Inclusion Body Myositis and Neoplasia: A Narrative Review
Abstract
1. Introduction
2. Is IBM a Paraneoplastic Myositis?
3. Genetic Susceptibility for IBM and Tumors
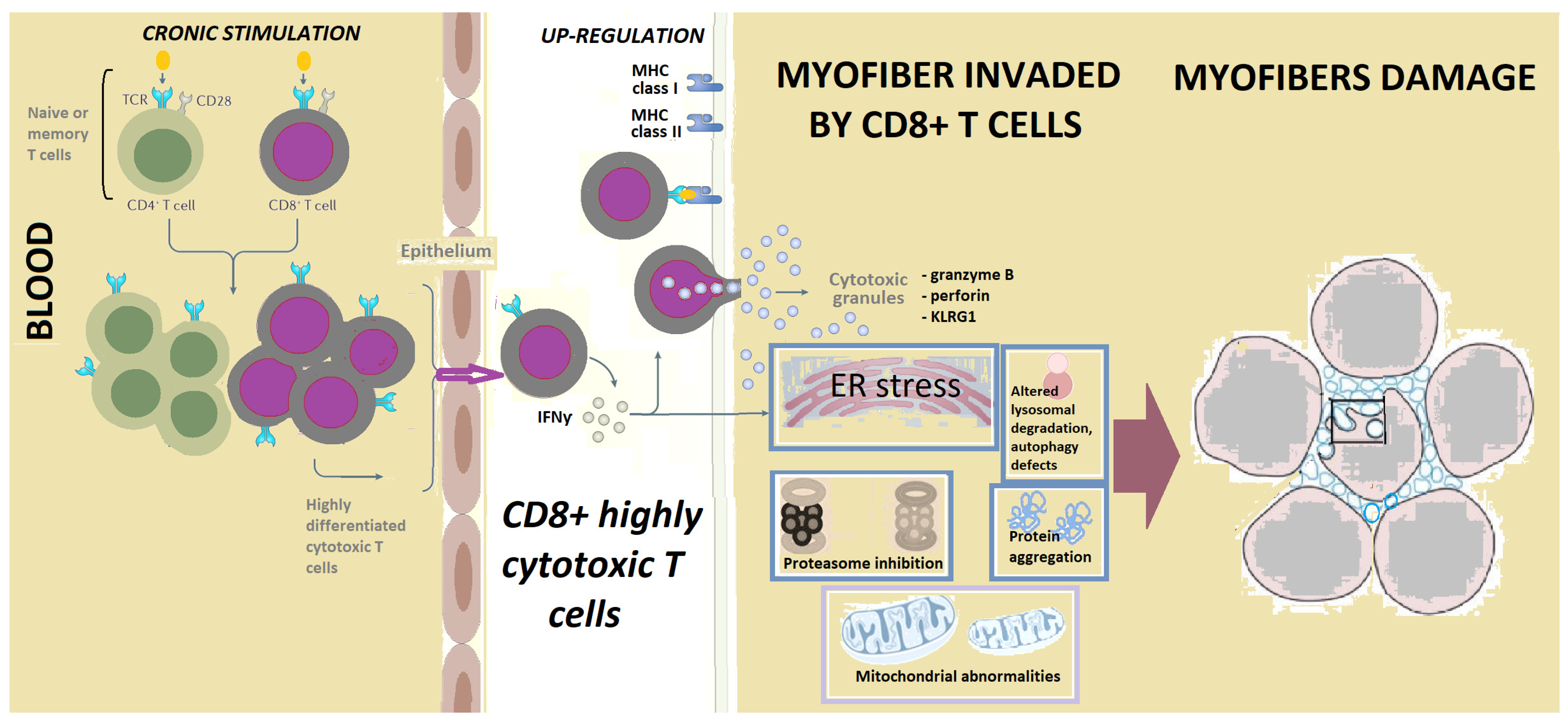
4. Inflammation in IBM, LGL and Other Cancers
4.1. CD8+CD28 Null CD57+ Lymphocytes
4.2. Interferons in Inclusion Body Myositis and Cancer
4.3. Stat Transcription Factors in IBM and Cancers/ LGLL
| Common Pathogenic Mechanisms | In IBM | In Cancers/T-LGL | References |
|---|---|---|---|
| Genetic susceptibility | DRB1*03 is associated with IBM. HLA DRA is elevated in IBM. CCR5 gene variants are found in IBM. A MYH2 gene variant increases IBM risk in Japanese | DRB1*03 is associated with T-LGLL. HLA DRA is elevated in bladder cancer. CCL5/CCR5 axis is involved in hematologic and solid tumor progression. MYH2 is involved in colorectal carcinogenesis. | [4,31,32,33,34,35,36,37,68] |
| CD8+CD28− cytotoxic T cells | In IBM these cytotoxic T cells are clonally expanded and produce IFNγ. | In T-LGLL these cytotoxic T cells are clonally expanded and produce IFNγ. | [4,39,44,69,70] |
| Interferon γ | IBM is associated with a prominent IFNγ signature, mostly early during the disease. | IFNγ is elevated in LGL. IFNγ is crucial for antitumoral effects, but low-dose IFNγ may favor tumorigenesis by impairing cytotoxic T cell responses. | [4,53,54,55,61] |
| STAT1, STAT3 | STAT1 in IBM muscle biopsies is elevated. STAT3 is involved in myogenesis. | pSTAT-1 in T-LGLL is elevated. STAT1, a tumor suppressor, may also promote tumorigenesis by sustaining inflammation. STAT3 constitutive activation is the hallmark of LGLL. STAT3 is involved in tumoral cachexia. | [4,39,40,53,56,59,62,63] |
| Anti-cN1A antibodies | In IBM anti-cN1A are associated with reduced muscle cN1A expression, mitochondrial abnormalities and myofiber intracytoplasmic protein aggregation of p62/SQSTM1. cN1A knockdown activates AMPK, which upregulates the muscle-specific ubiquitin ligases with muscle wasting. | Serum cN1A activity is decreased in breast cancer (possibly through inactivating antibodies), correlated with muscle damage parameters. | [7,64,71,72,73,74,75,76,77] |
| Mitochondrial abnormalities | In IBM mitochondrial size, dynamics, and function defects are progressive. SIRT1, regulating mitochondrial function, is low in IBM muscle, despite increased SIRT-1 mRNA. GDF15, a mitochondrial disease marker, is increased in IBM. | Mitochondria may favor cancer cells survival in oncogenesis. SIRT1 directly influences tumor progression, metastasis, and other oncogenic mechanisms. GDF15 is increased in cancers. | [6,78,79,80,81,82,83,84] |
| Autophagy | Sarcoplasmic aggregates of autophagy-associated proteins p62/SQSTM1, LC3 and TDP-43, involved in UPR and ER stress, are pathologic hallmarks of IBM. FYCO-1 missense variants are found in IBM vacuoles. | p62, a tumor suppressor, may accumulate in cancers due to autophagy defects. LC3, associated with autophagosome formation, is a marker of poor tumor differentiation. TDP-43 may function as tumor promoter or suppressor. FYCO-1 is associated with invasiveness and metastatic potential. | [59,73,85,86,87,88,89,90,91] |
| Chaperones | TCP-1 is overrepresented in IBM vacuoles. | Chaperonin-containing TCP-1 promotes tumor progression, chemoresistence and metastasis. | [85,92] |
| Ubiquitin-proteasome system | UPS dysfunctions are involved in IBM and in cancer. The ubiqutinase Atrogin-1/MAFbx is increased in IBM. | UPS dysfunctions are involved in cancer. Atrogin-1/MAFbx is increased in tumor cachexia. | [82,93,94,95] |
| Cell cycle | In IBM cell cycle markers Ki67, PCNA, cyclins D1, E are increased. | Ki67 and cyclins D1 and E are overexpressed in tumors. | [96,97,98] |
| MicroRNAs | MiR-133 is reduced in IBM. | MiR-133, a tumor suppressor, is reduced in acute myeloid leukemia and in other cancers. | [57,78,99] |
| Metabolic | In IBM metabolic profiles of activated cytotoxic CD8+T cells rely upon mitochondrial fatty acid oxidation. | Metabolic profiles of cancer cell and activated cytotoxic CD8+T cells are similar, relying upon mitochondrial fatty acid oxidation for survival. | [70] |
| Calcium homeostasis | In IBM Ca2+ homeostasis dysfunction is involved in the defective cytotoxic T cells apoptosis and mitochondrial defects. IBM may be a “functional calpainopathy”. | Aberrant calpain activation negatively impacts cancer prognosis. | [100,101,102] |
4.4. Anti-c5′N1A Antibodies
5. Mitochondrial Abnormalities
6. Autophagy in IBM and Cancers
7. Cell Cycle Abnormalities in IBM and Cancers
7.1. The Ubiquitin-Proteasome System in IBM and Cancers
7.2. Cell Cycle in IBM and Cancers
7.3. MicroRNAs in IBM
8. Other Mechanisms
9. Therapies in IBM and Cancers
10. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Prim. 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Zanframundo, G.; Faghihi-Kashani, S.; Scirè, C.A.; Bonella, F.; Corte, T.J.; Doyle, T.J.; Fiorentino, D.; Gonzalez-Gay, M.A.; Hudson, M.; Kuwana, M.; et al. Defiining anti-synthetase syndrome: A systematic literature review. Clin. Exp. Rheumatol. 2022, 40, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hilton-Jones, D.; Brady, S. Diagnostic criteria for inclusion body myositis. J. Intern. Med. 2016, 280, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.A. Inclusion body myositis: Clinical features and pathogenesis. Nat. Rev. Rheumatol. 2019, 15, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Ranque, B.; Maisonobe, T.; Dion, E.; Piette, J.-C.; Chauveheid, M.-P.; Amoura, Z.; Papo, T. Familial inflammatory inclusion body myositis. Ann. Rheum. Dis. 2005, 64, 634–637. [Google Scholar] [CrossRef]
- Rygiel, K.A.; Miller, J.; Grady, J.P.; Rocha, M.C.; Taylor, R.W.; Turnbull, U.M. Mitochondrial and inflammatory changes in sporadic inclusion body myositis. Neuropathol. Appl. Neurobiol. 2015, 41, 288–303. [Google Scholar] [CrossRef]
- Eura, N.; Sugie, K.; Kinugawa, K.; Nanaura, H.; Ohara, H.; Iwasa, N.; Shobatake, R.; Kiriyama, T. Anti-Cytosolic 5′-Nucleotidase 1A (cN1A) Positivity in Muscle is Helpful in the Diagnosis of Sporadic Inclusion Body Myositis: A Study of 35 Japanese Patients. J. Neurol. Neurosci. 2016, 7, 5. [Google Scholar] [CrossRef]
- Tasca, G.; Monforte, M.; De Fino, C.; Kley, R.A.; Ricci, E.; Mirabella, M. Magnetic resonance imaging pattern recognition in sporadic inclusion-body myositis. Muscle Nerve 2015, 52, 956–962. [Google Scholar] [CrossRef]
- Pluk, H.; Van Hoeve, B.J.A.; Van Dooren, S.H.J.; Stammen-Vogelzangs, J.; Van Der Heijden, A.; Schelhaas, H.J.; Verbeek, M.M.; Badrising, U.A.; Arnardottir, S.; Gheorghe, K.; et al. Autoantibodies to cytosolic 5′-nucleotidase 1A in inclusion body myositis. Ann. Neurol. 2013, 73, 397–407. [Google Scholar] [CrossRef]
- Callan, A.; Capkun, G.; Vasanthaprasad, V.; Freitas, R.; Needham, M. A Systematic Review and Meta-Analysis of Prevalence Studies of Sporadic Inclusion Body Myositis. J. Neuromuscul. Dis. 2017, 4, 127–137. [Google Scholar] [CrossRef]
- Needham, M.; Mastaglia, F.L. Sporadic inclusion body myositis: A review of recent clinical advances and current approaches to diagnosis and treatment. Clin. Neurophysiol. 2015, 127, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Dobloug, G.C.; Garen, T.; Brunborg, C.; Gran, J.T.; Molberg, Ø. Survival and cancer risk in an unselected and complete Norwegian idiopathic inflammatory myopathy cohort. Semin. Arthritis Rheum. 2015, 45, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.K.; Kim, W.B.; Baibergenova, A.; Alhusayen, R. Risk of Malignancy in Dermatomyositis and Polymyositis. J. Cutan. Med. Surg. 2016, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, A.G.S.; Allard, A.B.; Callen, J.P.; Chinoy, H.; Chung, L.; Fiorentino, D.; George, M.D.; Gordon, P.; Kolstad, K.; Kurtzman, D.J.B.; et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology 2021, 60, 2615–2628. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Ros, J.; Gil-Vila, A.; Vila-Pijoan, G.; Trallero-Araguás, E.; Pinal-Fernandez, I. Malignancy and myositis, from molecular mimicry to tumor infiltrating lymphocytes. Neuromuscul. Disord. 2019, 29, 819–825. [Google Scholar] [CrossRef]
- Antohe, M.; Nedelcu, R.I.; Nichita, L.; Popp, C.G.; Cioplea, M.; Brinzea, A.; Hodorogea, A.; Calinescu, A.; Balaban, M.; Ion, D.; et al. Tumor infiltrating lymphocytes: The regulator of melanoma evolution (Review). Oncol. Lett. 2019, 17, 4155–4161. [Google Scholar] [CrossRef]
- Ungprasert, P.; Bethina, N.K.; Jones, C.H. Malignancy and Idiopathic Inflammatory Myopathies. N. Am. J. Med Sci. 2013, 5, 569–572. [Google Scholar] [CrossRef]
- Cox, F.M.; Titulaer, M.; Sont, J.; Wintzen, A.R.; Verschuuren, J.; Badrising, U. A 12-year follow-up in sporadic inclusion body myositis: An end stage with major disabilities. Brain 2011, 134, 3167–3175. [Google Scholar] [CrossRef]
- Benveniste, O.; Guiguet, M.; Freebody, J.; Dubourg, O.; Squier, W.; Maisonobe, T.; Stojkovic, T.; Leite, M.I.; Allenbach, Y.; Herson, S.; et al. Long-term observational study of sporadic inclusion body myositis. Brain 2011, 134 Pt 11, 3176–3184. [Google Scholar] [CrossRef]
- Limaye, V.; Luke, C.; Tucker, G.; Hill, C.; Lester, S.; Blumbergs, P.; Roberts-Thomson, P. The incidence and associations of malignancy in a large cohort of patients with biopsy-determined idiopathic inflammatory myositis. Rheumatol. Int. 2013, 33, 965–971. [Google Scholar] [CrossRef]
- Buchbinder, R.; Forbes, A.; Hall, S.; Dennett, X.; Giles, G. Incidence of Malignant Disease in Biopsy-Proven Inflammatory Myopathy: A Population-Based Cohort Study. Ann. Intern. Med. 2001, 134, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Alverne, A.R.; Marie, S.K.; Levy-Neto, M.; de Souza, F.H.; de Carvalho, M.S.; Shinjo, S.K. Miosite de corpos de inclusão: Série de 30 casos de um centro terciário brasileiro [Inclusion body myositis: Series of 30 cases from a Brazilian tertiary center]. Acta Reumatol. Port. 2013, 38, 179–185. [Google Scholar] [PubMed]
- Naddaf, E.; Shelly, S.; Mandrekar, J.; Chamberlain, A.M.; Hoffman, E.M.; Ernste, F.C.; Liewluck, T. Survival and associated comorbidities in inclusion body myositis. Rheumatology 2021, 61, 2016–2024. [Google Scholar] [CrossRef]
- Jensen, M.L.; Wieting, J.; Andary, M.T.; Fankhauser, M.J.; Jones, M.J. Inclusion body myositis and transitional cell carcinoma of the bladder: Significant resolution of symptoms after tumor excision. Arch. Phys. Med. Rehabil. 1997, 78, 327–329. [Google Scholar] [CrossRef]
- Talanin, N.; Bushore, D.; Rasberry, R.; Rudolph, T.; Tuli, M.; Friedman-Musicante, R. Dermatomyositis with the features of inclusion body myositis associated with carcinoma of the bladder. Br. J. Dermatol. 1999, 141, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, D.T.; Bhagwati, N.S.; Fomberstein, B.; Wolfe, D.E.; Feliz, A.; Wiernik, P.H. Steroid-responsive inclusion body myositis associated with endometrial cancer. Clin. Exp. Rheumatol. 2005, 23, 93–96. [Google Scholar]
- Dardis, C.; Antezana, A.; Tanji, K.; Maccabee, P.J. Inclusion Body Myositis: A Case Presenting with Respiratory Failure and Autopsy Findings Leading to the Hypothesis of a Paraneoplastic Cause. Am. J. Case Rep. 2017, 18, 700–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greenberg, S.A.; Pinkus, J.L.; Amato, A.A.; Kristensen, T.; Dorfman, D.M. Association of inclusion body myositis with T cell large granular lymphocytic leukaemia. Brain 2016, 139, 1348–1360. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Illa, I.; Gallardo, E.; Juárez, C. Inclusion body myositis and paraproteinemia: Incidence and immunopathologic correlations. Ann. Neurol. 1997, 41, 100–104. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Kostov, B.; Fraile, G.; Caravia-Durán, D.; Maure, B.; Rascón, F.J.; Zamora, M.; Casanovas, A.; Lopez-Dupla, M.; Ripoll, M.; et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J. Hematol. Oncol. 2017, 10, 90. [Google Scholar] [CrossRef]
- Rojana-Udomsart, A.; Mitrpant, C.; James, I.; Witt, C.; Needham, M.; Day, T.; Kiers, L.; Corbett, A.; Martinez, P.; Wilton, S.D.; et al. Analysis of HLA-DRB3 alleles and supertypical genotypes in the MHC Class II region in sporadic inclusion body myositis. J. Neuroimmunol. 2013, 254, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Sanikommu, S.R.; Clemente, M.J.; Chomczynski, P.; Ii, M.G.A.; Jerez, A.; Thota, S.; Patel, B.; Hirsch, C.; Nazha, A.; Desamito, J.; et al. Clinical features and treatment outcomes in large granular lymphocytic leukemia (LGLL). Leuk. Lymphoma 2017, 59, 416–422. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Zhang, J.; Wang, Y.; Song, X. Identification of Hub Genes and Biological Pathways in Inclusion Body Myositis Using Bioinformatics Analysis. Int. J. Gen. Med. 2022, 15, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.-M.; Kang, H.W.; Jeong, P.; Byun, Y.J.; Lee, H.Y.; Kim, K.; Seo, S.P.; Kim, W.T.; Lee, J.-Y.; Ha, Y.-S.; et al. A prognostic immune predictor, HLA-DRA, plays diverse roles in non-muscle invasive and muscle invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 39, 237.e21–237.e29. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yabe, I.; Sato, K.; Kano, T.; Nakamura, M.; Hozen, H.; Sasaki, H. Clinical, pathological, and genetic mutation analysis of sporadic inclusion body myositis in Japanese people. J. Neurol. 2012, 259, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Kao, Y.; Zhang, C.; Sun, F.; Gong, Z.; Chen, J. Identification of Hub Genes Related to Carcinogenesis and Prognosis in Colorectal Cancer Based on Integrated Bioinformatics. Mediat. Inflamm. 2020, 2020, 5934821. [Google Scholar] [CrossRef]
- Friedman, J.; Schattner, A.; Shvidel, L.; Berrebi, A. Characterization of T-Cell Large Granular Lymphocyte Leukemia Associated with Sjogren’s Syndrome—An Important but Underrecognized Association. Semin. Arthritis Rheum. 2006, 35, 306–311. [Google Scholar] [CrossRef]
- Koskela, H.L.; Eldfors, S.; Ellonen, P.; van Adrichem, A.J.; Kuusanmäki, H.; Andersson, E.I.; Lagström, S.; Clemente, M.J.; Olson, T.; Jalkanen, S.E.; et al. Somatic STAT3 Mutations in Large Granular Lymphocytic Leukemia. N. Engl. J. Med. 2012, 366, 1905–1913. [Google Scholar] [CrossRef]
- Kulling, P.M.; Olson, K.C.; Hamele, C.E.; Toro, M.F.; Tan, S.F.; Feith, D.J.; Loughran, T.P., Jr. Dysregulation of the IFN-γ-STAT1 signaling pathway in a cell line model of large granular lymphocyte leukemia. PLoS ONE 2018, 13, e0193429. [Google Scholar] [CrossRef]
- Pandya, J.M.; Fasth, A.E.R.; Zong, M.; Arnardottir, S.; Dani, L.; Lindroos, E.; Malmström, V.; Lundberg, I.E. Expanded T cell receptor Vβ-restricted T cells from patients with sporadic inclusion body myositis are proinflammatory and cytotoxic CD28null T cells. Arthritis Care Res. 2010, 62, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Chaara, W.; Rosenzwajg, M.; Six, A.; Prevel, N.; Mingozzi, F.; Wanschitz, J.; Musset, L.; Charuel, J.-L.; Eymard, B.; et al. Th1 Response and Systemic Treg Deficiency in Inclusion Body Myositis. PLoS ONE 2014, 9, e88788. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Jersmann, H.; Tran, H.B.; Roscioli, E.; Holmes, M.; Reynolds, P.N.; Hodge, S. Lymphocyte senescence in COPD is associated with decreased histone deacetylase 2 expression by pro-inflammatory lymphocytes. Respir. Res. 2015, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Knauss, S.; Preusse, C.; Allenbach, Y.; Leonard-Louis, S.; Touat, M.; Fischer, N.; Radbruch, H.; Mothes, R.; Matyash, V.; Böhmerle, W.; et al. PD1 pathway in immune-mediated myopathies. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e558. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.M.; Macaulay, R.; Riddell, N.E.; Nunn, C.J.; Akbar, A.N. Blockade of PD-1 or p38 MAP kinase signaling enhances senescent human CD8+ T-cell proliferation by distinct pathways. Eur. J. Immunol. 2015, 45, 1441–1451. [Google Scholar] [CrossRef]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019, 19, 573–583. [Google Scholar] [CrossRef]
- Covre, L.P.; De Maeyer, R.P.H.; Gomes, D.C.O.; Akbar, A.N. The role of senescent T cells in immunopathology. Aging Cell 2020, 19, e13272. [Google Scholar] [CrossRef]
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 2020, 21, 684–694. [Google Scholar] [CrossRef]
- Choi, H.; Song, H.; Jung, Y.W. The Roles of CCR7 for the Homing of Memory CD8+ T Cells into Their Survival Niches. Immune Netw. 2020, 20, e20. [Google Scholar] [CrossRef]
- Steinway, S.; Loughran, T.P. Targeting IL-15 in large granular lymphocyte leukemia. Expert Rev. Clin. Immunol. 2013, 9, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Derfoul, A.; Pak, K.; Plotz, P.; Miller, F.W.; Milisenda, J.C.; Grau-Junyent, J.M.; Selva-O’Callaghan, A.; Paik, J.; et al. Identification of distinctive interferon gene signatures in different types of myositis. Neurology 2019, 93, e1193–e1204. [Google Scholar] [CrossRef]
- Rigolet, M.; Hou, C.; Amer, Y.B.; Aouizerate, J.; Periou, B.; Gherardi, R.K.; Lafuste, P.; Authier, F.J. Distinct interferon signatures stratify inflammatory and dysimmune myopathies. RMD Open 2019, 5, e000811. [Google Scholar] [CrossRef]
- Roos, A.; Preusse, C.; Hathazi, D.; Goebel, H.-H.; Stenzel, W. Proteomic Profiling Unravels a Key Role of Specific Macrophage Subtypes in Sporadic Inclusion Body Myositis. Front. Immunol. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Parkes, J.E.; Thoma, A.; Lightfoot, A.; Day, P.J.; Chinoy, H.; Lamb, J.A. MicroRNA and mRNA profiling in the idiopathic inflammatory myopathies. BMC Rheumatol. 2020, 4, 25. [Google Scholar] [CrossRef]
- Ivanidze, J.; Hoffmann, R.; Lochmüller, H.; Engel, A.G.; Hohlfeld, R.; Dornmair, K. Inclusion Body Myositis: Laser Microdissection Reveals Differential Up-Regulation of IFN-γ Signaling Cascade in Attacked versus Nonattacked Myofibers. Am. J. Pathol. 2011, 179, 1347–1359. [Google Scholar] [CrossRef]
- Bolko, L.; Jiang, W.; Tawara, N.; Landon-Cardinal, O.; Anquetil, C.; Benveniste, O.; Allenbach, Y. The role of interferons type I, II and III in myositis: A review. Brain Pathol. 2021, 31, e12955. [Google Scholar] [CrossRef]
- Benci, J.L.; Johnson, L.R.; Choa, R.; Xu, Y.; Qiu, J.; Zhou, Z.; Xu, B.; Ye, D.; Nathanson, K.L.; June, C.H.; et al. Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade. Cell 2019, 178, 933–948.e14. [Google Scholar] [CrossRef]
- Lane, R.S.; Femel, J.; Breazeale, A.P.; Loo, C.; Thibault, G.; Kaempf, A.; Mori, M.; Tsujikawa, T.; Chang, Y.H.; Lund, A.W. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 2018, 215, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Carpenter, R.L.; Cao, X.; Lo, H.-W. STAT1 gene expression is enhanced by nuclear EGFR and HER2 via cooperation With STAT3. Mol. Carcinog. 2012, 52, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Guadagnin, E.; Mázala, D.; Chen, Y.-W. STAT3 in Skeletal Muscle Function and Disorders. Int. J. Mol. Sci. 2018, 19, 2265. [Google Scholar] [CrossRef]
- Silva, K.A.S.; Dong, J.; Dong, Y.; Dong, Y.; Schor, N.; Tweardy, D.J.; Zhang, L.; Mitch, W.E. Inhibition of Stat3 Activation Suppresses Caspase-3 and the Ubiquitin-Proteasome System, Leading to Preservation of Muscle Mass in Cancer Cachexia. J. Biol. Chem. 2015, 290, 11177–11187. [Google Scholar] [CrossRef]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef]
- Muñoz-García, N.; Jara-Acevedo, M.; Caldas, C.; Bárcena, P.; López, A.; Puig, N.; Alcoceba, M.; Fernández, P.; Villamor, N.; Flores-Montero, J.A.; et al. STAT3 and STAT5B Mutations in T/NK-Cell Chronic Lymphoproliferative Disorders of Large Granular Lymphocytes (LGL): Association with Disease Features. Cancers 2020, 12, 3508. [Google Scholar] [CrossRef]
- Britson, K.A.; Yang, S.Y.; Lloyd, T.E. New Developments in the Genetics of Inclusion Body Myositis. Curr. Rheumatol. Rep. 2018, 20, 26. [Google Scholar] [CrossRef]
- Keller, C.W.; Schmidt, J.; Lünemann, J.D. Immune and myodegenerative pathomechanisms in inclusion body myositis. Ann. Clin. Transl. Neurol. 2017, 4, 422–445. [Google Scholar] [CrossRef]
- Allison, K.E.; Coomber, B.L.; Bridle, B.W. Metabolic reprogramming in the tumour microenvironment: A hallmark shared by cancer cells and T lymphocytes. Immunology 2017, 152, 175–184. [Google Scholar] [CrossRef]
- Rietveld, A.; Hoogen, L.L.V.D.; Bizzaro, N.; Blokland, S.L.M.; Dähnrich, C.; Gottenberg, J.-E.; Houen, G.; Johannsen, N.; Mandl, T.; Meyer, A.; et al. Autoantibodies to Cytosolic 5′-Nucleotidase 1A in Primary Sjögren’s Syndrome and Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 1200. [Google Scholar] [CrossRef] [PubMed]
- Lilleker, J.B.; Rietveld, A.; Pye, S.R.; Mariampillai, K.; Benveniste, O.; Peeters, M.T.J.; Miller, J.A.L.; Hanna, M.G.; Machado, P.; Parton, M.J.; et al. Cytosolic 5′-nucleotidase 1A autoantibody profile and clinical characteristics in inclusion body myositis. Ann. Rheum. Dis. 2017, 76, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Tawara, N.; Yamashita, S.; Zhang, X.; Korogi, M.; Zhang, Z.; Doki, T.; Matsuo, Y.; Nakane, S.; Maeda, Y.; Sugie, K.; et al. Pathomechanisms of anti-cytosolic 5′-nucleotidase 1A autoantibodies in sporadic inclusion body myositis. Ann. Neurol. 2017, 81, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Camici, M.; Garcia-Gil, M.; Allegrini, S.; Pesi, R.; Tozzi, M.G. Evidence for a Cross-Talk Between Cytosolic 5′-Nucleotidases and AMP-Activated Protein Kinase. Front. Pharmacol. 2020, 11, 609849. [Google Scholar] [CrossRef]
- Shabrokh, E.; Kavanaugh, J.; McMillan, R.; Pittman, J.; Hulver, M.; Frisard, M. Mitochondrial Dysregulation in Skeletal Muscle from Patients Diagnosed with Alzheimer’s Disease and Sporadic Inclusion Body Myositis. Open J. Mol. Integr. Physiol. 2014, 4, 11–19. [Google Scholar] [CrossRef]
- Khan, N.A.J.; Khalid, S.; Ullah, S.; Malik, M.U.; Makhoul, S. Necrotizing Autoimmune Myopathy: A Rare Variant of Idiopathic Inflammatory Myopathies. J. Investig. Med. High Impact Case Rep. 2017, 5, 2324709617709031. [Google Scholar] [CrossRef]
- Hardie, D.G.; Alessi, D.R. LKB1 and AMPK and the cancer-metabolism link—Ten years after. BMC Biol. 2013, 11, 36. [Google Scholar] [CrossRef]
- De Paepe, B. Sporadic Inclusion Body Myositis: An Acquired Mitochondrial Disease with Extras. Biomolecules 2019, 9, 15. [Google Scholar] [CrossRef]
- Oikawa, Y.; Izumi, R.; Koide, M.; Hagiwara, Y.; Kanzaki, M.; Suzuki, N.; Kikuchi, K.; Matsuhashi, T.; Akiyama, Y.; Ichijo, M.; et al. Mitochondrial dysfunction underlying sporadic inclusion body myositis is ameliorated by the mitochondrial homing drug MA-5. PLoS ONE 2020, 15, e0231064. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Sooyeon, L.; Go, K.L.; Kim, J.-S. Deacetylation of mitofusin-2 by sirtuin-1: A critical event in cell survival after ischemia. Mol. Cell. Oncol. 2015, 3, e1087452. [Google Scholar] [CrossRef] [PubMed]
- Askanas, V.; Engel, W.K.; Nogalska, A. Inclusion Body Myositis: A Degenerative Muscle Disease Associated with Intra-Muscle Fiber Multi-Protein Aggregates, Proteasome Inhibition, Endoplasmic Reticulum Stress and Decreased Lysosomal Degradation. Brain Pathol. 2009, 19, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.-H.; Kang, E.-B.; Cho, J.-Y. Resistance Exercise Improves Mitochondrial Quality Control in a Rat Model of Sporadic Inclusion Body Myositis. Gerontology 2019, 65, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Güttsches, A.; Jacobsen, F.; Schreiner, A.; Mertens-Rill, J.; Tegenthoff, M.; Marcus, K.; Vorgerd, M.; Kley, R.A. Chaperones in sporadic inclusion body myositis—Validation of proteomic data. Muscle Nerve 2020, 61, 116–121. [Google Scholar] [CrossRef]
- Emanuele, S.; Lauricella, M.; D’Anneo, A.; Carlisi, D.; De Blasio, A.; Di Liberto, D.; Giuliano, M. p62: Friend or Foe? Evidences for OncoJanus and NeuroJanus Roles. Int. J. Mol. Sci. 2020, 21, 5029. [Google Scholar] [CrossRef]
- Sánchez-Martín, P.; Saito, T.; Komatsu, M. p62/SQSTM 1: ‘Jack of all trades’ in health and cancer. FEBS J. 2019, 286, 8–23. [Google Scholar] [CrossRef]
- Schmitz, K.J.; Ademi, C.; Bertram, S.; Schmid, K.W.; Baba, H.A. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J. Surg. Oncol. 2016, 14, 189. [Google Scholar] [CrossRef]
- Güttsches, A.-K.; Brady, S.; Krause, K.; Msc, A.M.; Uszkoreit, J.; Eisenacher, M.; Schreiner, A.; Galozzi, S.; Mertens-Rill, J.; Tegenthoff, M.; et al. Proteomics of rimmed vacuoles define new risk allele in inclusion body myositis. Ann. Neurol. 2017, 81, 227–239. [Google Scholar] [CrossRef]
- Dionne, L.K.; Peterman, E.; Schiel, J.; Gibieža, P.; Skeberdis, V.A.; Jimeno, A.; Wang, X.-J.; Prekeris, R. FYCO1 regulates accumulation of post-mitotic midbodies by mediating LC3-dependent midbody degradation. J. Cell Sci. 2017, 130, 4051–4062. [Google Scholar] [CrossRef]
- Huntley, M.L.; Gao, J.; Termsarasab, P.; Wang, L.; Zeng, S.; Thammongkolchai, T.; Liu, Y.; Cohen, M.L.; Wang, X. Association between TDP-43 and mitochondria in inclusion body myositis. Lab. Investig. 2019, 99, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-X.; Lin, Y.-F.; Chen, C.-L.; Huang, M.-S.; Hsiao, M.; Liang, P.-H. Chaperonin-Containing TCP-1 Promotes Cancer Chemoresistance and Metastasis through the AKT-GSK3β-β-catenin and XIAP-Survivin Pathways. Cancers 2020, 12, 3865. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The ubiquitin–proteasome system in regulation of the skeletal muscle homeostasis and atrophy: From basic science to disorders. J. Physiol. Sci. 2020, 70, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-K.; Rocnik, E.; Fu, Q.; Kwon, B.; Zeng, L.; Walsh, K.; Querfurth, H. Foxo/Atrogin induction in human and experimental myositis. Neurobiol. Dis. 2012, 46, 463–475. [Google Scholar] [CrossRef]
- Ge, Z.; Leighton, J.S.; Wang, Y.; Peng, X.; Chen, Z.; Chen, H.; Sun, Y.; Yao, F.; Li, J.; Zhang, H.; et al. Integrated Genomic Analysis of the Ubiquitin Pathway across Cancer Types. Cell Rep. 2018, 23, 213–226.e3. [Google Scholar] [CrossRef]
- Kwon, B.; Kumar, P.; Lee, H.-K.; Zeng, L.; Walsh, K.; Fu, Q.; Barakat, A.; Querfurth, H.W. Aberrant cell cycle reentry in human and experimental inclusion body myositis and polymyositis. Hum. Mol. Genet. 2014, 23, 3681–3694. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Monteiro, L.S.; Diniz-Freitas, M.; Warnakulasuriya, S.; Garcia-Caballero, T.; Forteza-Vila, J.; Fraga, M. Prognostic Significance of Cyclins A2, B1, D1, and E1 and CCND1 Numerical Aberrations in Oral Squamous Cell Carcinomas. Anal. Cell. Pathol. 2018, 2018, 7253510. [Google Scholar] [CrossRef]
- Zheng, Z.-Z.; Ma, Y.-P.; Wu, R.-H.; Rong, G.; Li, C.; Li, G.-X.; Ren, F.-G.; Xu, L.-J. Serum miR-133 as a novel biomarker for predicting treatment response and survival in acute myeloid leukemia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 777–783. [Google Scholar]
- Amici, D.R.; Pinal-Fernandez, I.; Mázala, D.A.G.; Lloyd, T.E.; Corse, A.M.; Christopher-Stine, L.; Mammen, A.L.; Chin, E.R. Calcium dysregulation, functional calpainopathy, and endoplasmic reticulum stress in sporadic inclusion body myositis. Acta Neuropathol. Commun. 2017, 5, 24. [Google Scholar] [CrossRef]
- Johari, M.; Vihola, A.; Palmio, J.; Jokela, M.; Jonson, P.H.; Sarparanta, J.; Huovinen, S.; Savarese, M.; Hackman, P.; Udd, B. Comprehensive transcriptomic analysis shows disturbed calcium homeostasis and deregulation of T lymphocyte apoptosis in inclusion body myositis. J. Neurol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, I.; Harper, D.; Greer, P.A. Calpain as a therapeutic target in cancer. Expert Opin. Ther. Targets 2022, 26, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh, M.; Lam, T.; Greenberg, S.A. Autoantibodies against a 43 KDa Muscle Protein in Inclusion Body Myositis. PLoS ONE 2011, 6, e20266. [Google Scholar] [CrossRef] [PubMed]
- Amlani, A.; Choi, M.Y.; Tarnopolsky, M.; Brady, L.; Clarke, A.E.; La Torre, I.G.-D.; Mahler, M.; Schmeling, H.; Barber, C.; Jung, M.; et al. Anti-NT5c1A Autoantibodies as Biomarkers in Inclusion Body Myositis. Front. Immunol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Herbert, M.; Pruijn, G.J. Novel serology testing for sporadic inclusion body myositis. Curr. Opin. Rheumatol. 2015, 27, 595–600. [Google Scholar] [CrossRef]
- Jędrzejewska, A.; Kutryb-Zając, B.; Król, O.; Harasim, G.; Frańczak, M.; Jabłońska, P.; Słomińska, E.; Smoleński, R.T. The decreased serum activity of cytosolic 5′-nucleotidase IA as a potential marker of breast cancer-associated muscle inflammation. Nucleosides Nucleotides Nucleic Acids 2021, 41, 273–284. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Karlsson, H.K.; Szekeres, F.; Chibalin, A.V.; Krook, A.; Zierath, J.R. Suppression of 5′-Nucleotidase Enzymes Promotes AMP-activated Protein Kinase (AMPK) Phosphorylation and Metabolism in Human and Mouse Skeletal Muscle. J. Biol. Chem. 2011, 286, 34567–34574. [Google Scholar] [CrossRef]
- Meyer, A.; Laverny, G.; Allenbach, Y.; Grelet, E.; Ueberschlag, V.; Echaniz-Laguna, A.; Lannes, B.; Alsaleh, G.; Charles, A.L.; Singh, F.; et al. IFN-β-induced reactive oxygen species and mitochondrial damage contribute to muscle impairment and inflammation maintenance in dermatomyositis. Acta Neuropathol. 2017, 134, 655–666. [Google Scholar] [CrossRef]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell 2019, 178, 1231–1244.e11. [Google Scholar] [CrossRef]
- Lodi, R.S.; Yu, B.; Xia, L.; Liu, F. Roles and Regulation of Growth differentiation factor-15 in the Immune and tumor microenvironment. Hum Immunol. 2021, 82, 937–944. [Google Scholar] [CrossRef]
- Yousafzai, N.A.; Jin, H.; Ullah, M.; Wang, X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am. J. Cancer Res. 2021, 11, 5233–5248. [Google Scholar] [PubMed]
- Lin, Z.; Fang, D. The Roles of SIRT1 in Cancer. Genes Cancer 2013, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Ciccia, F.; Priori, R.; Astorri, E.; Guggino, G.; Alessandro, R.; Rizzo, A.; Conti, F.; Minniti, A.; Barbati, C.; et al. CD4 T lymphocyte autophagy is upregulated in the salivary glands of primary Sjögren’s syndrome patients and correlates with focus score and disease activity. Arthritis Res. Ther. 2017, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ying, Y.; Xie, H.; Liu, X.; Wang, X.; Li, J. The Regulatory Role of RNA Metabolism Regulator TDP-43 in Human Cancer. Front. Oncol. 2021, 11, 755096. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; Suharacb, T.; Rosenab, K.M.; McPhie, D.L.; Fujio, Y.; Tejadae, G.; Neve, R.L.; Adelman, L.S.; Walshcb, K. β-Amyloid Peptide Expression Is Sufficient for Myotube Death: Implications for Human Inclusion Body Myopathy. Mol. Cell. Neurosci. 2001, 17, 793–810. [Google Scholar] [CrossRef]
- Pandey, P.; Sliker, B.; Peters, H.L.; Tuli, A.; Herskovitz, J.; Smits, K.; Purohit, A.; Singh, R.K.; Dong, J.; Batra, S.K.; et al. Amyloid precursor protein and amyloid precursor-like protein 2 in cancer. Oncotarget 2016, 7, 19430–19444. [Google Scholar] [CrossRef]
- Pavliukeviciene, B.; Zentelyte, A.; Jankunec, M.; Valiuliene, G.; Talaikis, M.; Navakauskiene, R.; Niaura, G.; Valincius, G. Amyloid β oligomers inhibit growth of human cancer cells. PLoS ONE 2019, 14, e0221563. [Google Scholar] [CrossRef]
- Souter, S.; Lee, G. Microtubule-associated protein tau in human prostate cancer cells: Isoforms, phosphorylation, and interactions. J. Cell. Biochem. 2009, 108, 555–564. [Google Scholar] [CrossRef]
- Papin, S.; Paganetti, P. Emerging Evidences for an Implication of the Neurodegeneration-Associated Protein TAU in Cancer. Brain Sci. 2020, 10, 862. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Fratta, P.; Engel, W.K.; Van Leeuwen, F.W.; Hol, E.M.; Vattemi, G.; Askanas, V. Mutant ubiquitin UBB+1 is accumulated in sporadic inclusion-body myositis muscle fibers. Neurology 2004, 63, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, I.; Eran, A.; Nishino, I.; Moggio, M.; Lamperti, C.; Amato, A.A.; Lidov, H.G.; Kang, P.B.; North, K.N.; Mitrani-Rosenbaum, S.; et al. Distinctive patterns of microRNA expression in primary muscular disorders. Proc. Natl. Acad. Sci. USA 2007, 104, 17016–17021. [Google Scholar] [CrossRef] [PubMed]
- Zawit, M.; Bahaj, W.; Gurnari, C.; Maciejewski, J. Large Granular Lymphocytic Leukemia: From Immunopathogenesis to Treatment of Refractory Disease. Cancers 2021, 13, 4418. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.C.; Larkin, P.; Signorelli, R.; Hamele, C.; Olson, T.L.; Conaway, M.R.; Feith, D.J.; Loughran, T.P. Vitamin D pathway activation selectively deactivates signal transducer and activator of transcription (STAT) proteins and inflammatory cytokine production in natural killer leukemic large granular lymphocytes. Cytokine 2018, 111, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, S.; Lundberg, I.E. Current Treatment for Myositis. Curr. Treat. Options Rheumatol. 2018, 4, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef]
- Meyer, A.; Scirè, C.A.; Talarico, R.; Alexander, T.; Amoura, Z.; Avcin, T.; Barsotti, S.; Beretta, L.; Blagojevic, J.; Burmester, G.; et al. Idiopathic inflammatory myopathies: State of the art on clinical practice guidelines. RMD Open 2019, 4 (Suppl. 1), e000784. [Google Scholar] [CrossRef]
| Characteristics | Exhausted T Cell | Senescent T Cell |
|---|---|---|
| Cell cycle | Reversible block | Irreversible block |
| T cell markers | CD44+/− | Loss of CD27, CD28, +/−CCR7 Re-expression of CD45RA |
| NK markers | - | CD57++, KLRG1++ |
| Metabolic | PI3k/AKT/mTOR+/− MAPK++ | PI3k/AKT/mTOR+/− |
| Expression | PD-1, TIM1, LAG3, CTLA4, TIGIT | perforin, IFNγ, TNFα, granzyme B, KLRG1 |
| Function | Defective effector functions | Effector functions (SASP) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damian, L.; Login, C.C.; Solomon, C.; Belizna, C.; Encica, S.; Urian, L.; Jurcut, C.; Stancu, B.; Vulturar, R. Inclusion Body Myositis and Neoplasia: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 7358. https://doi.org/10.3390/ijms23137358
Damian L, Login CC, Solomon C, Belizna C, Encica S, Urian L, Jurcut C, Stancu B, Vulturar R. Inclusion Body Myositis and Neoplasia: A Narrative Review. International Journal of Molecular Sciences. 2022; 23(13):7358. https://doi.org/10.3390/ijms23137358
Chicago/Turabian StyleDamian, Laura, Cristian Cezar Login, Carolina Solomon, Cristina Belizna, Svetlana Encica, Laura Urian, Ciprian Jurcut, Bogdan Stancu, and Romana Vulturar. 2022. "Inclusion Body Myositis and Neoplasia: A Narrative Review" International Journal of Molecular Sciences 23, no. 13: 7358. https://doi.org/10.3390/ijms23137358
APA StyleDamian, L., Login, C. C., Solomon, C., Belizna, C., Encica, S., Urian, L., Jurcut, C., Stancu, B., & Vulturar, R. (2022). Inclusion Body Myositis and Neoplasia: A Narrative Review. International Journal of Molecular Sciences, 23(13), 7358. https://doi.org/10.3390/ijms23137358







