 | 1.49 ± 0.16 | 1.51 ± 0.11 | no data | no data |
| 0.5 mM 6-DMAP | 1.56 ± 0.11 | 1.54 ± 0.09 | 1.51 ± 0.11 | 1.54 ± 0.13 | 1.59 ± 0.09 | no data | no data |
| C +1g(16h) | sµg +1g(16h) | ols+sµg +1g(16h) | sµg+oll +1g(16h) | ols+sµg+oll +1g(16h) | S +1g(16h) | F +1g(16h) | |
| no inhibitors | 1.48 ± 0.13 | 1.47 ± 0.13 | 1.50 ± 0.15 | 1.50 ± 0.11 | 1.48 ± 0.13 | 1.57 ± 0.11 | 1.54 ± 0.08 |
| 200 µM Na3VO4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 nM CA | 1.49 ± 0.15 | 1.47 ± 0.17 | 1.52 ± 0.16 | 1.54 ± 0.13 | 1.53 ± 0.16 | 1.48 ± 0.13 | 1.49 ± 0.13 |
| 0.5 mM 6-DMAP | 1.56 ± 0.12 | 1.52 ± 0.17 | 1.59 ± 0.12 | 1.54 ± 0.11 | 1.58 ± 0.09 | 1.57 ± 0.10 | 1.58 ± 0.12 |
| Protein | Manufacturer with Catalog Number, Dilution |
|---|---|
| cyt c-1 (cytochrome c-1, 13.5 kDa) | Abcam, Cambridge, UK, #ab13575, 1 µg/mL |
| blw (bellwether, ATP synthase mitochondrial F1 complex, alpha subunit 1, 53 kDa) | Abcam, Cambridge, UK, #ab14748, 1 µg/mL |
| aTub (alpha-tubulin, 50 kDa) | Abcam, Cambridge, UK, #ab52866, 1:10,000 |
| acet-aTub (acetylated alpha-tubulin, 55 kDa) | Santa Cruz Biotechnology, Inc., Dallas, TX, USA, #sc-23950, 1:100–1:1000 |
| dynein (axonemal, intermediate chain 1, 74 kDa) | Invitrogen by Thermo Fisher Scientific, Waltham, MA, USA, eBioscienceTM #14-9772-80, 5 µg/mL |
| Gene | Primer Sequence, Forward/Reverse (5′…3′) | Product Size, bp |
|---|---|---|
| cyt c-1 | gcagcgacattgcgaagatt/actgctccagggcgtagata | 181 |
| blw | aataggagtagcggtgcgtg/aaccacggattgaaggcgat | 201 |
| alphaTub84D | aaggactacgaggaggtcgg/atgcgagtgggagcgtatga | 124 |
| alphaTub84B | cactggtacgttggtgaggg/cccatcgagcgttgaagtgg | 166 |
| dynein | agcaggtgaagggaaaaagg/accacgagattgcgaacgat | 208 |
| gapdh | atactcatcaaccctccccc/ggctgagttcctgctgtctt | 142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogneva, I.V.; Zhdankina, Y.S.; Kotov, O.V. Sperm of Fruit Fly Drosophila melanogaster under Space Flight. Int. J. Mol. Sci. 2022, 23, 7498. https://doi.org/10.3390/ijms23147498
Ogneva IV, Zhdankina YS, Kotov OV. Sperm of Fruit Fly Drosophila melanogaster under Space Flight. International Journal of Molecular Sciences. 2022; 23(14):7498. https://doi.org/10.3390/ijms23147498
Chicago/Turabian StyleOgneva, Irina V., Yulia S. Zhdankina, and Oleg V. Kotov. 2022. "Sperm of Fruit Fly Drosophila melanogaster under Space Flight" International Journal of Molecular Sciences 23, no. 14: 7498. https://doi.org/10.3390/ijms23147498
APA StyleOgneva, I. V., Zhdankina, Y. S., & Kotov, O. V. (2022). Sperm of Fruit Fly Drosophila melanogaster under Space Flight. International Journal of Molecular Sciences, 23(14), 7498. https://doi.org/10.3390/ijms23147498






