1. Introduction
Resorbable soft tissue fillers are currently popular for use in cosmetic treatment, as shown by an increase in use in the Netherlands between 2016 and 2019 estimated to be up to 160,000 in 2019 [
1]. In addition to Botox, a botulinum neurotoxin, exerting a temporary effect on facial contour, resorbable fillers such as polylactic acid, polycaprolacton, hydroxylapatite, or hyaluronic acid (HA) show a more prolonged effect period. The most popular types of fillers contain HA which is composed of a linear polysaccharide naturally present in the body [
2].
Injectable tissue fillers applied for aesthetic purposes may lead to severe complications including product migration, unexpected late swelling and inflammatory reactions such as granulomas [
3,
4,
5]. Unfortunately, the cause of the failure, i.e., being treatment- or product-related, is often not specified [
3]. The use of non-resorbable fillers, which has been recognized as problematic especially in the longer term [
4], has been greatly reduced. Since January 2015, non-resorbable fillers are only allowed for reconstructive purposes, and are therefore banned for cosmetic use in The Netherlands [
6]. An increasing number of resorbable fillers are becoming available, among which some may also unfortunately show long-lasting adverse effects somewhat similar to non-resorbable fillers. For example, both HA and calcium hydroxylapatite may induce nodules at various times during and after injection, with or without inflammation [
7,
8]. In contrast, an overview indicated limited adverse reactions after using calcium hydroxylapatite for unapproved indications [
9]. In an evaluation of more than 2000 patients injected with HA, poly-L-lactic acid or calcium hydroxylapatite, the most common complication was nodule or granuloma formation with a relatively low incidence [
10]. Even with a relatively low incidence, however, given the number of people treated with these fillers, a rather high number of patients will suffer complications.
In order to minimize the frequency and number of treatments, a more prolonged effect of injected cosmetic fillers is preferable. On the other hand, in view of the possibility for adverse responses, a prolonged presence may trigger more intense rejection responses and thus adverse reactions. The level of cross-linking and HA concentration are some of the features determining degradation and thus local persistence [
11]. Although HA is a naturally occurring molecule present in the body, it is the manipulation of the molecule (e.g., cross-linking, particulate physical form) that results in recognizing the filler as a foreign body [
12]. Thus, in addition to the initial inflammatory response by neutrophilic granulocytes immediately after injection, additionally, during prolonged presence, a foreign body response might develop. The extent and severity of such a foreign body response ultimately results in an adverse effect. Indeed, the biocompatibility of HA-based materials was reported to decrease with the number of modifications to which this polysaccharide was subjected [
13]. The product Hyacorp was removed from the market in 2012 due to a relatively high number of adverse effects. Keizers et al. [
14] showed that a high modification grade and cross-linking grade might be correlated with a higher risk of adverse effects. We hypothesized that manipulation by cross-linking HA chains may result in driving the macrophage immune responses into either a more fibrotic or a more inflammatory orientated response. The hypothesis is evaluated in this study by in vitro macrophage stimulation with extracts from various HA filler preparations. However, it should be realized that in addition to product characteristics, the injection procedure on its own could also lead to adverse events [
15].
Currently, fillers composed of HA have become one of the most popular materials used for soft tissue contouring [
16]. A large number of products are available that can differ in HA sources (primarily rooster comb or bacterial), cross-linkage (agent and degree), HA concentration, hardness, cohesivity, consistency, and longevity of the resulting correction [
16]. For a number of these products, advantages and disadvantages were reviewed [
16,
17,
18], whereas an overview of approved HA fillers is presented on the FDA website [
19]. Currently, FDA-approved dermal fillers containing an HA comprise up to 23 different products. However, due to the manipulation and formulation of these HA-based products, it is likely that adverse effects will also occur for these resorbable fillers. Transient local swelling but also more serious swelling has been reported [
20,
21]. An advantage of HA fillers is that potential excessive reactions and/or swelling can be treated with hyaluronidase, resulting in a decrease in the swelling [
22,
23]. Incidentally, even late occurring adverse events (e.g., delayed onset nodules, granulomatous foreign body reaction, severe edema) have been reported [
24,
25,
26]. However, the relation between product characteristics and adverse effects is not well understood. Thus, more knowledge is needed on the type of potential complications and the mechanism of the induction of these adverse responses.
The safety evaluation of soft tissue fillers should be risk-based and follow the principles used for medical devices. This depends on the type, use, and time of contact of the medical device with a patient, and the specific types of safety testing depend on the identified potential risks for the patient [
27]. In this context, there are still biological processes and reactions against implanted biomaterials that are far from understood. In addition, the prediction of long-term effects is not really possible. In order to make this possible, more knowledge needs to be generated on the mechanism of the interaction of the biomaterial with tissues and cells. Neutrophils followed by monocytes differentiating into macrophages are the first cells to respond to implanted medical devices initiating an acute inflammatory response that eventually results in the so-called macrophage directed foreign body response, the end-stage response of the inflammatory and wound healing reactions after the implantation of a medical device, prosthesis, or biomaterial [
28,
29]. Material characteristics including the source of the biomaterial, e.g., tissue-derived or synthetically manufactured, can affect the polarization of macrophages [
30]. By identifying the macrophage response both at the cellular and molecular levels, insight is obtained into the processes involved in the tissue response to an implanted material. The aim of this study was to identify potential cellular and/or molecular processes that may be predictive for HA-based filler material responses in patients.
Grotenhuis et al. [
31] previously reported on a human macrophage culture model that could be used to evaluate macrophage responses to biomaterials. In this study, we used the macrophages derived from a human monocyte cell line (THP-1) to evaluate cytokine induction by various HA fillers. We hypothesized that late adverse reactions after the injection of resorbable HA tissue fillers could be related to the degree of cross-linking of HA chains in injectable tissue fillers. This will have direct influence on the dynamics and kinetics of the degradation and absorption process of the injected product. Depending on the degree of cross-linking, further chemical and/or enzymatic breakdown may occur, or the fragments may locally activate macrophages, which then induce the inflammation/granuloma formation. A series of HA samples with different levels of cross-linking were prepared, and their reactivity was compared to a series of commercial HA fillers (Perfectha
®) with increasing sizes of the composing particulates resulting in a prolonged presence at the injection site.
3. Discussion
This study investigated the various aspects of HA fillers including the determination of the cross-linking degree of the HA fillers, the in vitro cytotoxicity of the HA fillers, possible induction of a macrophage pro-inflammatory/pro-fibrotic response, and the effect of the HA fillers on gene expression in macrophages. A series of HA fillers with an increasing degree of BDDE cross-linking was synthesized to validate the method for the analysis of the cross-linking grade of HA-based filler commercial products [
14]. Indeed, the RIVM 1–RIVM 5 preparations showed an increasing level of cross-linking of the HA chains. Our results show that both the experimentally synthesized RIVM preparations and Perfectha
® HA fillers did not elicit cytotoxic effects in a macrophage (RAW264.7) and fibroblast (L929) cell line (
Supplementary Figures S1 and S2), and THP-1-derived M0 macrophages.
Cytokine induction in macrophages can be used to evaluate a possible macrophage response and their further maturation into M1 and/or M2 macrophages after exposure to biomaterials [
31]. For the M1 phenotype associated with a more pro-inflammatory response the cytokines IL-1 β, IL-6, TNF-α, monocyte chemotactic protein (MCP)-3, and macrophage inflammatory protein (MIP)-1α can be evaluated, whereas for the M2 phenotype associated with a more fibrotic response, IL-1 receptor antagonist (IL-1RA), CCL18, regulated and normal T-cell expressed and secreted (RANTES), and macrophage-derived chemokine (MDC) can be used [
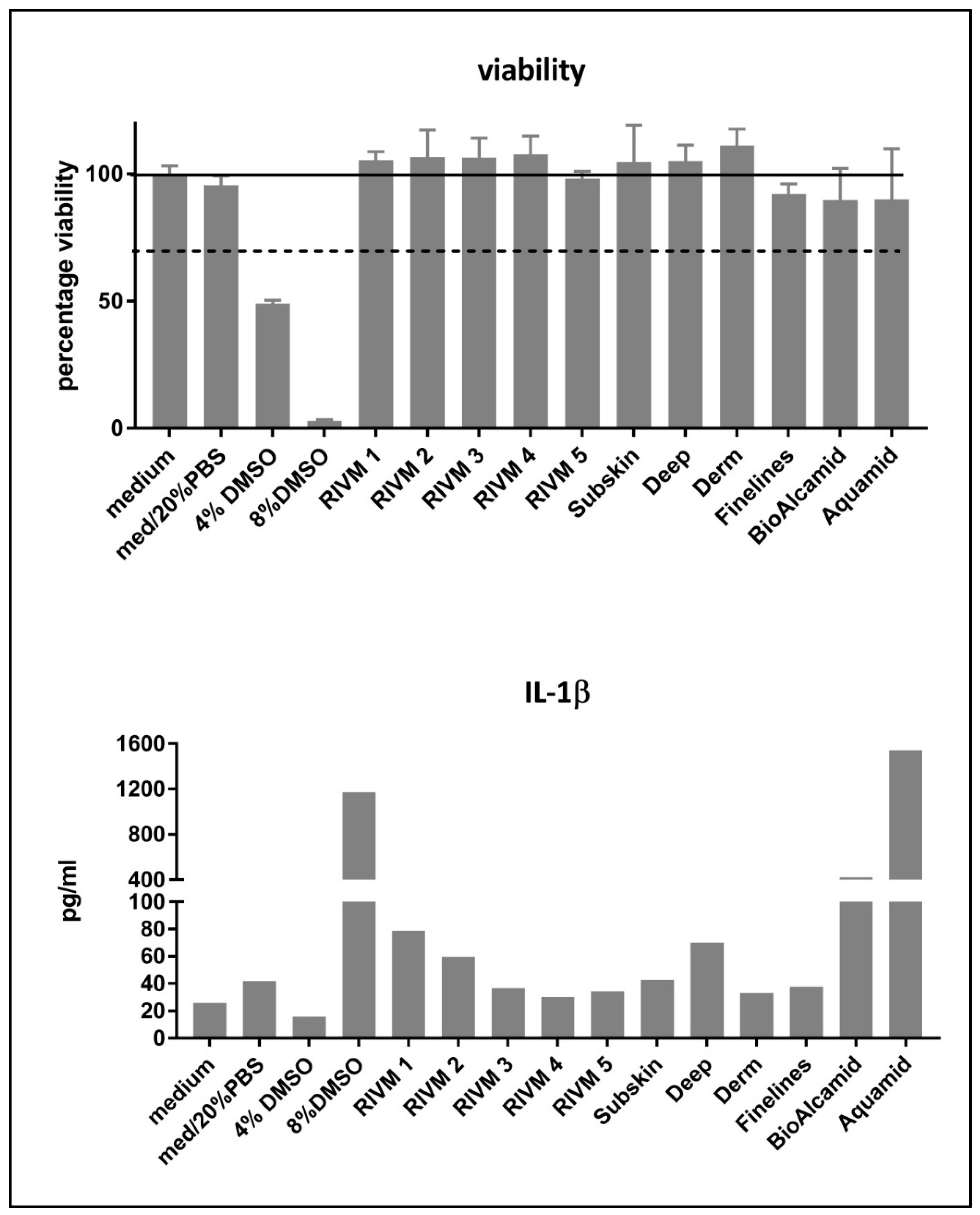
31]. We used IL-1β production as a marker for M1 macrophage induction and CCL-18 production as a marker for M2 macrophage induction. Only DMSO included as positive control was clearly toxic for the cells (
Figure 1). The induction of IL-1β and CCL18, as M1 and M2 markers, respectively, was evaluated in THP-1-derived macrophages. A high level of IL-1β production was observed for the DMSO positive control treatment. Additionally, the two non-resorbable fillers Bio-Alcamid
® and Aquamid
® induced a high IL-1β production at exposure levels that did not induce toxicity in M0 macrophages. In this respect, the M0 macrophages derived from the monocytic THP-1 cells might be less sensitive to the toxic effects of the non-resorbable filler Bio-Alcamid
® which was toxic for the murine RAW264.7 macrophage cell line. Bio-Alcamid
® induced both M1 (IL-1β) and M2 (CCL18) cytokines at high levels compared to the HA fillers evaluated (
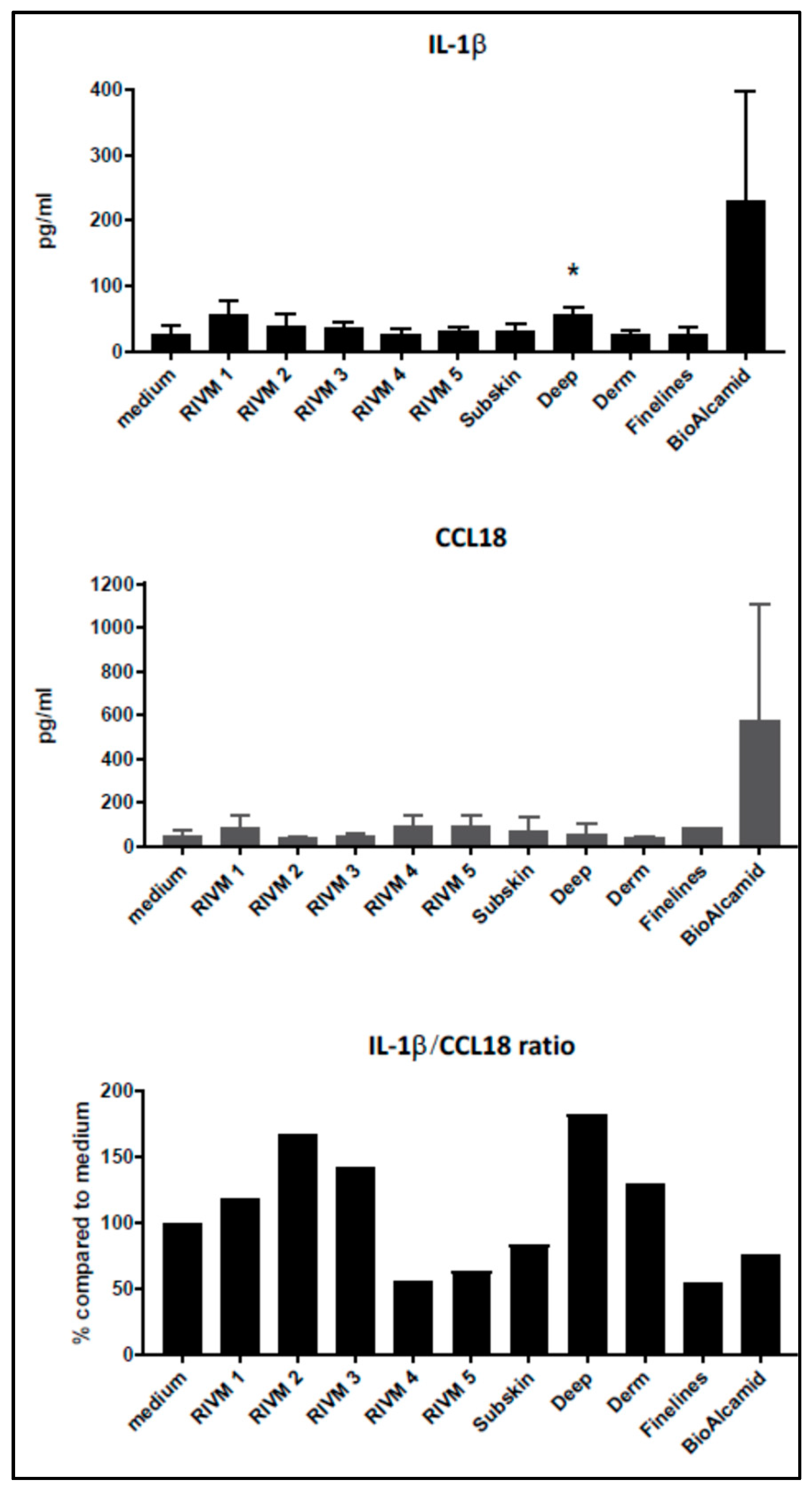
Figure 2). However, when we more closely considered the low levels of cytokine induction by the experimental and commercial HA fillers separately, some indications could be noted from the effects of the fillers prepared at RIVM. The two RIVM preparations with the highest cross-linking levels (RIVM 4 and 5) showed the lowest IL-1β/CCL18 ratio, suggesting that they might induce a pro-fibrotic response. The commercial fillers (Perfectha
®) show a gradual decrease in the IL-1β/CCL18 ratio in the order of Deep, Derm, SubSkin, and FineLines, suggesting an increase in a more pro-fibrotic response with a smaller particle size in the fillers. This phenomenon can also be observed for the gene expressions of
CCL18 and
CXCL8, where a higher up-regulation of these genes is evident for the fillers with a smaller particle size (
Table 3 and
Supplementary Table S1). An elevated expression of the pro-fibrotic cytokine
CXCL8 (also known as
IL-8) upon exposure to Bio-Alcamid
® and all Perfectha fillers is in line with the elevated expression of M2-macrophage-associated chemokine
CCL18, as
CCL18 induces an expression of
CXCL8 [
32].
Our results indicate that the evaluation of the levels of M1 and M2 cytokines after the exposure of M0 macrophages may possibly be a model to evaluate the pro-inflammatory/pro-fibrotic characteristics of biomaterials including fillers. The production of the M1 cytokine IL-1β and the M2 cytokine CCL18 can be considered indicative for either a more inflammatory- or fibrotic-oriented local response, respectively. Such characteristics could link to complications such as nodules or granulomas, which are among the most commonly reported complications. It is clear that these data should be complemented with other M1 and M2 markers before a more definitive conclusion can be drawn, and the effects of these fillers on differentiated M1 and M2 macrophages should be investigated. Nonetheless, evaluating the levels of M1 and M2 cytokines upon the incubation of THP-1-derived M0 macrophages may possibly be a first step to a cellular model to evaluate the pro-inflammatory/pro-fibrotic characteristics of biomaterials including fillers.
Remarkably, the resorbable RIVM HA preparations and Perfectha
® HA fillers hardly induced gene pathways related to immune activity (e.g., cytokine signaling and macrophage markers), while they did induce gene pathways related to cell cycle control. On the other hand, the non-resorbable filler Bio-Alcamid
® did induce pathways related to immune activity, and only a few pathways related to cell cycle control. A clear explanation of why the resorbable HA fillers did not show activation of immune-related pathways, whereas the non-resorbable Bio-Alcamid
® filler did activate the immune-related pathways, is not obvious as both types of fillers showed no effect on the viability of the exposed cells; in other words, they were not cytotoxic for the exposed macrophages. However, Bio-Alcamid
® also showed a much higher induction of cytokines as presented in
Figure 1 and
Figure 2 when compared to the resorbable RIVM HA fillers. Therefore, it could be expected that the immune-related gene expression would be also increased. Bio-Alcamid
® is known to cause a relatively high number of more severe complications that require clinical intervention [
33,
34,
35]. Thus, both the cytokine induction and altered gene expression could provide opportunities to develop methods for the preclinical safety evaluation of biomaterials. The in-house-made preparation RIVM 2 is the most reactive resorbable “filler” in our studies. This indicates that the gene expression response does not correlate with the number of cross-links. Bio-Alcamid
® is overall the most responsive filler in the macrophage cell model.
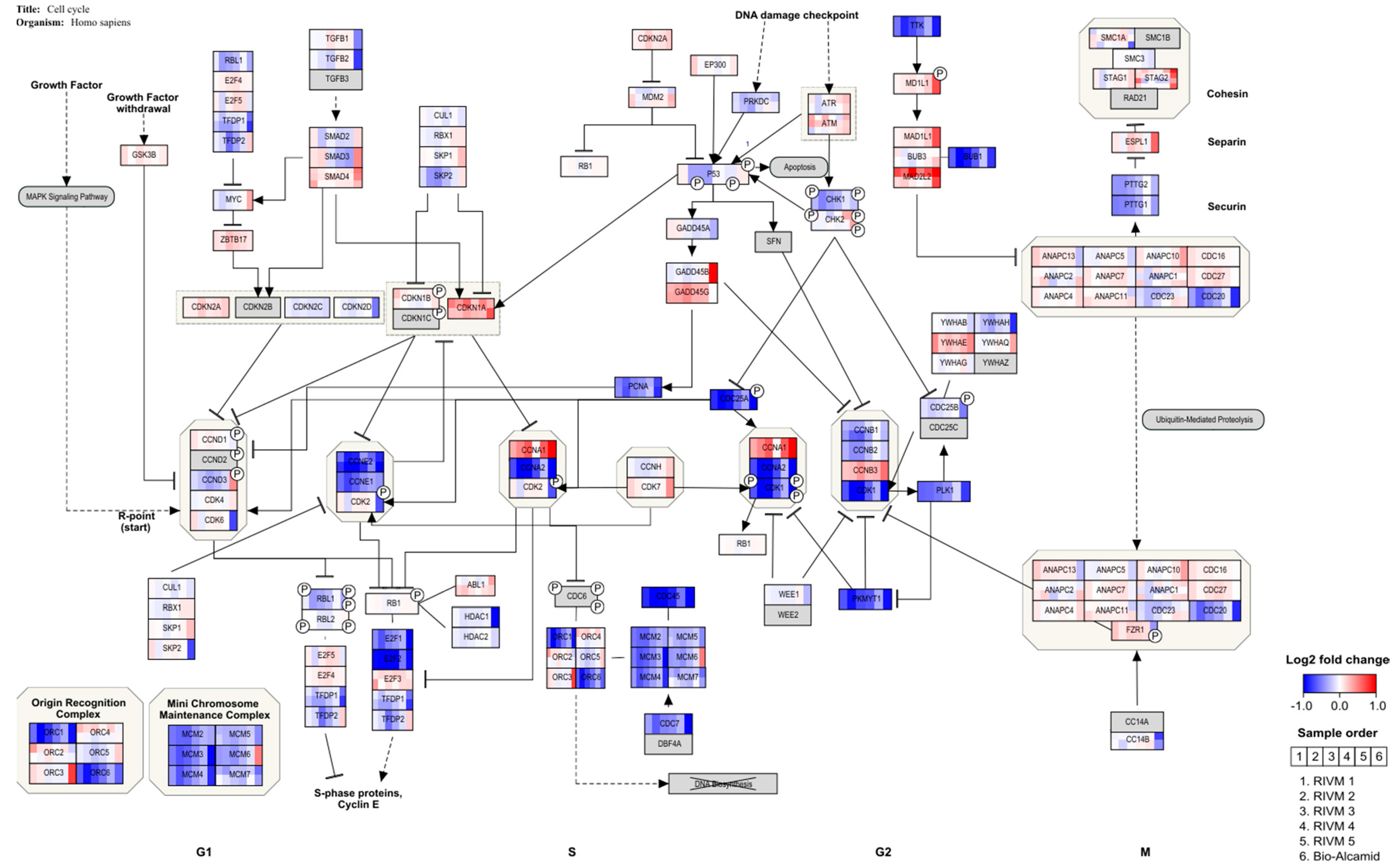
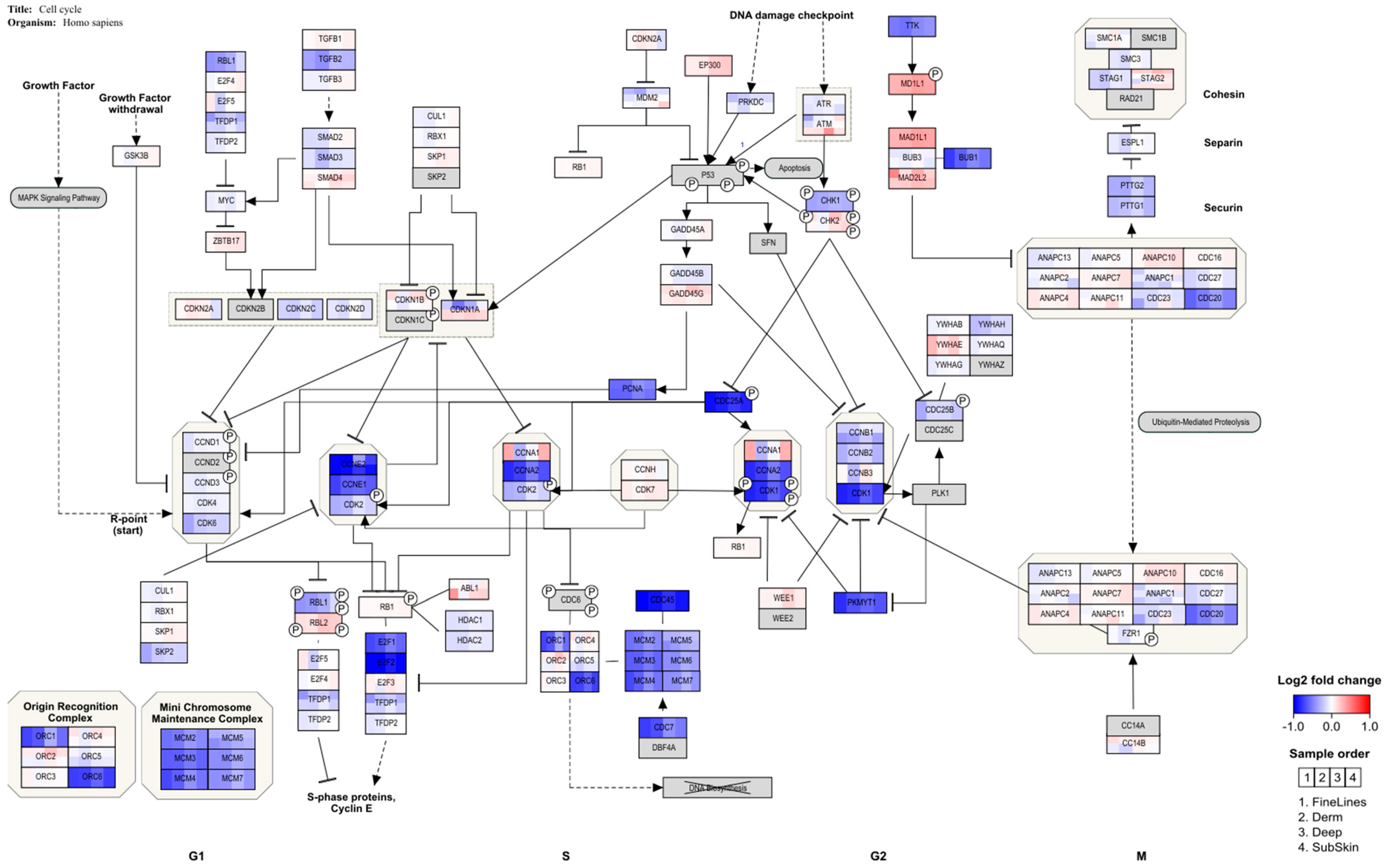
After HA filler exposure in the M0 macrophages derived from THP-1 cells, genes indicating cell cycle arrest at the G1 checkpoint are regulated, whereby
E2F and several cyclins are down-regulated (
Figure 3 and
Figure 4). This is in line with a study in which THP-1 macrophages were challenged with retinoic acid, resulting in the down-regulation of
E2F and
cyclin E and resulting in cell cycle arrest [
36]. In addition, this study also showed enhancement of the phagocytic activity of THP-1 cells. This would be in line with an adverse reaction to the dermal fillers. A clear inflammatory response, showing the up-regulation of several cytokines, is observed for a human full skin model after exposure to the same dermal fillers (Jennen et al., in preparation). This response can be considered to reflect an acute inflammatory response, as seen in patients injected with HA fillers [
37].
Although the in vitro experiments may give a good indication of the biological processes that underlie the exposure of the cell models to HA fillers, a direct comparison with reactions in patients is needed to indicate the value of the in vitro macrophage cell model. However, a comparison with the patient samples (biopsies) of local adverse effects of HA fillers might be difficult to achieve. Procedures to obtain biopsies are invasive and can lead to scarring or other severe complications [
38] and thus patients are reluctant to give consent for biopsies to be taken, especially coming from the facial area (Decates, personal communication). Additionally, adverse reactions can be clinically managed by applying hyaluronidase to the reaction site without causing (severe) complications [
22,
23]. The clinical relevance of our studies can be found in the predictive value of the evaluated test systems in the preclinical safety evaluation of biomaterials and/or medical devices. Both the fibroblast and macrophage responses, corresponding the formation of fibrotic tissue surrounding an implant and macrophages trying to phagocytize the implant as a foreign body, respectively, are normal biological responses to an implant that is not naturally present in a human body [
28,
29]. The extent of these reactions, i.e., severe fibrosis and/or capsule formation or a severe inflammation due to a massive foreign body response, determines whether these reactions should be seen as adverse.
In conclusion, our results could not confirm the hypothesis that the level of cross-linking in the experimental HA preparations or the particulate size in the commercial HA fillers is related to the biological (cytotoxic or immune) responses induced by the HA fillers. However, our results do present evidence that in vitro models either composed of a macrophage cell line or macrophages differentiated from a monocytic cell line may be used for the identification of immune pathways more prone for either an inflammatory response or a fibrotic response based on the cytokine patterns induced after exposure to the biomaterials used as soft tissue fillers. In addition, the observed alteration of cell cycle-related pathways and indication for cell cycle arrest could be an alarm response instigated by the different fillers to prevent the propagation of dysfunctional cells. Thus, the combination of evaluation of cytokine induction and effects on gene expression presents promising opportunities for the development of methods to identify potential cellular and/or molecular processes that may be predictive for biomaterial-induced responses in patients.