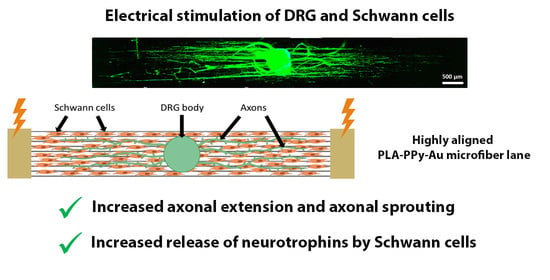
Electrical Stimulation Increases Axonal Growth from Dorsal Root Ganglia Co-Cultured with Schwann Cells in Highly Aligned PLA-PPy-Au Microfiber Substrates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stability and Degradation Study of PLA-PPy Microfibers
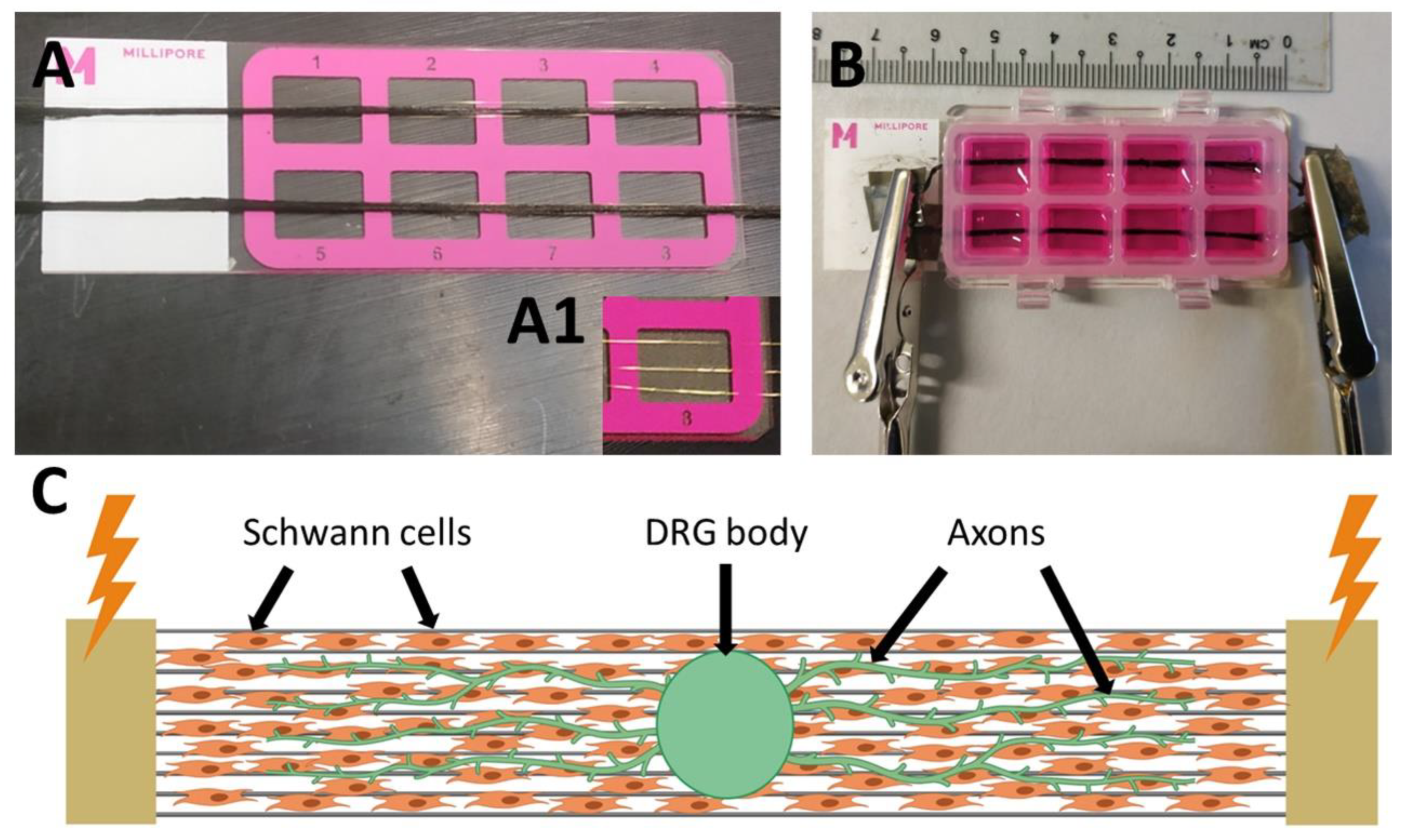
2.2. Bioreactor for the Electrical Stimulation
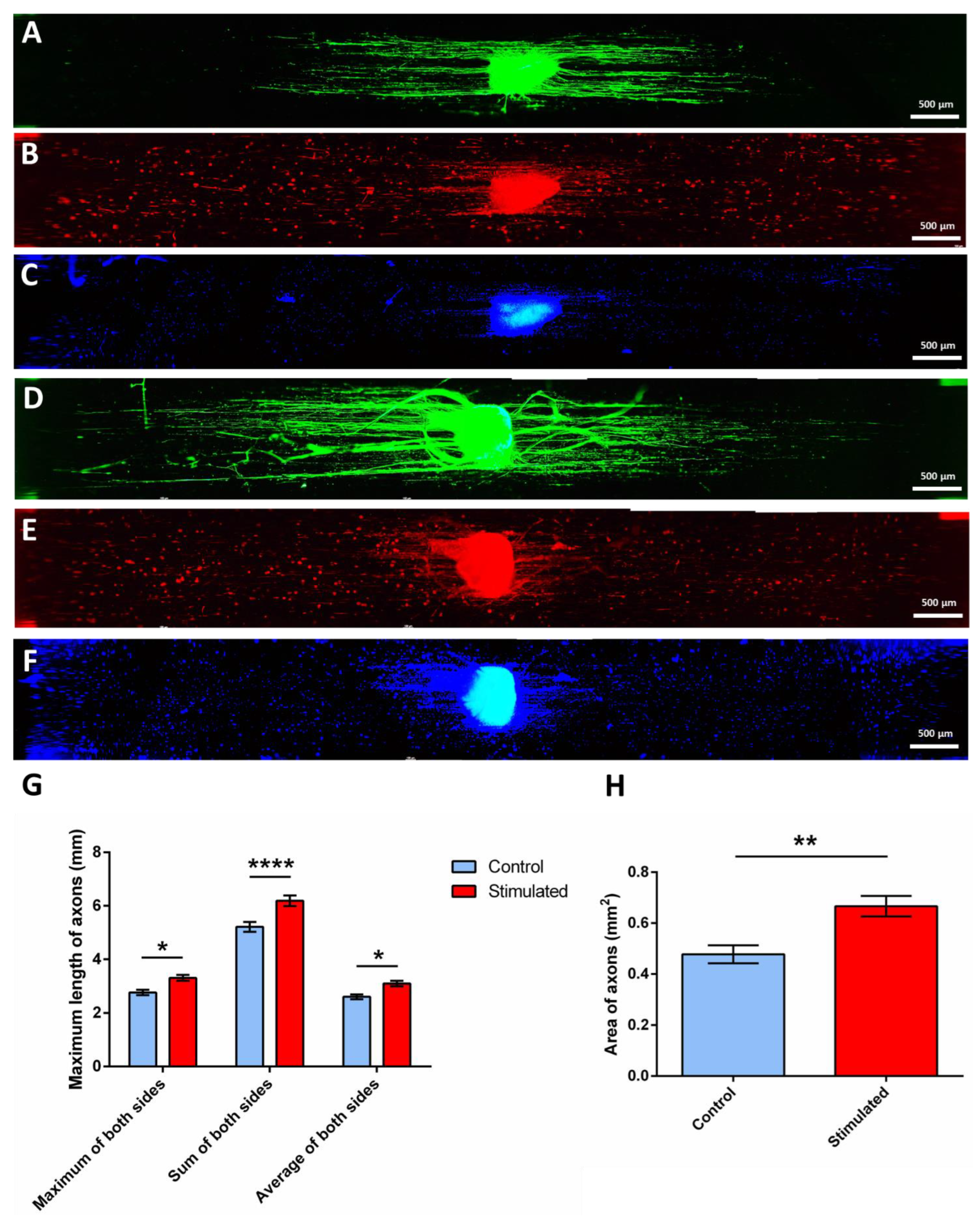
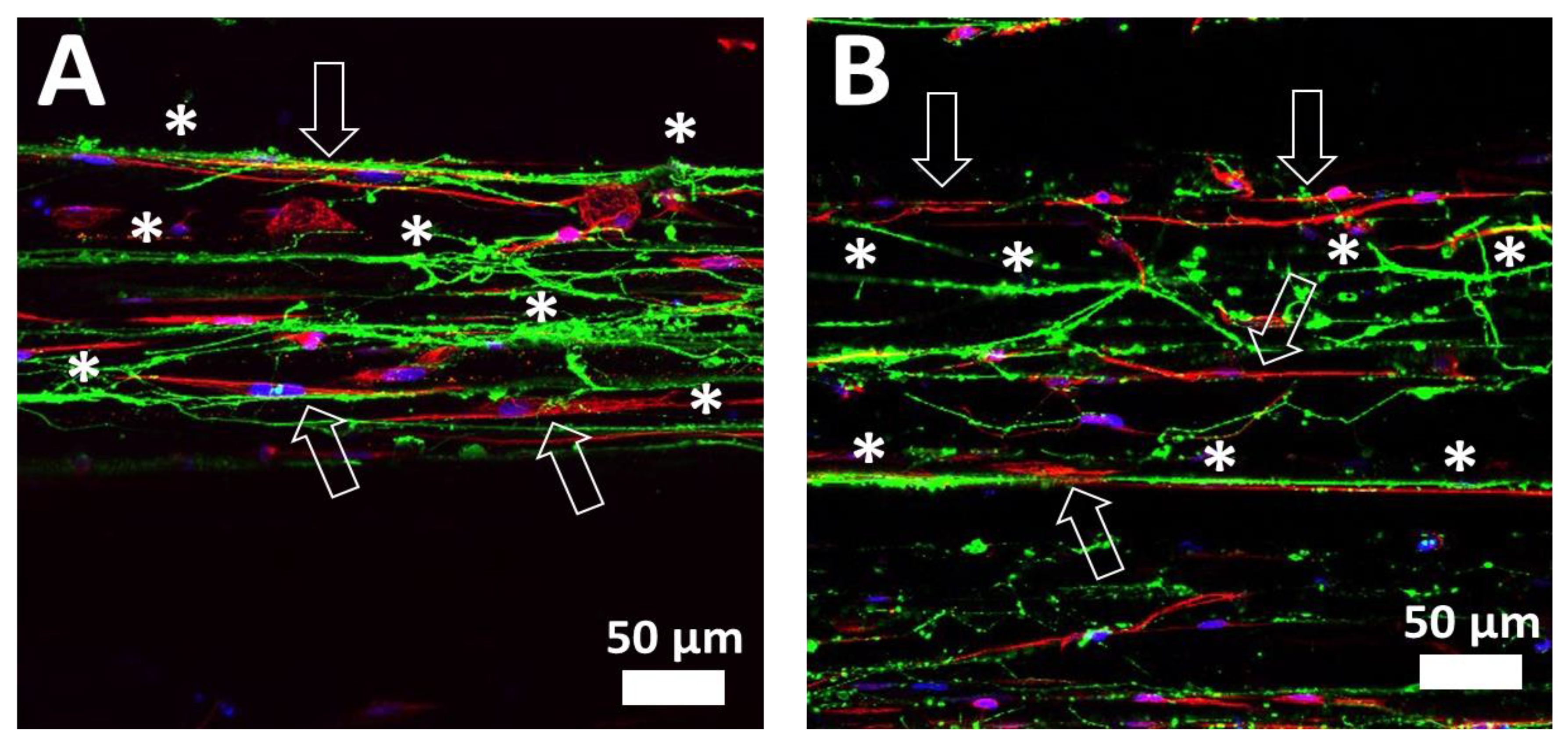
2.3. Electrical Stimulation of DRG and SC
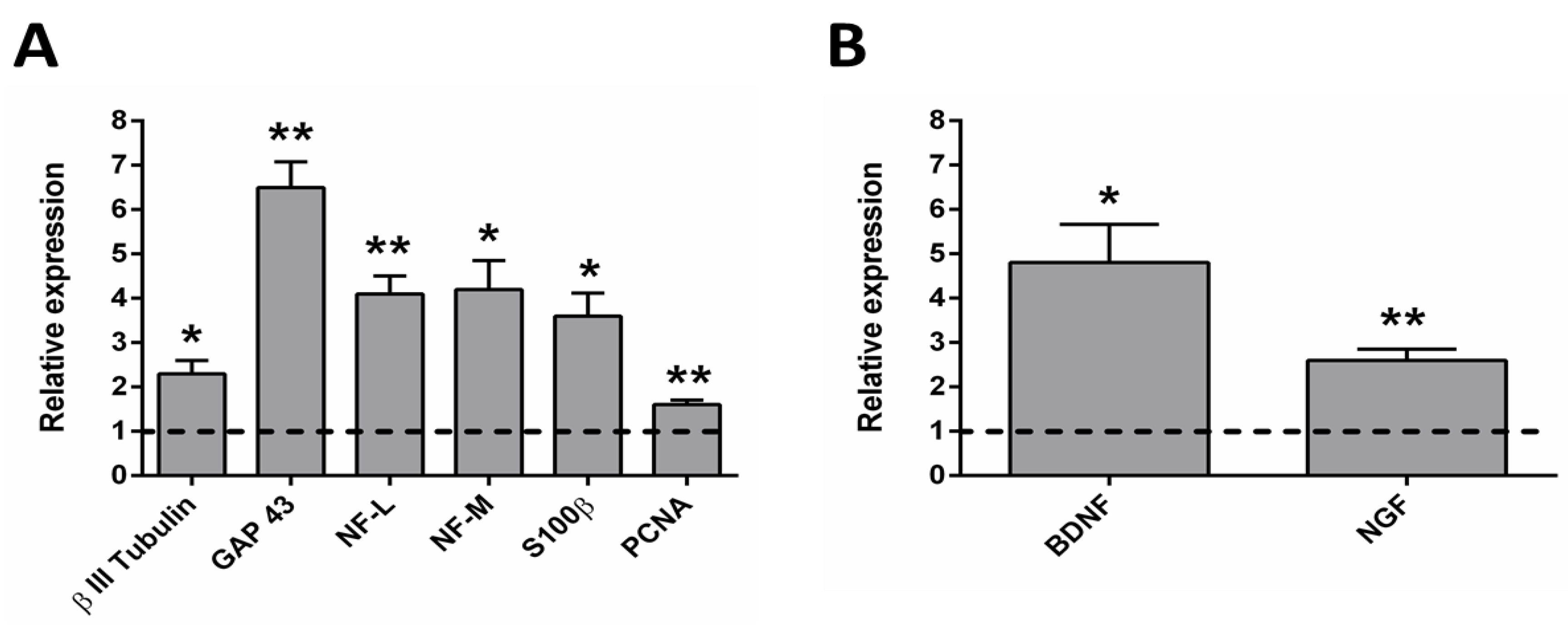
2.4. qRT-PCR Analysis after the Electrical Stimulation
3. Materials and Methods
3.1. Preparation of PLA Microfiber Bundles
3.2. Coating of PLA Microfibers with PPy
3.3. Bioreactor for Electrical Stimulation
3.4. Stability and Degradation of PLA-PPy Microfibers
3.5. Electrical Characterization
3.6. Field Emission Scanning Electron Microscopy (FESEM)
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
3.8. Thermogravimetric Analysis (TGA)
3.9. Substrates Sanitization and Preconditioning
3.10. Seeding of SCs and DRG
3.11. Electrical Stimulation Parameters
3.12. Immunostaining of SCs and DRG
3.13. Quantification of Axonal Extension and Axonal Sprouting
3.14. RNA Extraction and cDNA Synthesis
3.15. Quantitative Real-Time PCR
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Lim, S.H.; Hai-Quan, H.Q.M.; Chew, S.Y. Current Applications and Future Perspectives of Artificial Nerve Conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef]
- Zhu, W.; Masood, F.; O’Brien, J.; Zhang, L.G. Highly Aligned Nanocomposite Scaffolds by Electrospinning and Electrospraying for Neural Tissue Regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 693–704. [Google Scholar] [CrossRef]
- Lee, Y.S.; Collins, G.; Livingston Arinzeh, T. Neurite Extension of Primary Neurons on Electrospun Piezoelectric Scaffolds. Acta Biomater. 2011, 7, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, Z.; Zhao, B.; Qi, H.X.; Peng, J.; Zhang, L.; Xu, W.J.; Hu, P.; Lu, S.B. Biocompatibility Evaluation of Electrospun Aligned Poly(Propylene Carbonate) Nanofibrous Scaffolds with Peripheral Nerve Tissues and Cells in Vitro. Chin. Med. J. 2011, 124, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Qin, J.; Huang, Z.; Yin, G.; Pu, X.; He, D. Fabrication of Aligned Conducting PPy-PLLA Fiber Films and Their Electrically Controlled Guidance and Orientation for Neurites. ACS Appl. Mater. Interfaces 2016, 8, 12576–12582. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Z.; Pu, X.; Yin, G.; Zhang, J. Fabrication of Chitosan/Polypyrrole-Coated Poly(L-Lactic Acid)/Polycaprolactone Aligned Fibre Films for Enhancement of Neural Cell Compatibility and Neurite Growth. Cell Prolif. 2019, 52, e12588. [Google Scholar] [CrossRef]
- Henton, D.E.; Gruber, P.; Lunt, J.; Randall, J. Polylactic Acid Technology: A Review. Nat. Fibers Biopolym. Biocompos. 2005, 48674, 527–578. [Google Scholar] [CrossRef]
- Lunt, J. Large-Scale Production, Properties and Commercial App. Polym. Degrad. Stab. 1998, 3910, 145–152. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthetic Surg. J. 2018, 38, S13–S17. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior Cruciate Ligament Regeneration Using Braided Biodegradable Scaffolds: In Vitro Optimization Studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef]
- Zilberman, M.; Nelson, K.D.; Eberhart, R.C. Mechanical Properties and in Vitro Degradation of Bioresorbable Fibers and Expandable Fiber-Based Stents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 792–799. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(l-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Gisbert Roca, F.; Más Estellés, J.; Monleón Pradas, M.; Martínez-Ramos, C. Axonal Extension from Dorsal Root Ganglia on Fibrillar and Highly Aligned Poly(Lactic Acid)-Polypyrrole Substrates Obtained by Two Different Techniques: Electrospun Nanofibres and Extruded Microfibres. Int. J. Biol. Macromol. 2020, 163, 1959–1969. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Gordon, T. Effects of Short- and Long-Term Schwann Cell Denervation on Peripheral Nerve Regeneration, Myelination, and Size. Glia 2000, 32, 234–246. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical Engineering Strategies for Peripheral Nerve Repair: Surgical Applications, State of the Art, and Future Challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Katiyar, K.S.; Struzyna, L.A.; Morand, J.P.; Burrell, J.C.; Clements, B.; Laimo, F.A.; Browne, K.D.; Kohn, J.; Ali, Z.; Ledebur, H.C.; et al. Tissue Engineered Axon Tracts Serve as Living Scaffolds to Accelerate Axonal Regeneration and Functional Recovery Following Peripheral Nerve Injury in Rats. Front. Bioeng. Biotechnol. 2020, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of Neurite Outgrowth Using an Electrically Conducting Polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ye, Z.; Hu, X.; Lu, L.; Luo, Z. Electrical Stimulation Induces Calcium-Dependent Release of NGF from Cultured Schwann Cells. Glia 2010, 58, 622–631. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, H.; Shi, G. Conducting Polymer Nanomaterials: Electrosynthesis and Applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef]
- Sabouraud, G.; Sadki, S.; Brodie, N. The Mechanisms of Pyrrole Electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Wang, L.X.; Li, X.G.; Yang, Y.L. Preparation, Properties and Applications of Polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Z. Polypyrrole as Electrically Conductive Biomaterials: Synthesis, Biofunctionalization, Potential Applications and Challenges. Adv. Exp. Med. Biol. 2018, 1078, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Mattioli-Belmonte, M.; Gabbanelli, F.; Marcaccio, M.; Giantomassi, F.; Tarsi, R.; Natali, D.; Callegari, A.; Paolucci, F.; Biagini, G. Bio-Characterisation of Tosylate-Doped Polypyrrole Films for Biomedical Applications. Mater. Sci. Eng. C 2005, 25, 43–49. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.; Roca, M.I.; Martinez, J.G.; Meseguer-Olmo, L.; Cenis, J.L.; Moraleda, J.M.; Otero, T.F. Fabrication of Conductive Electrospun Silk Fibroin Scaffolds by Coating with Polypyrrole for Biomedical Applications. Bioelectrochemistry 2012, 85, 36–43. [Google Scholar] [CrossRef]
- Zhou, J.F.; Wang, Y.G.; Cheng, L.; Wu, Z.; Sun, X.D.; Peng, J. Preparation of Polypyrrole-Embedded Electrospun Poly(Lactic Acid) Nanofibrous Scaffolds for Nerve Tissue Engineering. Neural Regen. Res. 2016, 11, 1644–1652. [Google Scholar] [CrossRef]
- George, P.M.; Lyckman, A.W.; Lavan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Z.; Pu, X.; Chen, X.; Yin, G.; Wang, Y.; Miao, D.; Fan, J.; Mu, J. Polydopamine/Carboxylic Graphene Oxide-Composited Polypyrrole Films for Promoting Adhesion and Alignment of Schwann Cells. Colloids Surf. B Biointerfaces 2020, 191, 110972. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.Q.; Yang, Y. Application of Conductive PPy/SF Composite Scaffold and Electrical Stimulation for Neural Tissue Engineering. Biomaterials 2020, 255, 120164. [Google Scholar] [CrossRef] [PubMed]
- Quigley, A.F.; Razal, J.M.; Thompson, B.C.; Moulton, S.E.; Kita, M.; Kennedy, E.L.; Clark, G.M.; Wallace, G.G.; Kapsa, R.M.I. A Conducting-Polymer Platform with Biodegradable Fibers for Stimulation and Guidance of Axonal Growth. Adv. Mater. 2009, 21, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on Gold Nanoparticles and Their Applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications—A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef]
- Paviolo, C.; Stoddart, P.R. Gold Nanoparticles for Modulating Neuronal Behavior. Nanomaterials 2017, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.-R.; Sakuma, S. (Eds.) Nanomaterials in Pharmacology; Methods in Pharmacology and Toxicology; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3120-0. [Google Scholar]
- Markus, A.; Patel, T.D.; Snider, W.D. Neurotrophic Factors and Axonal Growth. Curr. Opin. Neurobiol. 2002, 12, 523–531. [Google Scholar] [CrossRef]
- Lu, P.; Tuszynski, M.H. Growth Factors and Combinatorial Therapies for CNS Regeneration. Exp. Neurol. 2008, 209, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Lykissas, M.; Batistatou, A.; Charalabopoulos, K.; Beris, A. The Role of Neurotrophins in Axonal Growth, Guidance, and Regeneration. Curr. Neurovasc. Res. 2007, 4, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bregman, B.S.; McAtee, M.; Dai, H.N.; Kuhn, P.L. Neurotrophic Factors Increase Axonal Growth after Spinal Cord Injury and Transplantation in the Adult Rat. Exp. Neurol. 1997, 148, 475–494. [Google Scholar] [CrossRef]
- Freeman, M.R. Sculpting the Nervous System: Glial Control of Neuronal Development. Curr. Opin. Neurobiol. 2006, 16, 119–125. [Google Scholar] [CrossRef]
- Pompili, E.; Ciraci, V.; Leone, S.; De Franchis, V.; Familiari, P.; Matassa, R.; Familiari, G.; Tata, A.M.; Fumagalli, L.; Fabrizi, C. Thrombin Regulates the Ability of Schwann Cells to Support Neuritogenesis and to Maintain the Integrity of the Nodes of Ranvier. Eur. J. Histochem. 2020, 64, 3109. [Google Scholar] [CrossRef] [Green Version]
- El Seblani, N.; Welleford, A.S.; Quintero, J.E.; van Horne, C.G.; Gerhardt, G.A. Invited Review: Utilizing Peripheral Nerve Regenerative Elements to Repair Damage in the CNS. J. Neurosci. Methods 2020, 335, 108623. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann Cell Update: Adaptive Reprogramming, EMT, and Stemness in Regenerating Nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann Cell Plasticity Involved in Peripheral Nerve Repair after Injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; Van Der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Gisbert Roca, F.; André, F.M.; Más Estellés, J.; Monleón Pradas, M.; Mir, L.M.; Martínez-Ramos, C. BDNF-Gene Transfected Schwann Cell-Assisted Axonal Extension and Sprouting on New PLA–PPy Microfiber Substrates. Macromol. Biosci. 2021, 21, 2000391. [Google Scholar] [CrossRef]
- Håkansson, E.; Lin, T.; Wang, H.; Kaynak, A. The Effects of Dye Dopants on the Conductivity and Optical Absorption Properties of Polypyrrole. Synth. Met. 2006, 156, 1194–1202. [Google Scholar] [CrossRef] [Green Version]
- Raudsepp, T.; Marandi, M.; Tamm, T.; Sammelselg, V.; Tamm, J. Influence of Ion-Exchange on the Electrochemical Properties of Polypyrrole Films. Electrochim. Acta 2014, 122, 79–86. [Google Scholar] [CrossRef]
- Chitte, H.K.; Shinde, G.N.; Bhat, N.V.; Walunj, V.E. Synthesis of Polypyrrole Using Ferric Chloride (FeCl3) as Oxidant Together with Some Dopants for Use in Gas Sensors. J. Sens. Technol. 2011, 1, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Fabela-Sánchez, O.; Salgado-Ceballos, H.; Medina-Torres, L.; Álvarez-Mejía, L.; Sánchez-Torres, S.; Mondragón-Lozano, R.; Morales-Guadarrama, A.; Díaz-Ruiz, A.; Olayo, M.-G.; Cruz, G.J.; et al. Effect of the Combined Treatment of Albumin with Plasma Synthesised Pyrrole Polymers on Motor Recovery after Traumatic Spinal Cord Injury in Rats. J. Mater. Sci. Mater. Med. 2018, 29, 13. [Google Scholar] [CrossRef]
- Gisbert Roca, F.; García-Bernabé, A.; Compañ Moreno, V.; Martínez-Ramos, C.; Monleón Pradas, M. Solid Polymer Electrolytes Based on Polylactic Acid Nanofiber Mats Coated with Polypyrrole. Macromol. Mater. Eng. 2020, 306, 2000584. [Google Scholar] [CrossRef]
- Wang, Z.; Roberge, C.; Dao, L.H.; Wan, Y.; Shi, G.; Rouabhia, M.; Guidoin, R.; Zhang, Z. In Vivo Evaluation of a Novel Electrically Conductive Polypyrrole/Poly(D,L-Lactide) Composite and Polypyrrole-Coated Poly(D,L-Lactide-Co-Glycolide) Membranes. J. Biomed. Mater. Res. Part A 2004, 70, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Roberge, C.; Wan, Y.; Dao, L.H.; Guidoin, R.; Zhang, Z. A Biodegradable Electrical Bioconductor Made of Polypyrrole Nanoparticle/Poly(D,L-Lactide) Composite: A Preliminary in Vitro Biostability Study. J. Biomed. Mater. Res. Part A 2003, 66, 738–746. [Google Scholar] [CrossRef]
- Huang, Z.B.; Yin, G.F.; Liao, X.M.; Gu, J.W. Conducting Polypyrrole in Tissue Engineering Applications. Front. Mater. Sci. 2014, 8, 39–45. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of Biocompatibility of Polypyrrole in Vitro and in Vivo. J. Biomed. Mater. Res. Part A 2004, 68, 411–422. [Google Scholar] [CrossRef]
- Vigneron, F.; Caps, V. Evolution in the Chemical Making of Gold Oxidation Catalysts. Comptes Rendus Chim. 2016, 19, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Domínguez-Bajo, A.; Rosa, J.M.; González-Mayorga, A.; Rodilla, B.L.; Arché-Núñez, A.; Benayas, E.; Ocón, P.; Pérez, L.; Camarero, J.; Miranda, R.; et al. Nanostructured Gold Electrodes Promote Neural Maturation and Network Connectivity. Biomaterials 2021, 279, 121186. [Google Scholar] [CrossRef] [PubMed]
- Lienemann, S.; Zötterman, J.; Farnebo, S.; Tybrandt, K. Stretchable Gold Nanowire-Based Cuff Electrodes for Low-Voltage Peripheral Nerve Stimulation. J. Neural Eng. 2021, 18, 045007. [Google Scholar] [CrossRef]
- Koppes, A.N.; Zaccor, N.W.; Rivet, C.J.; Williams, L.A.; Piselli, J.M.; Gilbert, R.J.; Thompson, D.M. Neurite Outgrowth On Electrospun PLLA Fibers Is Enhanced By Exogenous Electrical Stimulation. J. Neural Eng. 2014, 11, 046002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.J.; Adams, R.D.; Harkins, A.B.; Engeberg, E.D.; Willits, R.K. Stimulation Frequency Alters the Dorsal Root Ganglion Neurite Growth and Directionality In Vitro. IEEE Trans. Biomed. Eng. 2016, 63, 1257–1268. [Google Scholar] [CrossRef]
- Bertucci, C.; Koppes, R.; Dumont, C.; Koppes, A. Neural Responses to Electrical Stimulation in 2D and 3D in Vitro Environments. Brain Res. Bull. 2019, 152, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Imaninezhad, M.; Pemberton, K.; Xu, F.; Kalinowski, K.; Bera, R.; Zustiak, S.P. Directed and Enhanced Neurite Outgrowth Following Exogenous Electrical Stimulation on Carbon Nanotube-Hydrogel Composites. J. Neural Eng. 2018, 15, 056034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Wu, W.; Park, S.; Miller, A.L.; Terzic, A.; Lu, L. Effective Nerve Cell Modulation by Electrical Stimulation of Carbon Nanotube Embedded Conductive Polymeric Scaffolds. Biomater. Sci. 2018, 6, 2375–2385. [Google Scholar] [CrossRef]
- Yoon, S.H.; Mofrad, M.R.K. Cell Adhesion and Detachment on Gold Surfaces Modified with a Thiol-Functionalized RGD Peptide. Biomaterials 2011, 32, 7286–7296. [Google Scholar] [CrossRef]
- Chang, Y.J.; Hsu, C.M.; Lin, C.H.; Lu, M.S.C.; Chen, L. Electrical Stimulation Promotes Nerve Growth Factor-Induced Neurite Outgrowth and Signaling. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4130–4136. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of Conductive Polymers, Scaffolds and Electrical Stimulation for Nerve Tissue Engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef] [PubMed]
- Yadid, M.; Feiner, R.; Dvir, T. Gold Nanoparticle-Integrated Scaffolds for Tissue Engineering and Regenerative Medicine. Nano Lett. 2019, 19, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Zahmatkeshan, M.; Johari, B.; Kharrazi, S.; Mehdizadeh, M.; Bolouri, B.; Rezayat, S.M. Investigating the Effects of Electrical Stimulation via Gold Nanoparticles on in Vitro Neurite Outgrowth: Perspective to Nerve Regeneration. Microelectron. Eng. 2017, 173, 1–5. [Google Scholar] [CrossRef]
- Graham, R.D.; Bruns, T.M.; Duan, B.; Lempka, S.F. The Effect of Clinically Controllable Factors on Neural Activation During Dorsal Root Ganglion Stimulation. Neuromodulation 2021, 24, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Vuka, I.; Vučić, K.; Repić, T.; Ferhatović Hamzić, L.; Sapunar, D.; Puljak, L. Electrical Stimulation of Dorsal Root Ganglion in the Context of Pain: A Systematic Review of In Vitro and In Vivo Animal Model Studies. Neuromodulation 2018, 21, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.J.; Bruns, T.M.; Wagenaar, J.B.; Gaunt, R.A.; Weber, D.J. Online Feedback Control of Functional Electrical Stimulation Using Dorsal Root Ganglia Recordings. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 7246–7249. [Google Scholar] [CrossRef] [Green Version]
- Franken, G.; Douven, P.; Debets, J.; Joosten, E.A.J. Conventional Dorsal Root Ganglion Stimulation in an Experimental Model of Painful Diabetic Peripheral Neuropathy: A Quantitative Immunocytochemical Analysis of Intracellular γ-Aminobutyric Acid in Dorsal Root Ganglion Neurons. Neuromodulation 2021, 24, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; McCarthy, C.W.; Gilbert, R.J. Varying the Diameter of Aligned Electrospun Fibers Alters Neurite Outgrowth and Schwann Cell Migration. Acta Biomater. 2010, 6, 2970–2978. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Ciardelli, G.; Zanetti, M.; Geuna, S.; Perroteau, I. The Influence of Electrospun Fibre Size on Schwann Cell Behaviour and Axonal Outgrowth. Mater. Sci. Eng. C 2015, 48, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Cheng, L.; DeLisle, M.; Wu, C.; Chew, S.; Hutchinson, E.B.; Sheridan, A.; Alexandre, C.; Latremoliere, F.; Sheu, S.H.; et al. Neuronal-Specific TUBB3 Is Not Required for Normal Neuronal Function but Is Essential for Timely Axon Regeneration. Cell Rep. 2018, 24, 1865–1879.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, M.; Karki, R.; Spennato, M.; Pandya, D.; He, S.; Andreoli, M.; Fiedler, P.; Ferlini, C. Clas-III β-Tubulin in Normal and Cancer Tissues. Gene 2015, 563, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Strata, P. Structural Plasticity of Climbing Fibers and the Growth-Associated Protein GAP-43. Front. Neural Circuits 2013, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, C.J.; Park, M.; Spillane, M.; Yoo, S.; Pacheco, A.; Gomes, C.; Vuppalanchi, D.; Mcdonald, M.; Kim, H.K.; Merianda, T.T.; et al. Axonally Synthesized β-Actin and GAP-43 Proteins Support Distinct Modes of Axonal Growth. J. Neurosci. 2013, 33, 3311–3322. [Google Scholar] [CrossRef] [Green Version]
- Denny, J. Molecular Mechanisms, Biological Actions, and Neuropharmacology of the Growth-Associated Protein GAP-43. Curr. Neuropharmacol. 2006, 4, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Bocquet, A.; Berges, R.; Frank, R.; Robert, P.; Peterson, A.C.; Eyer, J. Neurofilaments Bind Tubulin and Modulate Its Polymerization. J. Neurosci. 2009, 29, 11043–11054. [Google Scholar] [CrossRef]
- Sorci, G. S100B Protein in Tissue Development, Repair and Regeneration. World J. Biol. Chem. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, X.; Xiao, Z.; Gao, X.; Liu, Z.; Zhang, Z.; Wang, K.; Zhu, Y.; Ren, H.; Wang, T. Functional Expression, Characterization, and Application of Human S100B. Oncol. Rep. 2017, 38, 2309–2316. [Google Scholar] [CrossRef] [Green Version]
- Maga, G.; Hübscher, U. Proliferating Cell Nuclear Antigen (PCNA): A Dancer with Many Partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naryzhny, S.N. Proliferating Cell Nuclear Antigen: A Proteomics View. Cell. Mol. Life Sci. 2008, 65, 3789–3808. [Google Scholar] [CrossRef]
- Paunesku, T.; Mittal, S.; Protić, M.; Oryhon, J.; Korolev, S.V.; Joachimiak, A.; Woloschak, G.E. Proliferating Cell Nuclear Antigen (PCNA): Ringmaster of the Genome. Int. J. Radiat. Biol. 2001, 77, 1007–1021. [Google Scholar] [CrossRef]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; Dechiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF Autocrine Loop in Adult Sensory Neurons Prevents Cell Death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2003, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigova, T.; Pencea, V.; Wiegand, S.J.; Luskin, M.B. Intraventricular Administration of BDNF Increases the Number of Newly Generated Neurons in the Adult Olfactory Bulb. Mol. Cell. Neurosci. 1998, 11, 234–245. [Google Scholar] [CrossRef]
- Benraiss, A.; Chmielnicki, E.; Lerner, K.; Roh, D.; Goldman, S.A. Adenoviral Brain-Derived Neurotrophic Factor Induces Both Neostriatal and Olfactory Neuronal Recruitment from Endogenous Progenitor Cells in the Adult Forebrain. J. Neurosci. 2001, 21, 6718–6731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pencea, V.; Bingaman, K.D.; Wiegand, S.J.; Luskin, M.B. Infusion of Brain-Derived Neurotrophic Factor into the Lateral Ventricle of the Adult Rat Leads to New Neurons in the Parenchyma of the Striatum, Septum, Thalamus, and Hypothalamus. J. Neurosci. 2001, 21, 6706–6717. [Google Scholar] [CrossRef] [Green Version]
- Ernfors, P.; Kucera, J.; Lee, K.F.; Loring, J.; Jaenisch, R. Studies on the Physiological Role of Brain-Derived Neurotrophic Factor and Neurotrophin-3 in Knockout Mice. Int. J. Dev. Biol. 2003, 39, 799–807. [Google Scholar] [CrossRef]
- Freeman, R.S.; Burch, R.L.; Crowder, R.J.; Lomb, D.J.; Schoell, M.C.; Straub, J.A.; Xie, L. NGF Deprivation-Induced Gene Expression: After Ten Years, Where Do We Stand? Prog. Brain Res. 2004, 146, 111–126. [Google Scholar] [CrossRef]
- Wiesmann, C.; De Vos, A.M. Nerve Growth Factor: Structure and Function. Cell. Mol. Life Sci. C 2001, 58, 748–759. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]




| Experiment Number | Current per Lane (mA) | Voltage per Lane (mV) | Frequency (Hz) | Effect on Axonal Growth |
|---|---|---|---|---|
| #1 | 3 | 30.6 | 250 | Adverse effect |
| #2 | 1 | 10.2 | 250 | Adverse effect |
| #3 | 0.5 | 5.1 | 50 | No effect |
| #4 | 0.5 | 5.1 | 3 | Favourable effect |
| Species | Gen | Primer Forward | Primer Reverse |
|---|---|---|---|
| Chicken (Gallus Gallus) | β III Tubulin | TCCTCTCACAAGTACGTGCC | CCCCGCTCTGACCGAAAAT |
| NF-L | AAGACGCTGGAGATCGAAGC | CACCTTCCAGCAGTTTCCTGT | |
| NF-M | CACCACCTATCAGGACACGAT | GGGTCCAGTGATGCTTCCAG | |
| GAP 43 | CATAAGGCAGCCACCAAAAT | CGGAAGCCTCACTCTCTTTG | |
| S100β | TGCTTGCCATGAGTTCTTTG | GCACTGTCCAAGAGGCTTTC | |
| PCNA | GAGACCTCAGCCACATTGGT | AGTCAGCTGGACTGGCTCAT | |
| GAPDH | AGTCAACGGATTTGGCCGTA | ACAGTGCCCTTGAAGTGTCC | |
| Rat (Rattus norvegicus) | BDNF | GCGGCAGATAAAAAGACTGC | GTAGTTCGGCATTGCGAGTT |
| NGF | TGATCGGCGTACAGGCAGA | GAGGGCTGTGTCAAGGGAAT | |
| GAPDH | AGACAGCCGCATCTTCTTGT | GACCAGCTTCCCATTCTCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisbert Roca, F.; Serrano Requena, S.; Monleón Pradas, M.; Martínez-Ramos, C. Electrical Stimulation Increases Axonal Growth from Dorsal Root Ganglia Co-Cultured with Schwann Cells in Highly Aligned PLA-PPy-Au Microfiber Substrates. Int. J. Mol. Sci. 2022, 23, 6362. https://doi.org/10.3390/ijms23126362
Gisbert Roca F, Serrano Requena S, Monleón Pradas M, Martínez-Ramos C. Electrical Stimulation Increases Axonal Growth from Dorsal Root Ganglia Co-Cultured with Schwann Cells in Highly Aligned PLA-PPy-Au Microfiber Substrates. International Journal of Molecular Sciences. 2022; 23(12):6362. https://doi.org/10.3390/ijms23126362
Chicago/Turabian StyleGisbert Roca, Fernando, Sara Serrano Requena, Manuel Monleón Pradas, and Cristina Martínez-Ramos. 2022. "Electrical Stimulation Increases Axonal Growth from Dorsal Root Ganglia Co-Cultured with Schwann Cells in Highly Aligned PLA-PPy-Au Microfiber Substrates" International Journal of Molecular Sciences 23, no. 12: 6362. https://doi.org/10.3390/ijms23126362
APA StyleGisbert Roca, F., Serrano Requena, S., Monleón Pradas, M., & Martínez-Ramos, C. (2022). Electrical Stimulation Increases Axonal Growth from Dorsal Root Ganglia Co-Cultured with Schwann Cells in Highly Aligned PLA-PPy-Au Microfiber Substrates. International Journal of Molecular Sciences, 23(12), 6362. https://doi.org/10.3390/ijms23126362