Engineering a 3D In Vitro Model of Human Gingival Tissue Equivalent with Genipin/Cytochalasin D
Abstract
:1. Introduction
2. Results
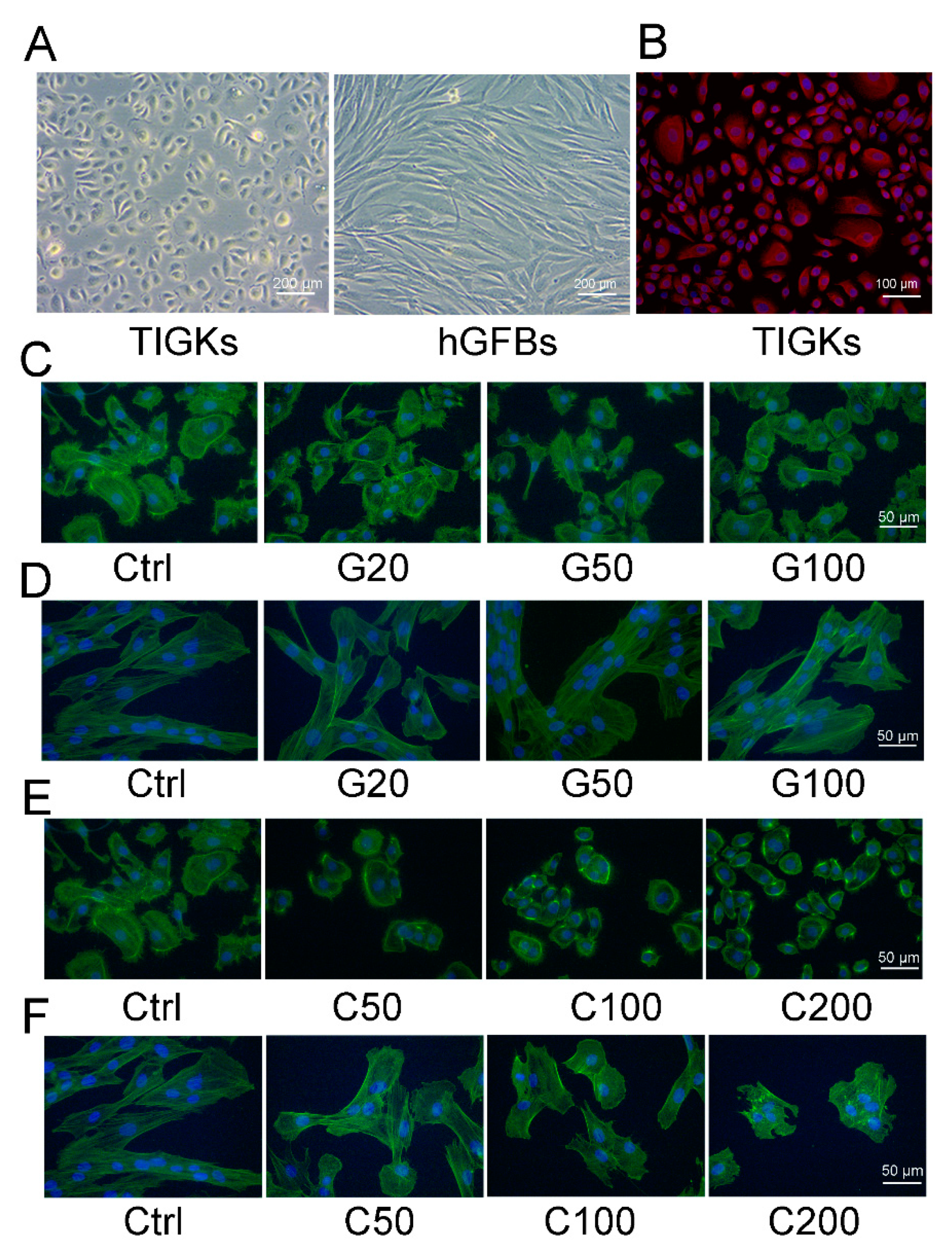
2.1. Determination of Noncytotoxic Concentrations of Genipin or Cytochalasin D to the TIGKs and the hGFBs Cultured in 2D Monolayer Cultures
2.1.1. Evaluation of Cellular Metabolic Activity of the TIGKs and the hGFBs
2.1.2. Morphological Evaluation of the TIGKs and the hGFBs Cultured in the 2D Monolayer Culture
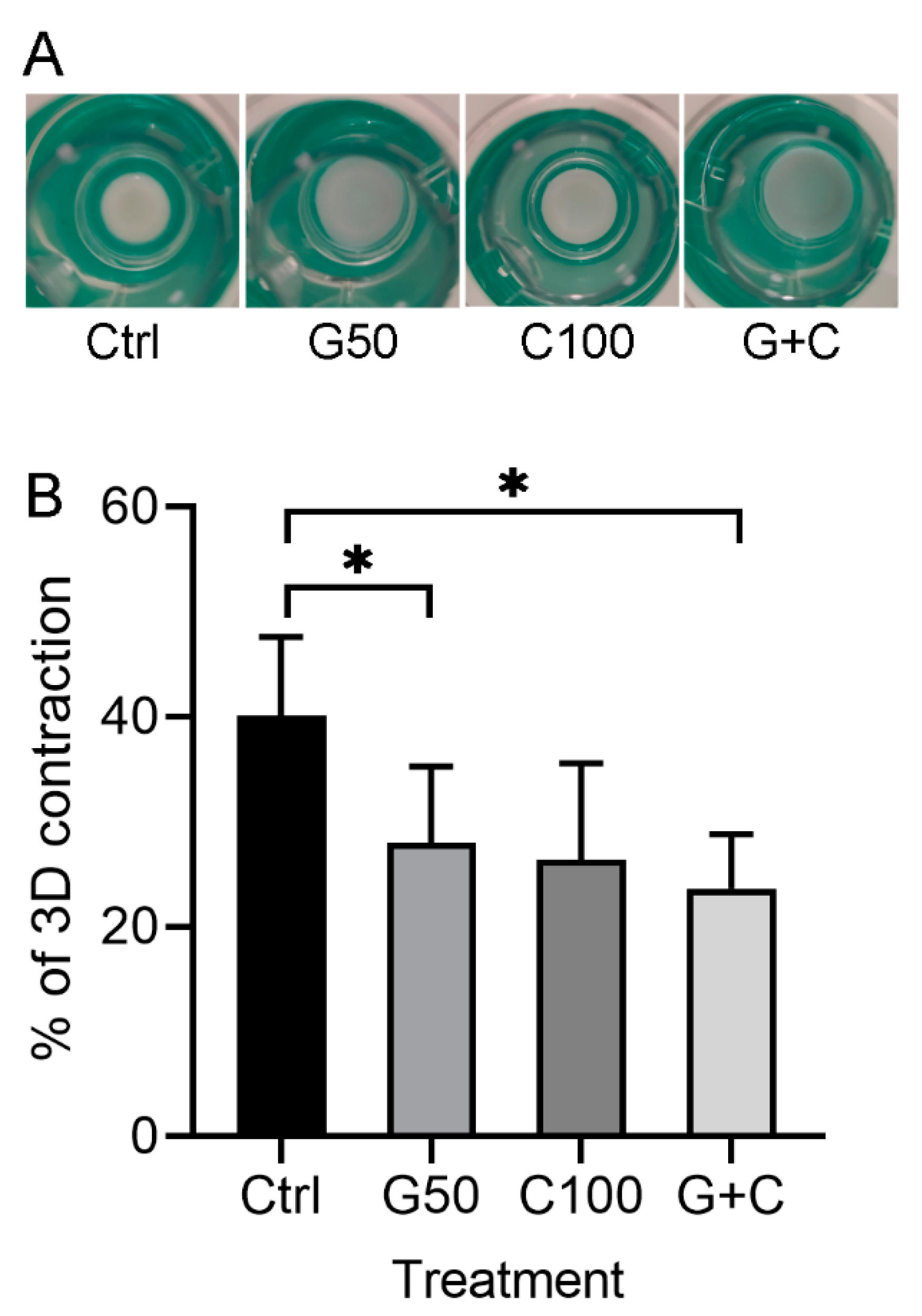
2.2. Effects of Genipin or/and Cytochalasin D on 3D Gingival Tissue Equivalents
2.2.1. Positive Effects of Genipin or Combination of Genipin and Cytochalasin D on the Contraction of the 3D Gingival Tissue Equivalent
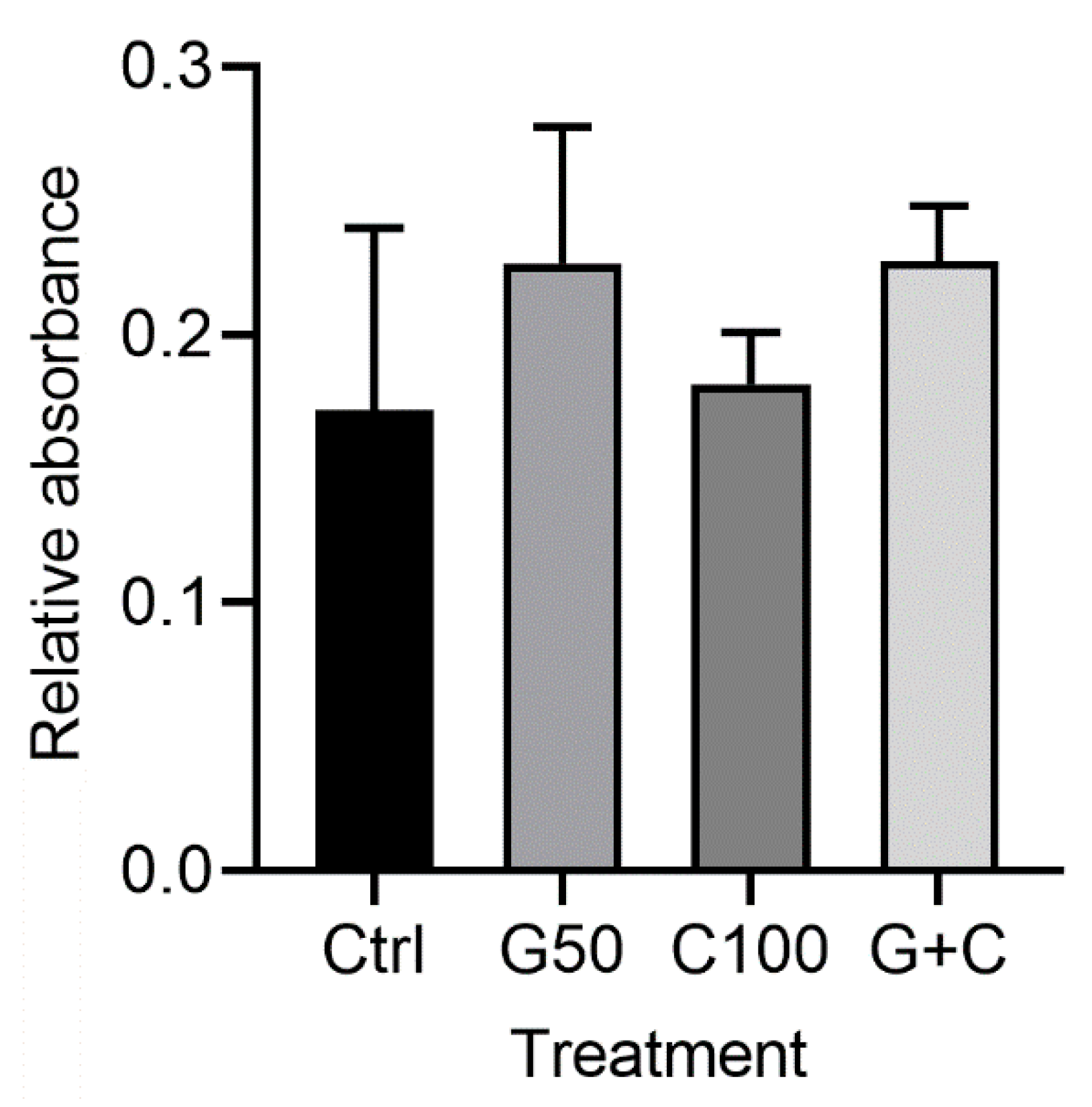
2.2.2. Effects of Genipin or/and Cytochalasin D on Cellular Metabolic Activity in the 3D Gingival Tissue Equivalents
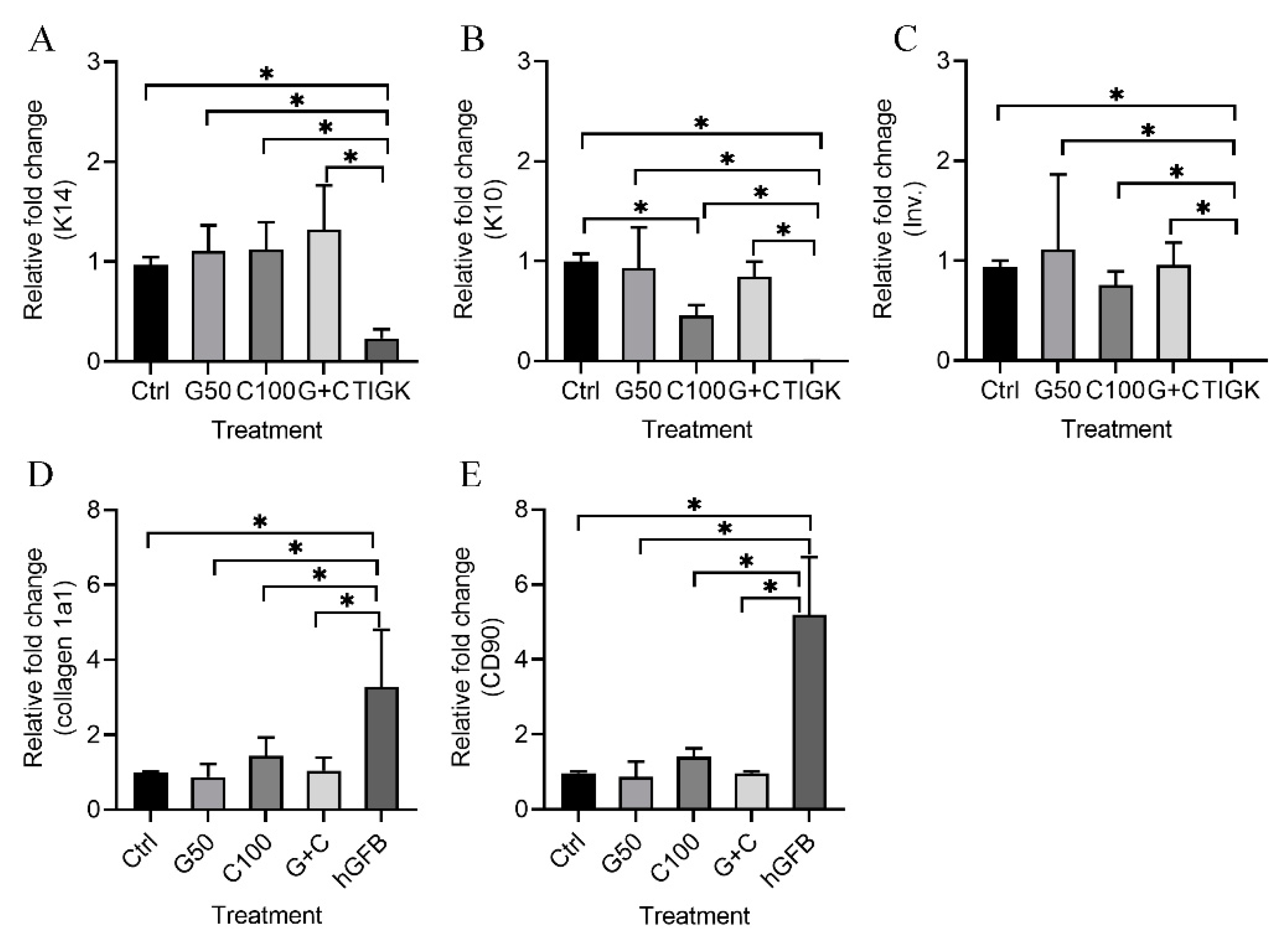
2.2.3. mRNA Expression of Keratinocyte and Fibroblast Genes in the 3D Gingival Tissue Equivalents
2.2.4. Histological Evaluation of the 3D Gingival Tissue Equivalents
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Detection of Genipin/Cytochalasin D Toxicity on the TIGKs and the hGFBs in the 2D Monolayer Culture
4.3. Actin Filament Staining of the TIGKs or the hGFBs in the 2D Monolayer Culture
4.4. Immunocytochemical Staining of the TIGKs/hGFBs with Ki-67 and p53 in the 2D Monolayer Culture
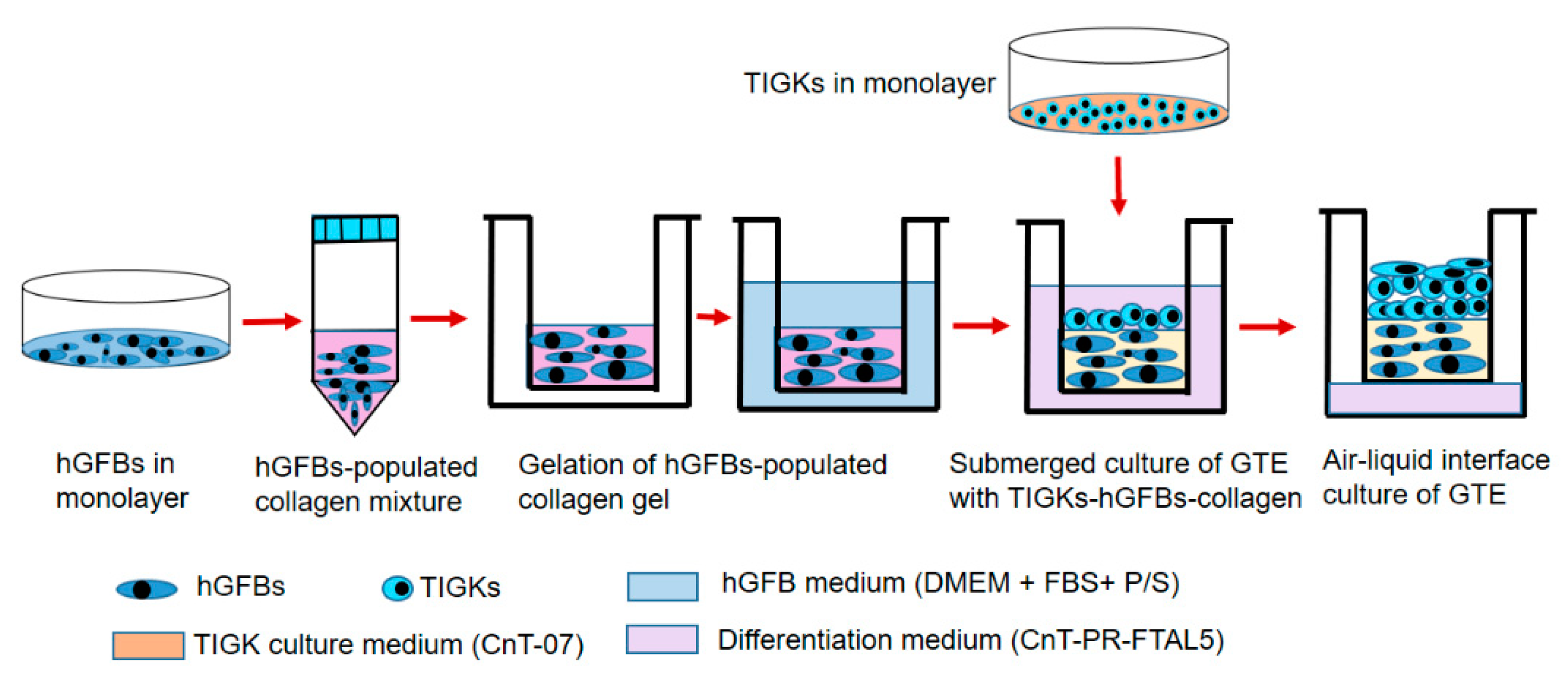
4.5. Construction of the 3D Gingival Tissue Equivalents
4.6. Cytotoxicity Analysis of the 3D Gingival Tissue Equivalents
4.7. Histological and Immunohistological Assay of the 3D Gingival Tissue Equivalents
4.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, Q.; Shen, C. Construction of low contracted 3D skin equivalents by genipin cross-linking. Exp. Dermatol. 2018, 27, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, C.M.; van Lier, A.; Roffel, S.; Kramer, D.; Scheper, R.J.; Gibbs, S. Development of a full-thickness human skin equivalent in vitro model derived from TERT-immortalized keratinocytes and fibroblasts. Tissue Eng. Part A 2015, 21, 2448–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, J.P.H.; Niehues, H.; Rikken, G.; van Vlijmen-Willems, I.; van de Zande, G.; Zeeuwen, P.; Schalkwijk, J.; van den Bogaard, E.H. Immortalized N/TERT keratinocytes as an alternative cell source in 3D human epidermal models. Sci. Rep. 2017, 7, 11838. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, L.; Jederka, K.; Cios, A.; Ciepelak, M.; Lewicka, A.; Stankiewicz, W.; Lewicki, S. A simple method for the production of human skin equivalent in 3D, multi-cell culture. Int. J. Mol. Sci. 2020, 21, 4644. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Pinnock, A.; Murdoch, C.; Moharamzadeh, K.; Whawell, S.; Douglas, C.W. Characterisation and optimisation of organotypic oral mucosal models to study Porphyromonas gingivalis invasion. Microbes Infect. 2014, 16, 310–319. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Cox, S.C.; Sammons, R.L.; Jabbari, S.; Shelton, R.M.; Kuehne, S.A. A review of co-culture models to study the oral microenvironment and disease. J. Oral Microbiol. 2020, 12, 1773122. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-assisted regenerative medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Riacci, L.; Sorriento, A.; Ricotti, L. Genipin-based crosslinking of jellyfish collagen 3D hydrogels. Gels 2021, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Tsukahara, K.; Moriwaki, S.; Kitahara, T.; Takema, Y. Effects of natural product extracts on contraction and mechanical properties of fibroblast populated collagen gel. Biol. Pharm. Bull. 2000, 23, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Kosten, I.J.; Buskermolen, J.K.; Spiekstra, S.W.; de Gruijl, T.D.; Gibbs, S. Gingiva equivalents secrete negligible Aamounts of key chemokines involved in langerhans cell migration compared to skin equivalents. J. Immunol. Res. 2015, 2015, 627125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J. Oral Biol. Craniofac. Res. 2020, 10, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Z.; Day, J.H.; Su, X.; Guadarrama, A.G.; Sandbo, N.K.; Esnault, S.; Denlinge, L.C.; Berthier, E.; Theberge, A.B. Investigating fibroblast-induced collagen gel contraction using a dynamic microscale platform. Front. Bioeng. Biotechnol. 2019, 7, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue Integration and Degradation of a Porous Collagen-Based Scaffold Used for Soft Tissue Augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Larsen, J.; Krasieva, T.B.; Lyubovitsky, J.G. Effect of genipin crosslinking on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 2011, 3, 2579–2584. [Google Scholar] [CrossRef] [Green Version]
- Macaya, D.; Ng, K.K.; Spector, M. Injectable Collagen–Genipin Gel for the Treatment of Spinal Cord Injury: In Vitro Studies. Adv. Funct. Mater. 2011, 21, 4788–4797. [Google Scholar] [CrossRef]
- Výborný, K.; Vallová, J.; Kočí, Z.; Kekulová, K.; Jiráková, K.; Jendelová, P.; Hodan, J.; Kubinová, S. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci. Rep. 2019, 9, 10674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-based trilayer scaffold formultitissue periodontal regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Del Gaudio, C.; Lucatelli, E.; Kuevda, E.; Boieri, M.; Mazzanti, B.; Bianco, A.; Macchiarini, P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014, 35, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.S.; Lim, E.S.; Kim, H.M.; Hwang, Y.C.; Lee, K.W.; Min, K.S. Genipin, a cross-linking agent, promotes odontogenic differentiation of human dental pulp cells. J. Endod. 2015, 41, 501–507. [Google Scholar] [CrossRef]
- Zhou, X.; Tao, Y.; Chen, E.; Wang, J.; Fang, W.; Zhao, T.; Liang, C.; Li, F.; Chen, Q. Genipin-cross-linked type II collagen scaffold promotes the differentiation of adipose-derived stem cells into nucleus pulposus-like cells. J. Biomed. Mater. Res. A 2018, 106, 1258–1268. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Baiguera, S.; Boieri, M.; Mazzanti, B.; Ribatti, D.; Bianco, A.; Macchiarini, P. Induction of angiogenesis using VEGF releasing genipin-crosslinked electrospun gelatin mats. Biomaterials 2013, 34, 7754–7765. [Google Scholar] [CrossRef]
- Feng, Z.; Matsumoto, T.; Nakamura, T. Measurements of the mechanical properties of contracted collagen gels populated with rat fibroblasts or cardiomyocytes. Artif. Organs 2003, 6, 192–196. [Google Scholar] [CrossRef]
- Feng, Z.; Yamato, M.; Akutsu, T.; Nakamura, T.; Okano, T.; Umezu, M. Investigation on the mechanical properties of contracted collagen gels as a scaffold for tissue engineering. Artif. Organs 2003, 27, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Dongari-Bagtzoglou, A.; Kashleva, H. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat. Protoc. 2006, 1, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Casella, J.F.; Flanagan, M.D.; Lin, S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 1981, 293, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Apine, A.A.; Prasad, B.M. Current status of animal experimentation in the study of periodontal diseases and therapeutics. Dent. Sci. 2014, 2, 51–56. [Google Scholar]
- Oz, H.S.; Puleo, D.A. Animal models for periodontal disease. J. Biomed. Biotechnol. 2011, 2011, 754857. [Google Scholar] [CrossRef] [Green Version]
- Struillou, X.; Boutigny, H.; Soueidan, A.; Layrolle, P. Experimental animal models in periodontology: A review. Open Dent. J. 2010, 4, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.W.; Shi, W.; Huang, G.T.; Kinder Haake, S.; Park, N.H.; Kuramitsu, H.; Genco, R.J. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 2000, 68, 3140–3146. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, O.; Watanabe, K.; Lamont, R.J. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 2002, 4, 305–314. [Google Scholar] [CrossRef]
- Yilmaz, O.; Young, P.A.; Lamont, R.J.; Kenny, G.E. Gingival epithelial cell signalling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology 2003, 149, 2417–2426. [Google Scholar] [CrossRef] [Green Version]
- Bao, K.; Akguel, B.; Bostanci, N. Establishment and characterization of immortalized gingival epithelial and fibroblastic cell lines for the development of organotypic cultures. Cells Tissues Organs 2014, 199, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Okamura, H.; Kumazawa, Y. Three-dimensional inflammatory human tissue equivalents of gingiva. J. Vis. Exp. 2018, 134, 57157. [Google Scholar] [CrossRef] [PubMed]
- Kasugai, S.; Ogura, H. The effects of cytoskeletal inhibitors on the collagen gel contraction by dog periodontal ligament fibroblasts in vitro. Arch. Oral Biol. 1993, 38, 785–792. [Google Scholar] [CrossRef]
- Ibusuki, S.; Halbesma, G.J.; Randolph, M.A.; Redmond, R.W.; Kochevar, I.E.; Gill, T.J. Photochemically cross-linked collagen gels as three-dimensional scaffolds for tissue engineering. Tissue Eng. 2007, 13, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Buskermolen, J.K.; Reijnders, C.M.; Spiekstra, S.W.; Steinberg, T.; Kleverlaan, C.J.; Feilzer, A.J.; Bakker, A.D.; Gibbs, S. Development of a full-thickness human gingiva equivalent constructed fromimmortalized keratinocytes and fibroblasts. Tissue Eng. Part. C Methods 2016, 22, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Eun-Kyung, Y.; Lee, D.H.; Park, S.N.; Choe, T.B.; Park, J.K. Contraction behavior of collagen gel and fibroblasts activity in dermal equivalent model. J. Microbiol. Biotechnol. 1997, 7, 267–271. [Google Scholar]
- Kanta, J. Collagen matrix as a tool in studying fibroblastic cell behavior. Cell Adh. Migr. 2015, 9, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Oliveros Anerillas, L.; Kingham, P.J.; Lammi, M.J.; Wiberg, M.; Kelk, P. Three-dimensional osteogenic differentiation of bone marrow mesenchymal stem cellspromotes matrix metallopeptidase 13 (MMP13) expression in type I collagen hydrogels. Int. J. Mol. Sci. 2021, 22, 13594. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Umino, T.; Liu, X.D.; Wang, H.J.; Romberger, D.J.; Spurzem, J.R.; Rennard, S.I. Effect of initial collagen concentration on fibroblast mediated contraction of collagen gels. Chest 2000, 117, 234S–235S. [Google Scholar] [CrossRef]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. A 2008, 87, 308–320. [Google Scholar] [CrossRef]
- Liu, T.X.; Wang, Z. Collagen crosslinking of porcine sclera using genipin. Acta Ophthalmol. 2013, 91, e253–e257. [Google Scholar] [CrossRef] [PubMed]
- Ujihara, Y.; Miyazaki, H.; Wada, S. Morphological study of fibroblasts treated with cytochalasin D and colchicine using a confocal laser scanning microscopy. J. Physiol. Sci. 2008, 58, 499–506. [Google Scholar] [CrossRef]
- Loty, S.; Forest, N.; Boulekbache, H.; Sautier, J.M. Cytochalasin D induces changes in cell shape and promotes in vitro chondrogenesis: A morphological study. Biol. Cell 1995, 83, 149–161. [Google Scholar] [CrossRef]
- Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Hu, J.; Xu, J.; Liechty, K.W.; Zgheib, C. Mesenchymal stromal cells contract collagen more efficiently than dermal fibroblasts: Implications for cytotherapy. PLoS ONE 2019, 14, e0218536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Ghalbzouri, A.; Lamme, E.; Ponec, M. Crucial role of fibroblasts in regulating epidermal morphogenesis. Cell Tissue Res. 2002, 310, 189–199. [Google Scholar] [CrossRef]
- El Ghalbzouri, A.; Ponec, M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004, 12, 359–367. [Google Scholar] [CrossRef]
- Fernandez, T.L.; Van Lonkhuyzen, D.R.; Dawson, R.A.; Kimlin, M.G.; Upton, Z. In vitro investigations on the effect of dermal fibroblasts on keratinocyte responses to ultraviolet B radiation. Photochem. Photobiol. 2014, 90, 1332–1339. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J. Cell Physiol. 2004, 200, 134–145. [Google Scholar] [CrossRef]
- Yadav, S.; Jingran, R.; Srivastava, R.; Madan, R. Role of fibroblast in periodontal heath and disease: An overview. Asian J. Oral Health Allied Sci. 2017, 7, 22. [Google Scholar]
- Ghaffari, A.; Kilani, R.T.; Ghahary, A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J. Investig. Dermatol. 2009, 129, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Koskela, A.; Engstrom, K.; Hakelius, M.; Nowinski, D.; Ivarsson, M. Regulation of fibroblast gene expression by keratinocytes in organotypic skin culture provides possible mechanisms for the antifibrotic effect of reepithelialization. Wound Repair Regen. 2010, 18, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.A.; Gossiel, F.; Bullock, A.J.; Sun, T.; Blumsohn, A.; Mac Neil, S. Investigation of keratinocyte regulation of collagen I synthesis by dermal fibroblasts in a simple in vitro model. Br. J. Derm. 2006, 154, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Tandara, A.A.; Kloeters, O.; Mogford, J.E.; Mustoe, T.A. Hydrated keratinocytes reduce collagen synthesis by fibroblasts via paracrine mechanisms. Wound Repair Regen. 2007, 15, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, T.; He, S.; Sa, G. Regulatory mechanism of oral mucosal rete peg formation. J. Mol. Histol. 2021, 52, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Kaitainen, S.; Mähönen, A.J.; Lappalainen, R.; Kröger, H.; Lammi, M.J.; Qu, C. TiO2 coating promotes human mesenchymal stem cell proliferation without the loss of their capacity for chondrogenic differentiation. Biofabrication 2013, 5, 025009. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Prat, A.; Sedic, M.; Proia, T.; Wronski, A.; Mazumdar, S.; Skibinski, A.; Shirley, S.H.; Perou, C.M.; Gill, G.; et al. Cell-state transitions regulated by SLUG are critical for tissue regeneration and tumor initiation. Stem Cell Rep. 2014, 2, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Strudwick, X.L.; Lang, D.L.; Smith, L.E.; Cowin, A.J. Combination of low calcium with Y-27632 rock inhibitor increases the proliferative capacity, expansion potential and lifespan of primary human keratinocytes while retaining their capacity to differentiate into stratified epidermis in a 3D skin model. PLoS ONE 2015, 10, e0123651. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Jo, H.S.; Shim, W.S.; Kwon, Y.W.; Bae, S.; Kwon, Y.; Azamov, B.; Hur, J.; Lee, D.; Ryu, S.H.; et al. Emodin induces collagen type I synthesis in Hs27 human dermal fibroblasts. Exp. Ther. Med. 2021, 21, 420. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]


| Gene | Primer Pairs 5′→3′ | Product Size (bp) | Reference |
|---|---|---|---|
| RPLP0 | F: AGATGCAGCAGATCCGCAT R: GTGGTGATACCTAAAGCCTG | 319 | [65] |
| K14 | F: CATGAGTGTGGAAGCCGACAT R: GCCTCTCAGGGCATTCATCTC | 154 | [66] |
| K10 | F: TTGCTGAACAAAACCGCAAAG R: GCCAGTTGGGACTGTAGTTCT | 169 | [67] |
| Inv. | F: GACTGCTGTAAAGGGACTGCC R: CATTCCCAGTTGCTCATCTCTC | 250 | [67] |
| Coll 1a1 | F: GAACGCGTGTCATCCCTTGT R: GAACGAGGTAGTCTTTCAGCAACA | 94 | [68] |
| CD90 | F: TCGCTCTCCTGCTAACAGTCT R: CTCGTACTGGATGGGTGAACT | 134 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koskinen Holm, C.; Qu, C. Engineering a 3D In Vitro Model of Human Gingival Tissue Equivalent with Genipin/Cytochalasin D. Int. J. Mol. Sci. 2022, 23, 7401. https://doi.org/10.3390/ijms23137401
Koskinen Holm C, Qu C. Engineering a 3D In Vitro Model of Human Gingival Tissue Equivalent with Genipin/Cytochalasin D. International Journal of Molecular Sciences. 2022; 23(13):7401. https://doi.org/10.3390/ijms23137401
Chicago/Turabian StyleKoskinen Holm, Cecilia, and Chengjuan Qu. 2022. "Engineering a 3D In Vitro Model of Human Gingival Tissue Equivalent with Genipin/Cytochalasin D" International Journal of Molecular Sciences 23, no. 13: 7401. https://doi.org/10.3390/ijms23137401
APA StyleKoskinen Holm, C., & Qu, C. (2022). Engineering a 3D In Vitro Model of Human Gingival Tissue Equivalent with Genipin/Cytochalasin D. International Journal of Molecular Sciences, 23(13), 7401. https://doi.org/10.3390/ijms23137401





