Recent Development of Polydopamine Anti-Bacterial Nanomaterials
Abstract
:1. Introduction
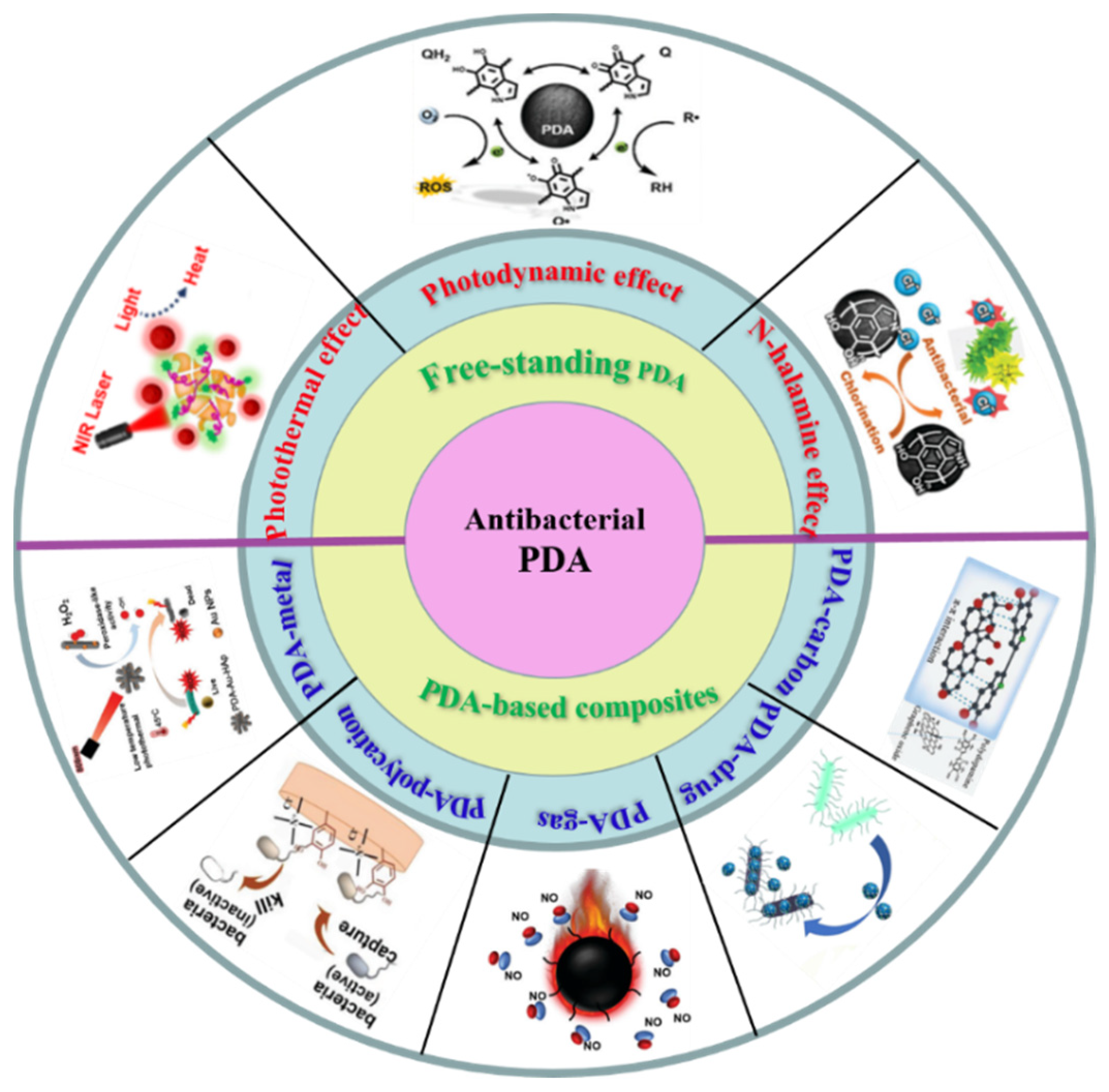
2. Free-Standing PDA Anti-Bacteria
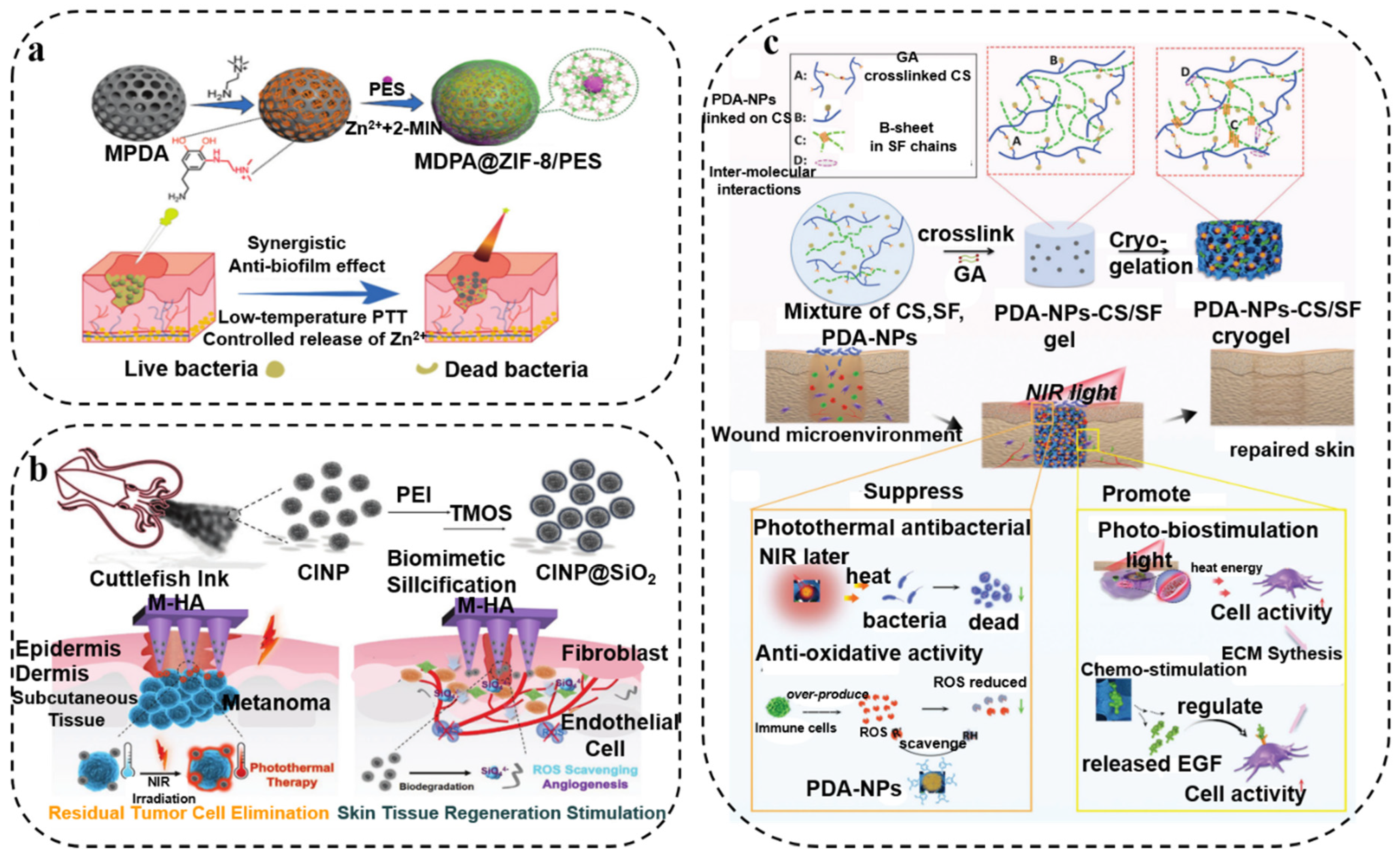
2.1. Photothermal Antibacterial Therapy
2.2. Photodynamic Antibacterial Therapy
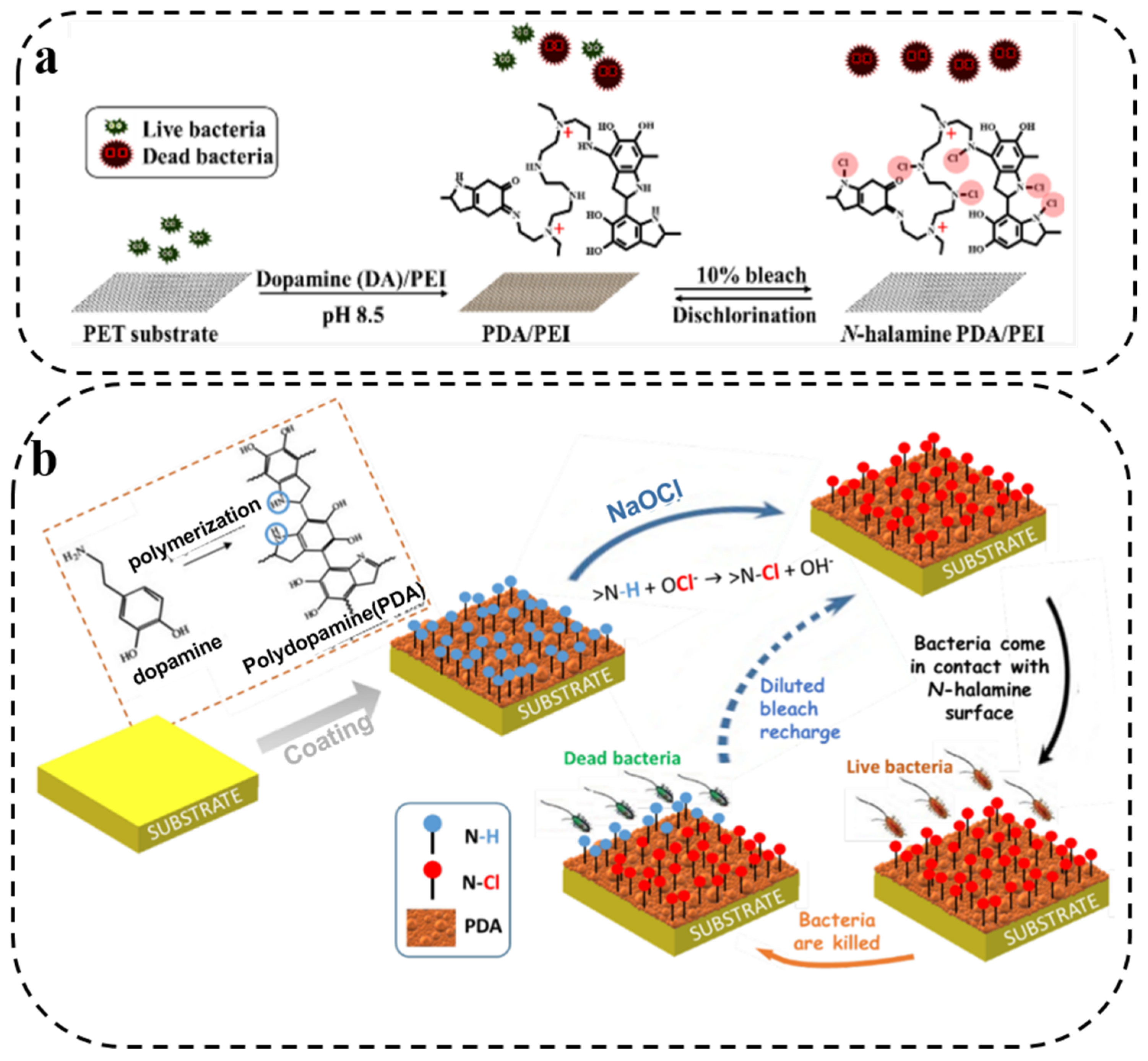
2.3. N-Halamine Antibacterial Therapy
3. PDA Composite Synergistic Antibacterial Therapy
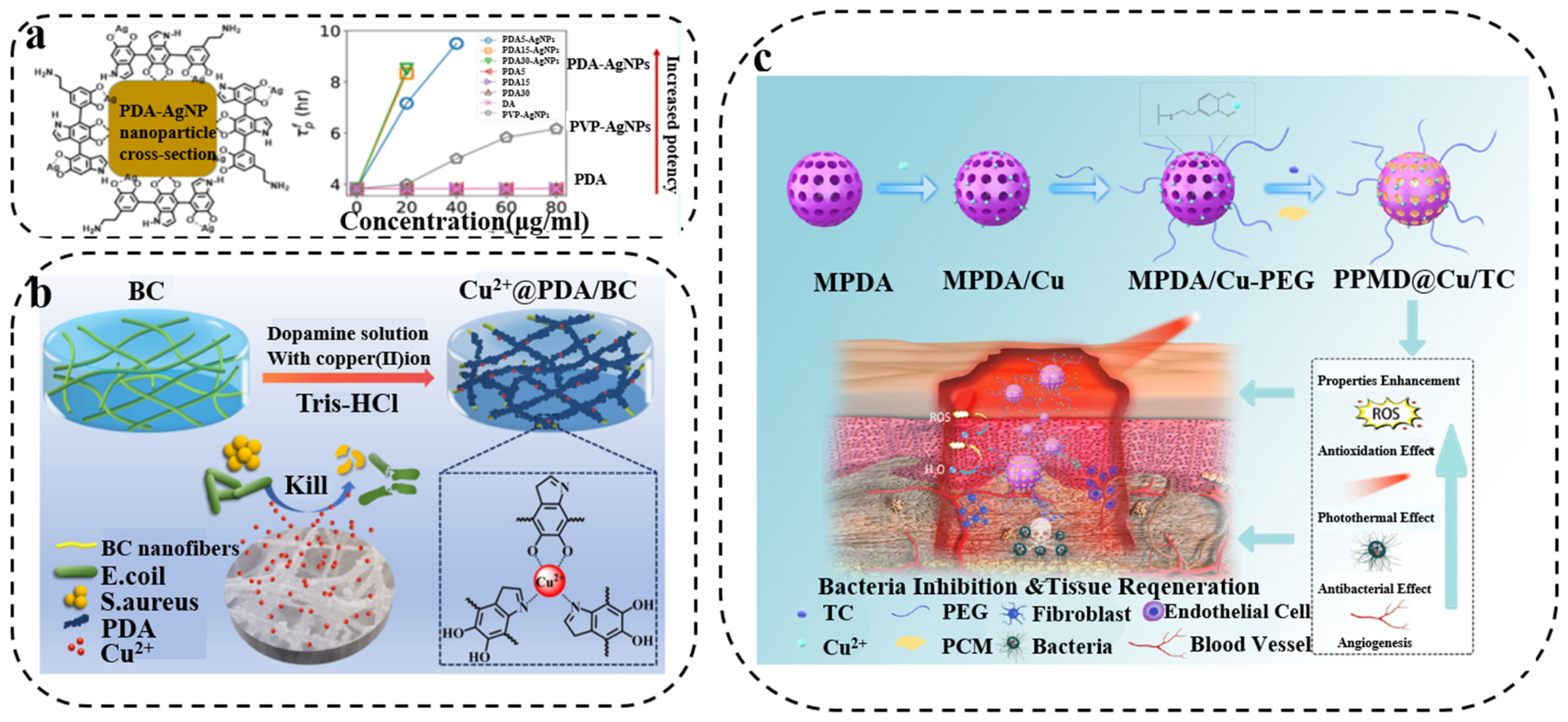
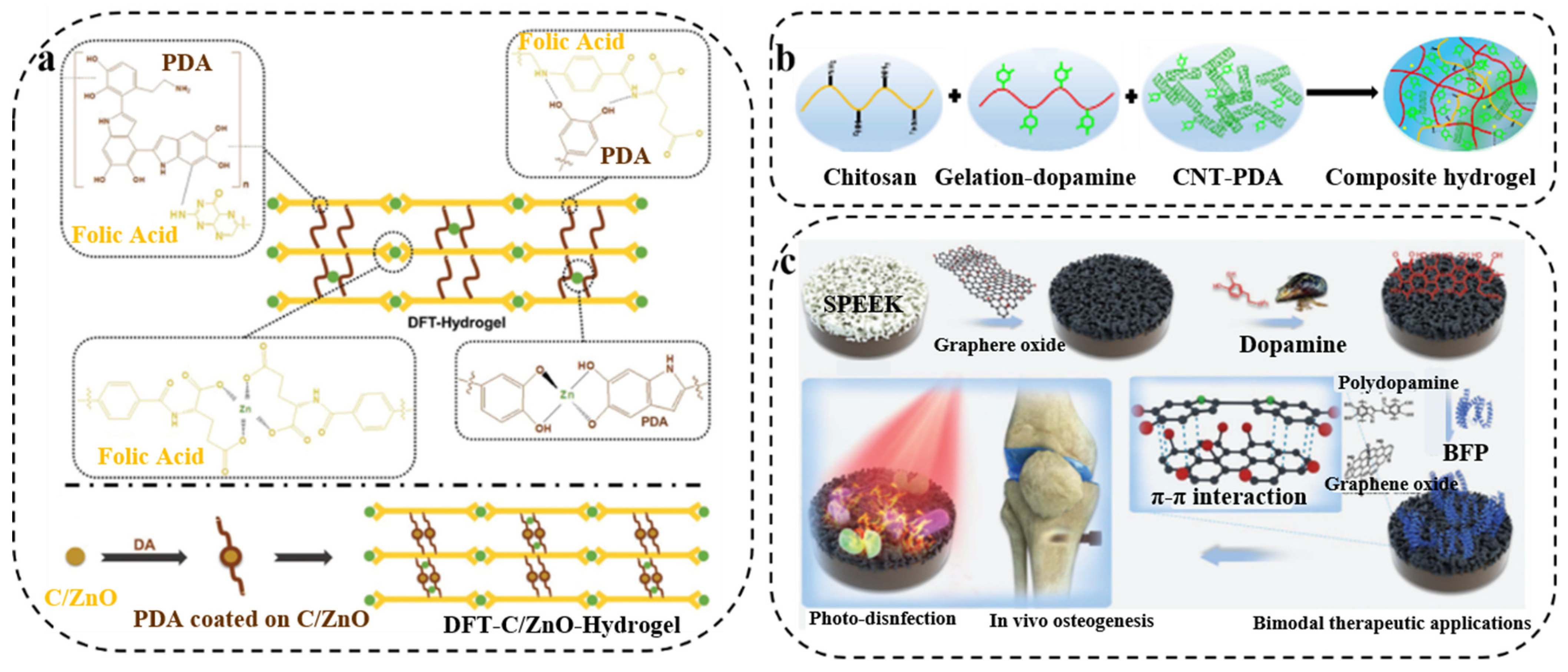
3.1. PDA/Metal Composite Synergistic Antibacterial Therapy
3.2. PDA/Polycation Composite Synergistic Antibacterial Therapy
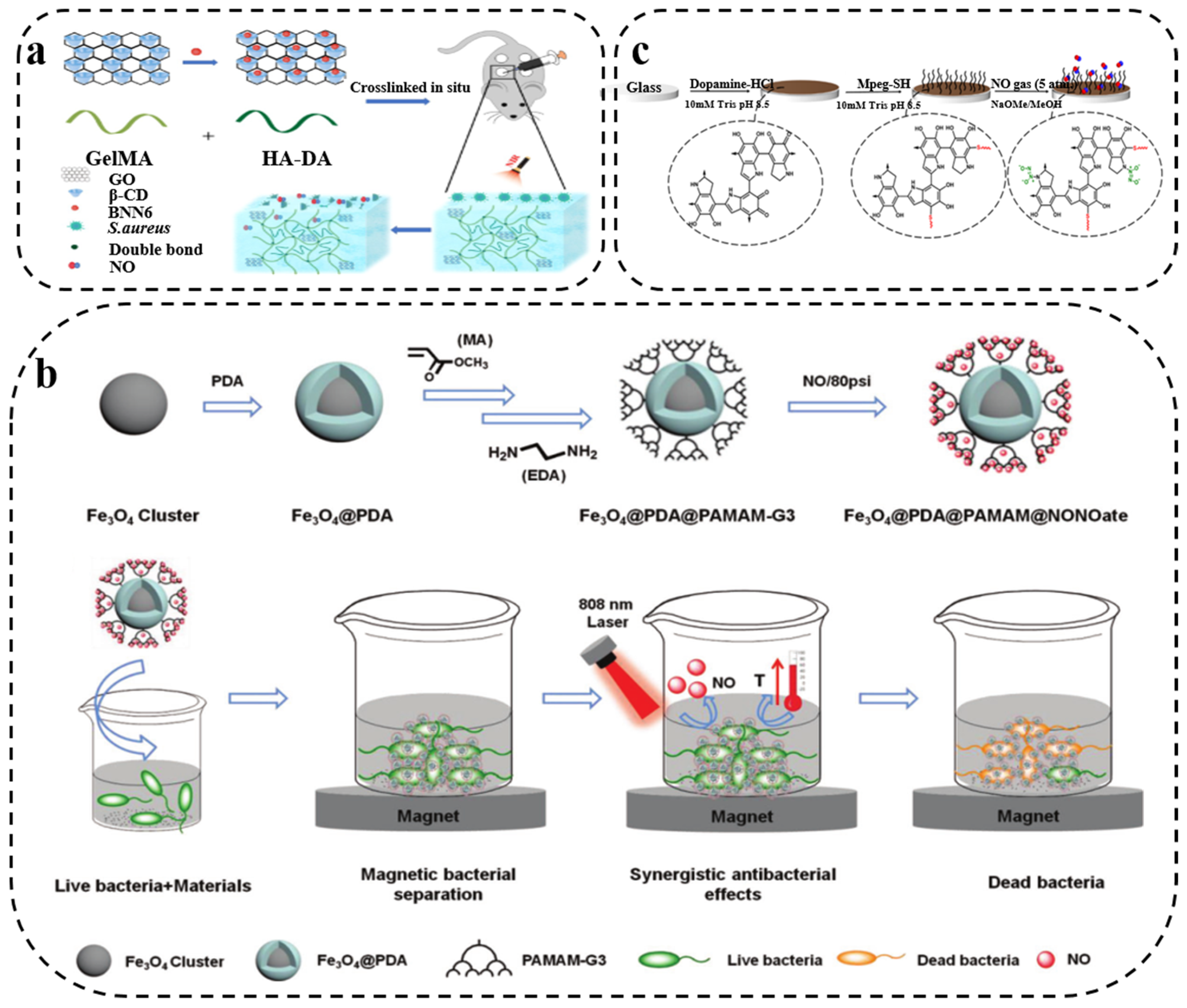
3.3. PDA/Gas Composite Synergistic Antibacterial Therapy
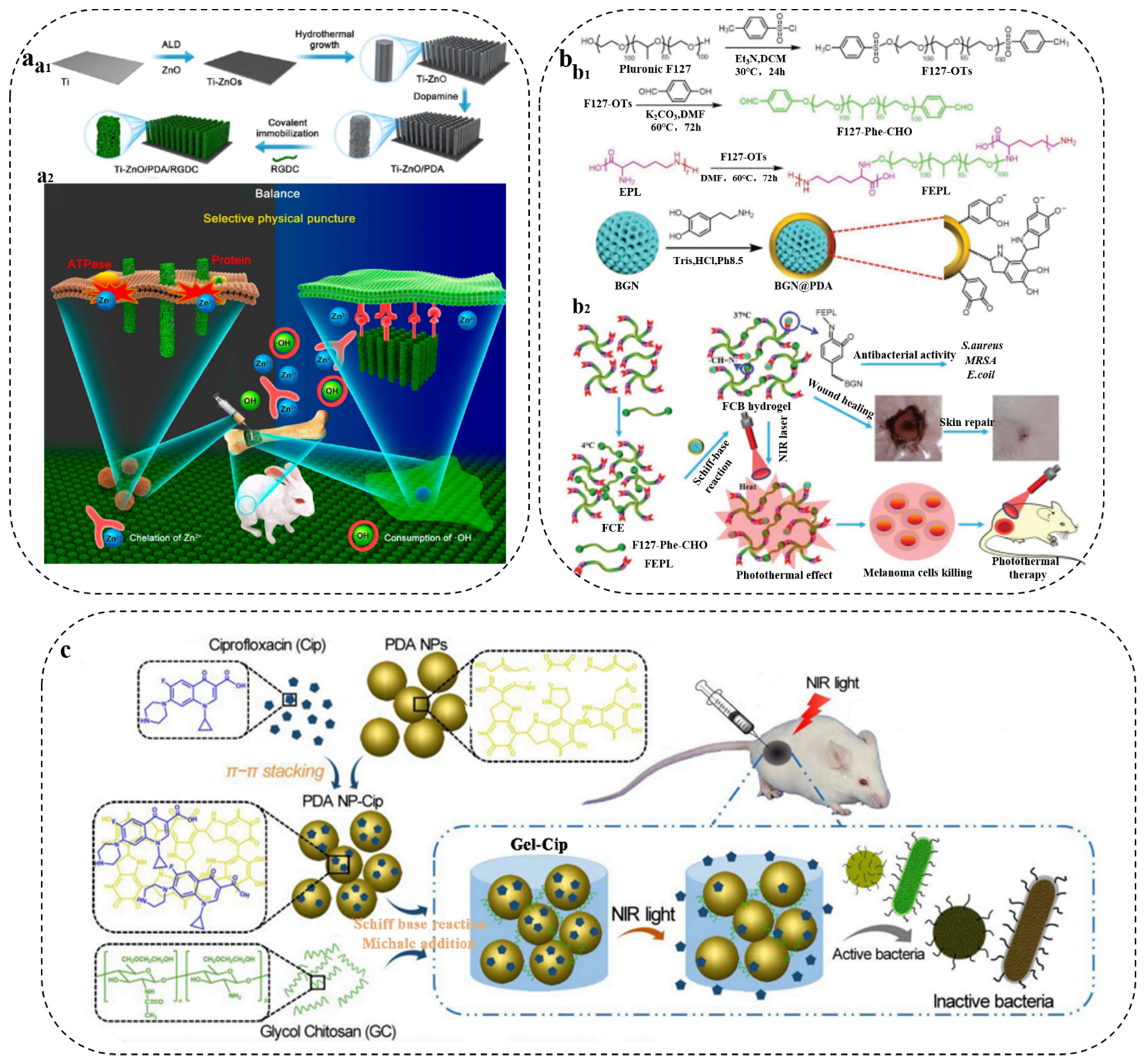
3.4. PDA/Antibacterial Drug Composite Synergistic Antibacterial Therapy
3.5. PDA/Carbon Composite Synergistic Antibacterial Therapy
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, H.; Yang, Y.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 2019, 31, 1805092. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Molecular mechanisms of antibiotic resistance. Chem. Commun. 2011, 47, 4055–4061. [Google Scholar] [CrossRef]
- Davies, S.C.; Fowler, T.; Watson, J.; Livermore, D.M.; Walker, D. Annual Report of the Chief Medical Officer: Infection and the rise of antimicrobial resistance. Lancet 2013, 381, 1606–1609. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Chen, W.; Wang, J.; Chen, H.; Sun, L.; Li, X.; Ji, J.; Yu, Q.; Shen, L.; et al. Construction of nanomaterials with targeting phototherapy properties to inhibit resistant bacteria and biofilm infections. Chem. Eng. J. 2019, 358, 74–90. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Hu, J.; Hu, J.; Zhang, S.; Yang, Z.; Li, Y.; Cheng, Y. Smart hydrogels with antibacterial properties built from all natural building blocks. Chem. Mater. 2019, 31, 7678–7685. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-free antibacterial strategies enabled by nanomaterials: Progress and perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.-K.; Koo, B.; Zheng, J.; Aghaloo, T.; Lee, M. Chitosan–lysozyme conjugates for enzyme-triggered hydrogel degradation in tissue engineering applications. ACS Appl. Mater. Inter. 2018, 10, 41138–41145. [Google Scholar] [CrossRef]
- Gu, J.; Su, Y.; Liu, P.; Li, P.; Yang, P. An environmentally benign antimicrobial coating based on a protein supramolecular assembly. ACS Appl. Mater. Inter. 2017, 9, 198–210. [Google Scholar] [CrossRef]
- Gao, Q.; Li, P.; Zhao, H.; Chen, Y.; Jiang, L.; Ma, P.X. Methacrylate-ended polypeptides and polypeptoids for antimicrobial and antifouling coatings. Polym. Chem. 2017, 8, 6386–6397. [Google Scholar] [CrossRef]
- Gao, Q.; Yu, M.; Su, Y.; Xie, M.; Zhao, X.; Li, P.; Ma, P.X. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta. Biomater. 2017, 51, 112–124. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.-Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011, 10, 149–156. [Google Scholar] [CrossRef]
- Wang, Y.; El-Deen, A.G.; Li, P.; Oh, B.H.L.; Guo, Z.; Khin, M.M.; Vikhe, Y.S.; Wang, J.; Hu, R.G.; Boom, R.M.; et al. High-Performance Capacitive Deionization Disinfection of Water with Graphene Oxide-graft-Quaternized Chitosan Nanohybrid Electrode Coating. ACS Nano 2015, 9, 10142–10157. [Google Scholar] [CrossRef] [Green Version]
- Natalio, F.; André, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef]
- Qiu, J.; Geng, H.; Wang, D.; Qian, S.; Zhu, H.; Qiao, Y.; Qian, W.; Liu, X. Layer-number dependent antibacterial and osteogenic behaviors of graphene oxide electrophoretic deposited on titanium. ACS Appl. Mater. Inter. 2017, 9, 12253–12263. [Google Scholar] [CrossRef]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Edit. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Yang, T.; Qiu, H.; Lu, L.; Tu, Q.; Xiong, K.; Huang, N.; Yang, Z. Mussel-inspired “built-up” surface chemistry for combining nitric oxide catalytic and vascular cell selective properties. Biomaterials 2020, 241, 119904. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Li, X.; Huang, N.; Zhao, X.; Yang, Z. Mussel-inspired dopamine-Cu(II) coatings for sustained in situ generation of nitric oxide for prevention of stent thrombosis and restenosis. Biomaterials 2019, 194, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Q.; He, X.; Chen, H.; Zou, Y.; Li, Y.; Lin, K.; Cai, X.; Xiao, J.; Zhang, Q.; et al. Multifunctional melanin-like nanoparticles for bone-targeted chemo-photothermal therapy of malignant bone tumors and osteolysis. Biomaterials 2018, 183, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Edit. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-Containing Polydopamine Nanomaterials: Catalysis, Energy, and Theranostics. Small 2020, 16, e1907042. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Hu, J.; Yang, L.; Yang, P.; Jiang, S.; Liu, X.; Li, Y. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8, 4940–4950. [Google Scholar] [CrossRef]
- Huang, S.; Liang, N.; Hu, Y.; Zhou, X.; Abidi, N. Polydopamine-Assisted Surface Modification for Bone Biosubstitutes. Biomed. Res. Int. 2016, 2016, 2389895. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lan, Y.; Zhu, W.; Li, W.; Xu, D.; Cui, J.; Shen, D.; Li, G. Polydopamine-coated nanofibrous mats as a versatile platform for producing porous functional membranes. J. Mater. Chem. 2012, 22, 16994–17001. [Google Scholar] [CrossRef]
- Geng, H.; Zhuang, L.; Li, M.; Liu, H.; Caruso, F.; Hao, J.; Cui, J. Interfacial Assembly of Metal-Phenolic Networks for Hair Dyeing. ACS Appl. Mater. Inter. 2020, 12, 29826–29834. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Fu, Y.; Yang, L.; Zhu, F.; Liu, X.; Gu, Z.; Li, Y. Antioxidant shape amphiphiles for accelerated wound healing. J. Mater. Chem. 2020, 8, 7018–7023. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.; Xia, W.; Li, H.; He, X.; Zhou, Y.; Liu, X.; Zhang, C.; Li, Y. Tea stain-inspired solar energy harvesting polyphenolic nanocoatings with tunable absorption spectra. Nano Res. 2021, 14, 969–975. [Google Scholar] [CrossRef]
- Lu, Z.; Xiao, J.; Wang, Y.; Meng, M. In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid. Interf. Sci. 2015, 452, 8–14. [Google Scholar] [CrossRef]
- Patel, K.; Kushwaha, P.; Kumar, S.; Kumar, R. Lysine and α-Aminoisobutyric Acid Conjugated Bioinspired Polydopamine Surfaces for the Enhanced Antibacterial Performance of the Foley Catheter. ACS Appl. Bio Mater. 2019, 2, 5799–5809. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Inter. 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Zhang, L.; Wang, W. Highly conductive one-dimensional nanofibers: Silvered electrospun silica nanofibers via poly(dopamine) functionalization. ACS Appl. Mater. Inter. 2014, 6, 5105–5112. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta. Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Chiu, T.-H. Stable N-halamine on polydopamine coating for high antimicrobial efficiency. Eur. Polym. J. 2020, 130, 109654. [Google Scholar] [CrossRef]
- Xu, J.W.; Yao, K.; Xu, Z.K. Nanomaterials with a photothermal effect for antibacterial activities: An overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef]
- Li, J.; Cui, D.; Jiang, Y.; Huang, J.; Cheng, P.; Pu, K. Near-Infrared Photoactivatable Semiconducting Polymer Nanoblockaders for Metastasis-Inhibited Combination Cancer Therapy. Adv. Mater. 2019, 31, e1905091. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, H.; Wang, Y.; Sevencan, C.; Li, B.L. Functionalized MoS2-Based Nanomaterials for Cancer Phototherapy and Other Biomedical Applications. ACS Mater. Lett. 2021, 3, 462–496. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Q.; Zha, Z.; Zhu, D.; Zheng, L.; Shi, L.; Wei, X.; Lian, L.; Wu, K.; Cheng, L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 2021, 6, 4389–4401. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Zhou, L.; Su, Y.; Dong, C.M. A sweet polydopamine nanoplatform for synergistic combination of targeted chemo-photothermal therapy. Macromol. Rapid Comm. 2015, 36, 916–922. [Google Scholar] [CrossRef]
- Tran, H.Q.; Batul, R.; Bhave, M.; Yu, A. Current Advances in the Utilization of Polydopamine Nanostructures in Biomedical Therapy. Biotechnol. J. 2019, 14, e1900080. [Google Scholar] [CrossRef]
- Hu, D.; Zou, L.; Li, B.; Hu, M.; Ye, W.; Ji, J. Photothermal Killing of Methicillin-Resistant Staphylococcus aureus by Bacteria-Targeted Polydopamine Nanoparticles with Nano-Localized Hyperpyrexia. ACS Biomater. Sci. Eng. 2019, 5, 5169–5179. [Google Scholar] [CrossRef]
- Peng, D.; Liu, G.; He, Y.; Gao, P.; Gou, S.; Wu, J.; Yu, J.; Liu, P.; Cai, K. Fabrication of a pH-responsive core-shell nanosystem with a low-temperature photothermal therapy effect for treating bacterial biofilm infection. Biomater. Sci. 2021, 9, 7483–7491. [Google Scholar] [CrossRef]
- Lei, Q.; He, D.; Ding, L.; Kong, F.; He, P.; Huang, J.; Guo, J.; Brinker, C.J.; Luo, G.; Zhu, W. Microneedle Patches Integrated with Biomineralized Melanin Nanoparticles for Simultaneous Skin Tumor Photothermal Therapy and Wound Healing. Adv. Funct. Mater. 2022, 2113269. [Google Scholar] [CrossRef]
- Ye, Y.; Zheng, L.; Wu, T.; Ding, X.; Chen, F.; Yuan, Y.; Fan, G.C.; Shen, Y. Size-Dependent Modulation of Polydopamine Nanospheres on Smart Nanoprobes for Detection of Pathogenic Bacteria at Single-Cell Level and Imaging-Guided Photothermal Bactericidal Activity. ACS Appl. Mater. Inter. 2020, 12, 35626–35637. [Google Scholar] [CrossRef]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef]
- Jia, Q.; Song, Q.; Li, P.; Huang, W. Rejuvenated Photodynamic Therapy for Bacterial Infections. Adv. Healthc. Mater. 2019, 8, e1900608. [Google Scholar] [CrossRef]
- Wu, M.; Chen, C.; Liu, Z.; Tian, J.; Zhang, W. Regulating the bacterial oxygen microenvironment via a perfluorocarbon-conjugated bacteriochlorin for enhanced photodynamic antibacterial efficacy. Acta. Biomater. 2022, 142, 242–252. [Google Scholar] [CrossRef]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect. Biochem. Molec. 2005, 35, 443–459. [Google Scholar] [CrossRef]
- Hao, L.; Jiang, R.; Fan, Y.; Xu, J.-n.; Tian, L.; Zhao, J.; Ming, W.; Ren, L. Formation and Antibacterial Performance of Metal–Organic Framework Films via Dopamine-Mediated Fast Assembly under Visible Light. ACS Sustain. Chem. Eng. 2020, 8, 15834–15842. [Google Scholar] [CrossRef]
- Su, R.; Yan, H.; Li, P.; Zhang, B.; Zhang, Y.; Su, W. Photo-enhanced antibacterial activity of polydopamine-curcumin nanocomposites with excellent photodynamic and photothermal abilities. Photodiagn. Photodyn. 2021, 35, 102417. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hai, L.; Li, Y.; Li, H.; Gong, M.; Wang, Z.; Tang, Z.; Deng, L.; He, D. An Ultrasmall Fe3O4 -Decorated Polydopamine Hybrid Nanozyme Enables Continuous Conversion of Oxygen into Toxic Hydroxyl Radical via GSH-Depleted Cascade Redox Reactions for Intensive Wound Disinfection. Small 2022, 18, e2105465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, X.; Jie, Z.; Yang, H.; Yang, S.; Liang, J. Regenerable antimicrobial N-halamine/silica hybrid nanoparticles. J. Nanopart. Res. 2014, 16, 2454. [Google Scholar] [CrossRef]
- Wan, X.; Zhuang, L.; She, B.; Deng, Y.; Chen, D.; Tang, J. In-situ reduction of monodisperse nanosilver on hierarchical wrinkled mesoporous silica with radial pore channels and its antibacterial performance. Mat. Sci. Eng. C 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Song, C.; Chang, Y.; Cheng, L.; Xu, Y.; Chen, X.; Zhang, L.; Zhong, L.; Dai, L. Preparation, characterization, and antibacterial activity studies of silver-loaded poly(styrene-co-acrylic acid) nanocomposites. Mat. Sci. Eng. C 2014, 36, 146–151. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Pu, Y.; Chen, Y.; Zhao, J.; Tang, J. Ultra-fine silver nanoparticles dispersed in mono-dispersed amino functionalized poly glycidyl methacrylate based microspheres as an effective anti-bacterial agent. React. Funct. Polym. 2016, 103, 92–98. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Y.J.; Gao, Y.; Gao, T.; Gao, G. Chemical Insights into Antibacterial N-Halamines. Chem. Rev. 2017, 117, 4806–4862. [Google Scholar] [CrossRef]
- Chien, H.W.; Chiu, T.H.; Lee, Y.L. Rapid Biocidal Activity of N-Halamine-Functionalized Polydopamine and Polyethylene Imine Coatings. Langmuir 2021, 37, 8037–8044. [Google Scholar] [CrossRef]
- Nazi, N.; Humblot, V.; Debiemme-Chouvy, C. A New Antibacterial N-Halamine Coating Based on Polydopamine. Langmuir 2020, 36, 11005–11014. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, Y.; Teng, G.; Wang, L.; Jin, X.; Qiang, Z.; Ma, W. Fabrication of a Double-Shell Ag/AgCl/G-ZnFe2O4 Nanocube with Enhanced Light Absorption and Superior Photocatalytic Antibacterial Activity. ACS Appl. Mater. Inter. 2020, 12, 29883–29898. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone). ACS Appl. Mater. Inter. 2013, 5, 12895–12904. [Google Scholar] [CrossRef]
- He, S.; Zhou, P.; Wang, L.; Xiong, X.; Zhang, Y.; Deng, Y.; Wei, S. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Wang, S.-N.; Zhao, T.-T.; Li, Y. Surface characteristics, corrosion behavior, and antibacterial property of Ag-implanted NiTi alloy. Rare Met. 2013, 32, 113–121. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Huang, J.; Mao, L.; Chen, J.; Li, M. Preparation and characterization of antifouling and antibacterial polysulfone ultrafiltration membranes incorporated with a silver–polydopamine nanohybrid. J. Appl. Polym. Sci. 2018, 135, 46430. [Google Scholar] [CrossRef]
- Islam, M.S.; Akter, N.; Rahman, M.M.; Shi, C.; Islam, M.T.; Zeng, H.; Azam, M.S. Mussel-inspired immobilization of silver nanoparticles toward antimicrobial cellulose paper. ACS Sustain. Chem. Eng. 2018, 6, 9178–9188. [Google Scholar] [CrossRef]
- Coelho, D.; Sampaio, A.; Silva, C.; Felgueiras, H.P.; Amorim, M.T.P.; Zille, A. Antibacterial Electrospun Poly(vinyl alcohol)/Enzymatic Synthesized Poly(catechol) Nanofibrous Midlayer Membrane for Ultrafiltration. ACS Appl. Mater. Inter. 2017, 9, 33107–33118. [Google Scholar] [CrossRef] [Green Version]
- Niyonshuti, I.I.; Krishnamurthi, V.R.; Okyere, D.; Song, L.; Benamara, M.; Tong, X.; Wang, Y.; Chen, J. Polydopamine Surface Coating Synergizes the Antimicrobial Activity of Silver Nanoparticles. ACS. Appl. Mater. Inter. 2020, 12, 40067–40077. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta. Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: Implications for a new antibacterial mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Feng, F.; Jiang, W.; Ma, L.; Liu, M.; Li, K.; Wan, Y.; Ao, H. Designment of polydopamine/bacterial cellulose incorporating copper (II) sulfate as an antibacterial wound dressing. Biomater. Adv. 2022, 134, 112591. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.N.; Wang, D.; Yu, Q.P.; Yu, Z.P.; Wang, H.Y.; Wu, C.Y.; Du, S.W.; Chen, X.Y.; Li, J.F.; Zhou, Z.K.; et al. Near-Infrared Light-Controllable Multifunction Mesoporous Polydopamine Nanocomposites for Promoting Infected Wound Healing. ACS Appl. Mater. Inter. 2022, 14, 2534–2550. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Das, P.; Natan, M.; Jacobi, G.; Banin, E.; Luong, J.H.T.; Gedanken, A. Antimicrobial Activities of Zn-Doped CuO Microparticles Decorated on Polydopamine against Sensitive and Antibiotic-Resistant Bacteria. ACS Appl. Polym. Mater. 2020, 2, 5878–5888. [Google Scholar] [CrossRef]
- Du, Y.; Yu, M.; Ge, J.; Ma, P.X.; Chen, X.; Lei, B. Development of a Multifunctional Platform Based on Strong, Intrinsically Photoluminescent and Antimicrobial Silica-Poly(citrates)-Based Hybrid Biodegradable Elastomers for Bone Regeneration. Adv. Funct. Mater. 2015, 25, 5016–5029. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Qian, Y.; Hu, J.; Liu, S. Amphiphilic star copolymer-based bimodal fluorogenic/magnetic resonance probes for concomitant bacteria detection and inhibition. Adv. Mater. 2014, 26, 6734–6741. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Z.; Liu, L.; Lv, F.; Wang, Y.; Wang, S. Cationic conjugated polymers for discrimination of microbial pathogens. Adv. Mater. 2014, 26, 4333–4338. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.P.; Miller, K.P.; Ganewatta, M.S.; Bam, M.; Yan, Y.; Nagarkatti, M.; Decho, A.W.; Tang, C. Antimicrobial metallopolymers and their bioconjugates with conventional antibiotics against multidrug-resistant bacteria. J. Am. Chem. Soc. 2014, 136, 4873–4876. [Google Scholar] [CrossRef]
- Gan, D.; Xu, T.; Xing, W.; Ge, X.; Fang, L.; Wang, K.; Ren, F.; Lu, X. Mussel-Inspired Contact-Active Antibacterial Hydrogel with High Cell Affinity, Toughness, and Recoverability. Adv. Funct. Mater. 2019, 29, 1805964. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yan, B.; Yang, J.; Huang, W.; Chen, L.; Zeng, H. Injectable Self-Healing Hydrogel with Antimicrobial and Antifouling Properties. ACS Appl. Mater. Inter. 2017, 9, 9221–9225. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, C.; Zhang, N.; Ding, X.; Yu, B.; Xu, F.-J. Polycationic Synergistic Antibacterial Agents with Multiple Functional Components for Efficient Anti-Infective Therapy. Adv. Funct. Mater. 2018, 28, 1706709. [Google Scholar] [CrossRef]
- Jin, X.; Yuan, J.; Shen, J. Zwitterionic polymer brushes via dopamine-initiated ATRP from PET sheets for improving hemocompatible and antifouling properties. Colloid. Surf. B Biointerfaces 2016, 145, 275–284. [Google Scholar] [CrossRef]
- Yu, L.; Hu, P.; Chen, Y. Gas-Generating Nanoplatforms: Material Chemistry, Multifunctionality, and Gas Therapy. Adv. Mater. 2018, 30, 1801964. [Google Scholar] [CrossRef]
- Rong, F.; Tang, Y.; Wang, T.; Feng, T.; Song, J.; Li, P.; Huang, W. Nitric Oxide-Releasing Polymeric Materials for Antimicrobial Applications. Antioxidants 2019, 8, 556. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Deng, G.; Jiang, K.; Wang, H.; Song, Z.; Han, H. Photothermally triggered nitric oxide nanogenerator targeting type IV pili for precise therapy of bacterial infections. Biomaterials 2021, 268, 120588. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano 2020, 14, 347–359. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Inter. 2020, 12, 28952–28964. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Liu, R.; Ma, D.; Xue, W. Dendritic Fe3O4@Poly(dopamine)@PAMAM Nanocomposite as Controllable NO-Releasing Material: A Synergistic Photothermal and NO Antibacterial Study. Adv. Funct. Mater. 2018, 28, 1707440. [Google Scholar] [CrossRef]
- Sadrearhami, Z.; Shafiee, F.N.; Ho, K.K.K.; Kumar, N.; Krasowska, M.; Blencowe, A.; Wong, E.H.H.; Boyer, C. Antibiofilm Nitric Oxide-Releasing Polydopamine Coatings. ACS Appl. Mater. Inter. 2019, 11, 7320–7329. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, C.; Webster, T.J. Self-assembled peptide nanomaterials for biomedical applications: Promises and pitfalls. Int. J. Nanomed. 2017, 12, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Balancing Bacteria-Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine Nanorods. ACS Nano 2017, 11, 11250–11263. [Google Scholar] [CrossRef]
- Fan, X.L.; Li, H.Y.; Ye, W.Y.; Zhao, M.Q.; Huang, D.N.; Fang, Y.; Zhou, B.Q.; Ren, K.F.; Ji, J.; Fu, G.S. Magainin-modified polydopamine nanoparticles for photothermal killing of bacteria at low temperature. Colloid. Surface B 2019, 183, 110423. [Google Scholar] [CrossRef]
- Zhou, L.; Xi, Y.; Xue, Y.; Wang, M.; Liu, Y.; Guo, Y.; Lei, B. Injectable Self-Healing Antibacterial Bioactive Polypeptide-Based Hybrid Nanosystems for Efficiently Treating Multidrug Resistant Infection, Skin-Tumor Therapy, and Enhancing Wound Healing. Adv. Funct. Mater. 2021, 31, 2107158. [Google Scholar] [CrossRef]
- Sakoulas, G.; Alder, J.; Thauvin-Eliopoulos, C.; Moellering, R.C., Jr.; Eliopoulos, G.M. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 2006, 50, 1581–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Chen, W.C.; Hsieh, P.H.; Chen, D.W.; Lee, M.S.; Shih, H.N.; Ueng, S.W. In vitro activities of daptomycin-, vancomycin-, and teicoplanin-loaded polymethylmethacrylate against methicillin-susceptible, methicillin-resistant, and vancomycin-intermediate strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 5480–5484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, S.J.; Yang, S.B.; Lee, D.; Nah, H.; Heo, D.N.; Moon, H.-J.; Hwang, Y.-S.; Reis, R.L.; Moon, J.-H.; et al. Facile preparation of mussel-inspired antibiotic-decorated titanium surfaces with enhanced antibacterial activity for implant applications. Appl. Surf. Sci. 2019, 496, 143675. [Google Scholar] [CrossRef]
- Ran, H.H.; Cheng, X.; Gao, G.; Sun, W.; Jiang, Y.W.; Zhang, X.; Jia, H.R.; Qiao, Y.; Wu, F.G. Colistin-Loaded Polydopamine Nanospheres Uniformly Decorated with Silver Nanodots: A Nanohybrid Platform with Improved Antibacterial and Antibiofilm Performance. ACS Appl. Bio Mater. 2020, 3, 2438–2448. [Google Scholar] [CrossRef]
- Ma, K.; Dong, P.; Liang, M.; Yu, S.; Chen, Y.; Wang, F. Facile Assembly of Multifunctional Antibacterial Nanoplatform Leveraging Synergistic Sensitization between Silver Nanostructure and Vancomycin. ACS Appl. Mater. Inter. 2020, 12, 6955–6965. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Luo, Z.; Ni, Y.; Sun, H.; Gao, X.; Li, Y.; Zhang, S.; Li, Y.; Wei, S. Facile and Versatile Strategy for Construction of Anti-Inflammatory and Antibacterial Surfaces with Polydopamine-Mediated Liposomes Releasing Dexamethasone and Minocycline for Potential Implant Applications. ACS Appl. Mater. Inter. 2017, 9, 43300–43314. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The Antibacterial Applications of Graphene and Its Derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Natan, M.; Jacobi, G.; Banin, E.; Luong, J.H.T.; Gedanken, A. Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus of Colloidal Polydopamine Prepared by Carbon Dot Stimulated Polymerization of Dopamine. Nanomaterials 2019, 9, 1731. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Rapid and Superior Bacteria Killing of Carbon Quantum Dots/ZnO Decorated Injectable Folic Acid-Conjugated PDA Hydrogel through Dual-Light Triggered ROS and Membrane Permeability. Small 2019, 15, e1900322. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Wang, S.; Duan, C.; Yang, W.; Gao, X.; Shi, J.; Kang, J.; Deng, Y.; Shi, X.L.; Chen, Z.G. Two-dimensional nanocoating-enabled orthopedic implants for bimodal therapeutic applications. Nanoscale 2020, 12, 11936–11946. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zhang, B.; Tang, J. Mussel-inspired functionalization of graphene for synthesizing Ag-polydopamine-graphene nanosheets as antibacterial materials. Nanoscale 2013, 5, 118–123. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Ghosh, A.K.; Sarkar, P.; Kundu, P.P. A Mussel Mimetic, Bioadhesive, Antimicrobial Patch Based on Dopamine-Modified Bacterial Celluloser/GO/Ag NPs: A Green Approach toward Wound-Healing Applications. ACS Sustain. Chem. Eng. 2019, 7, 12083–12097. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Wang, T.; Liu, J. Recent Development of Polydopamine Anti-Bacterial Nanomaterials. Int. J. Mol. Sci. 2022, 23, 7278. https://doi.org/10.3390/ijms23137278
Xu Z, Wang T, Liu J. Recent Development of Polydopamine Anti-Bacterial Nanomaterials. International Journal of Molecular Sciences. 2022; 23(13):7278. https://doi.org/10.3390/ijms23137278
Chicago/Turabian StyleXu, Zhengwei, Tingting Wang, and Junqiu Liu. 2022. "Recent Development of Polydopamine Anti-Bacterial Nanomaterials" International Journal of Molecular Sciences 23, no. 13: 7278. https://doi.org/10.3390/ijms23137278
APA StyleXu, Z., Wang, T., & Liu, J. (2022). Recent Development of Polydopamine Anti-Bacterial Nanomaterials. International Journal of Molecular Sciences, 23(13), 7278. https://doi.org/10.3390/ijms23137278





