Y192 within the SH2 Domain of Lck Regulates TCR Signaling Downstream of PLC-γ1 and Thymic Selection
Abstract
:1. Introduction
2. Results
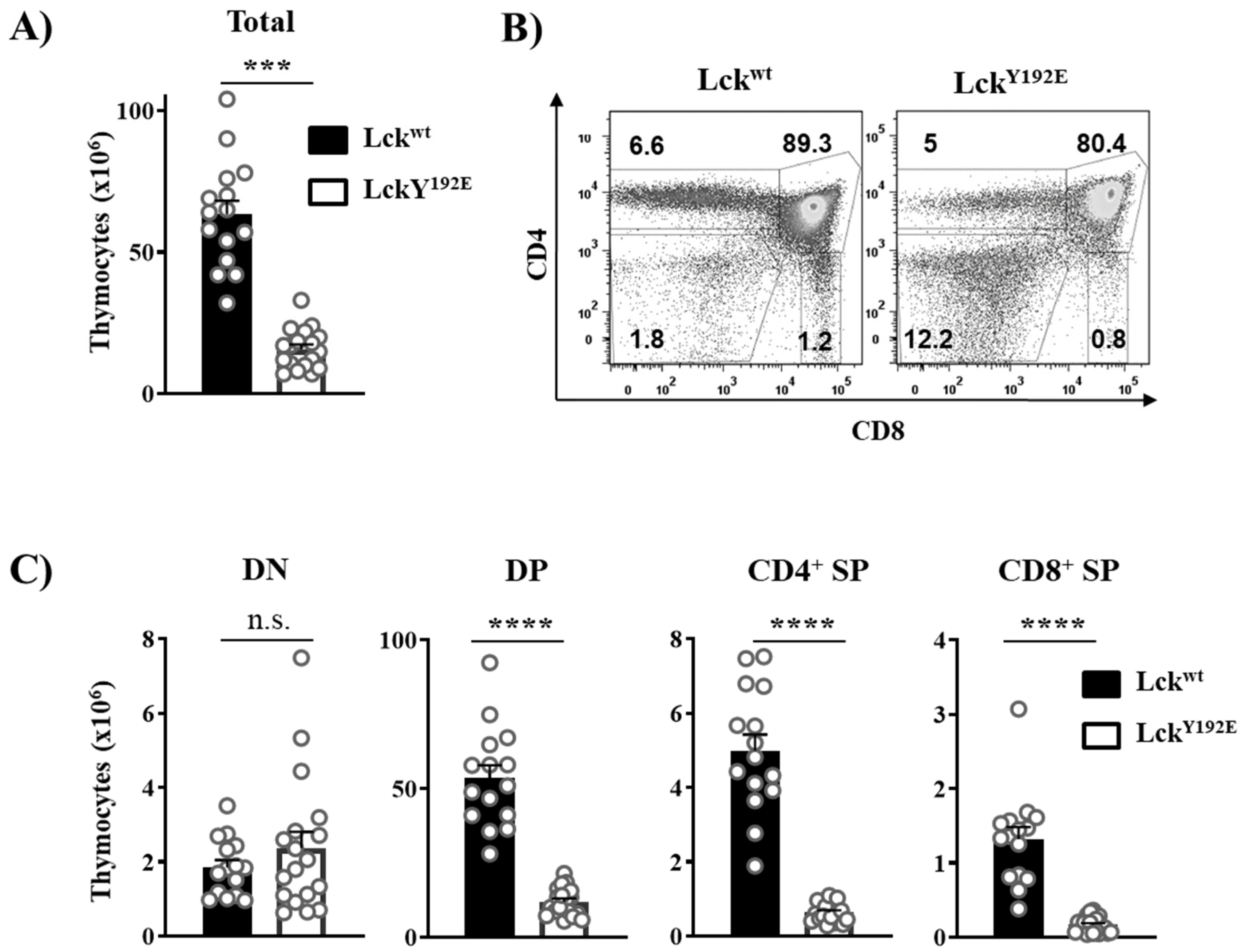
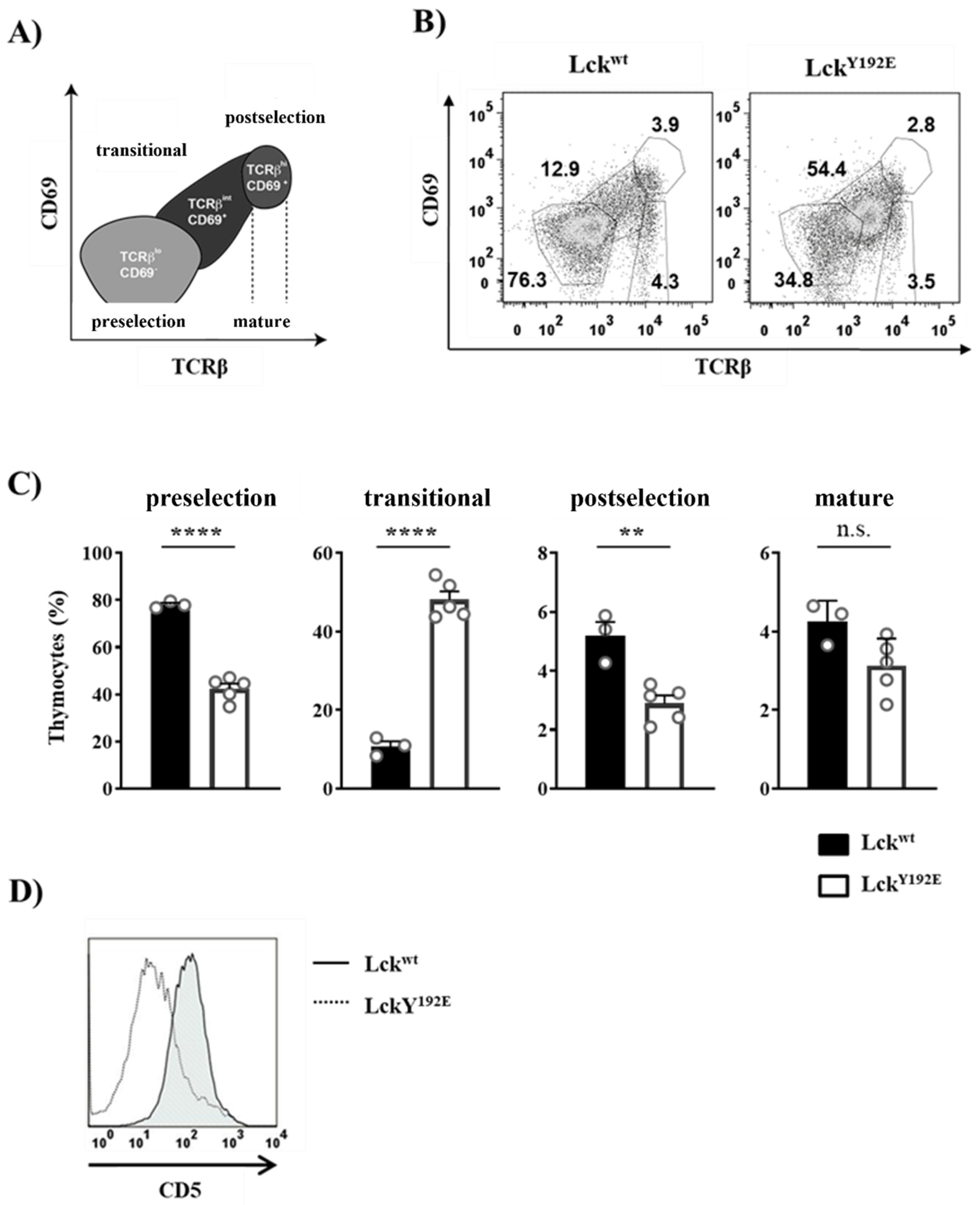
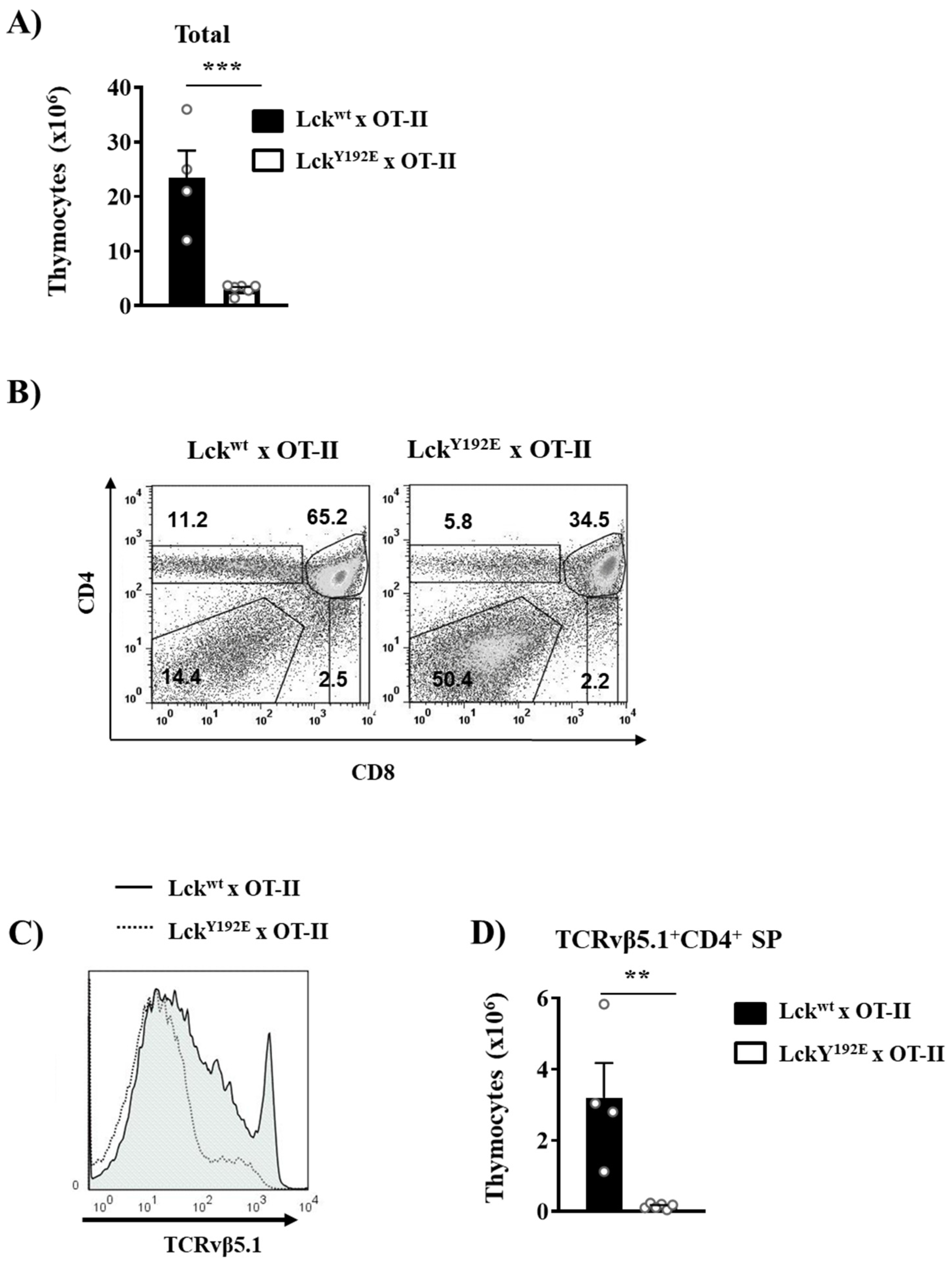
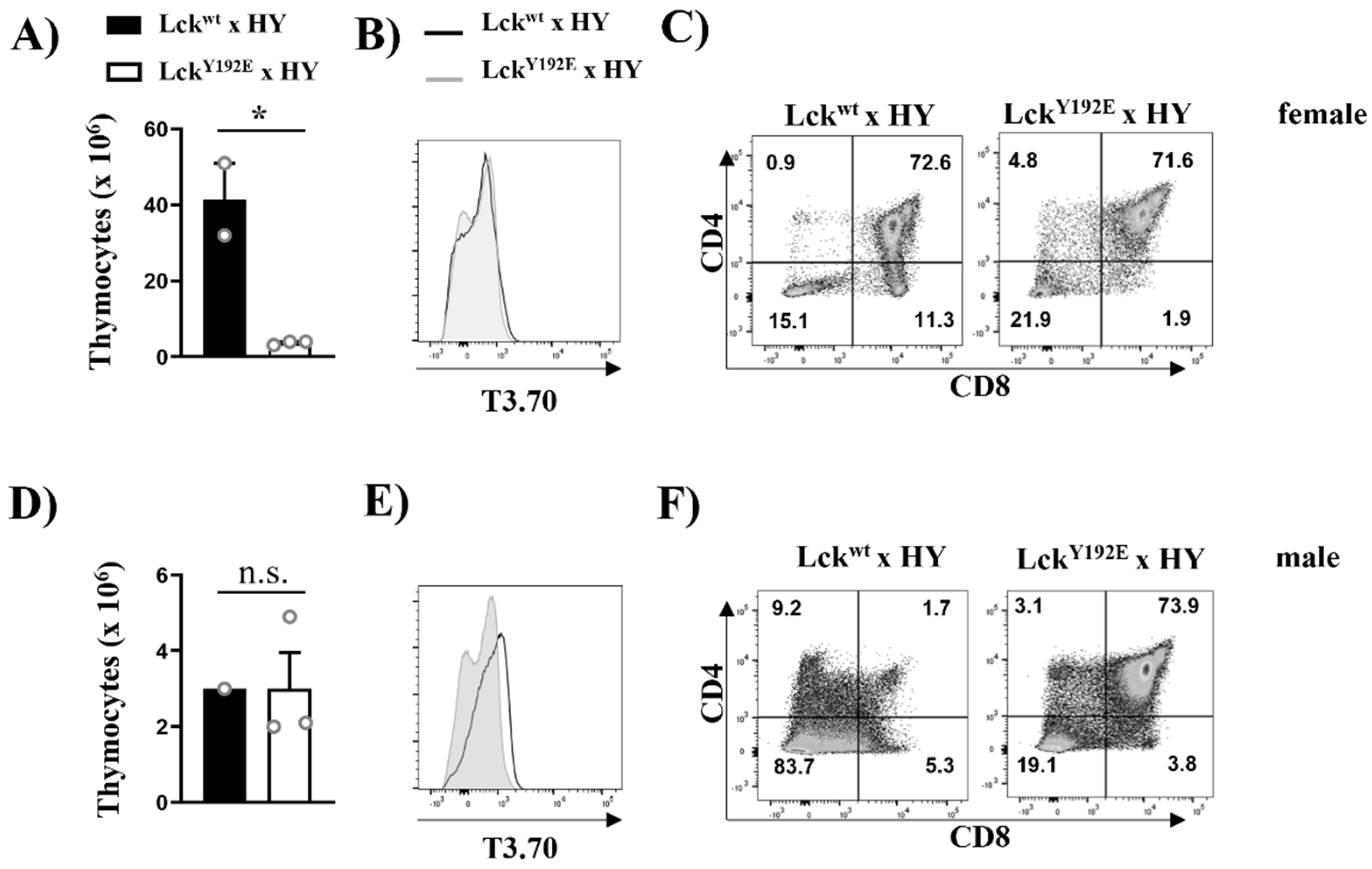
2.1. Defective Thymocyte Selection in LckY192E Mice
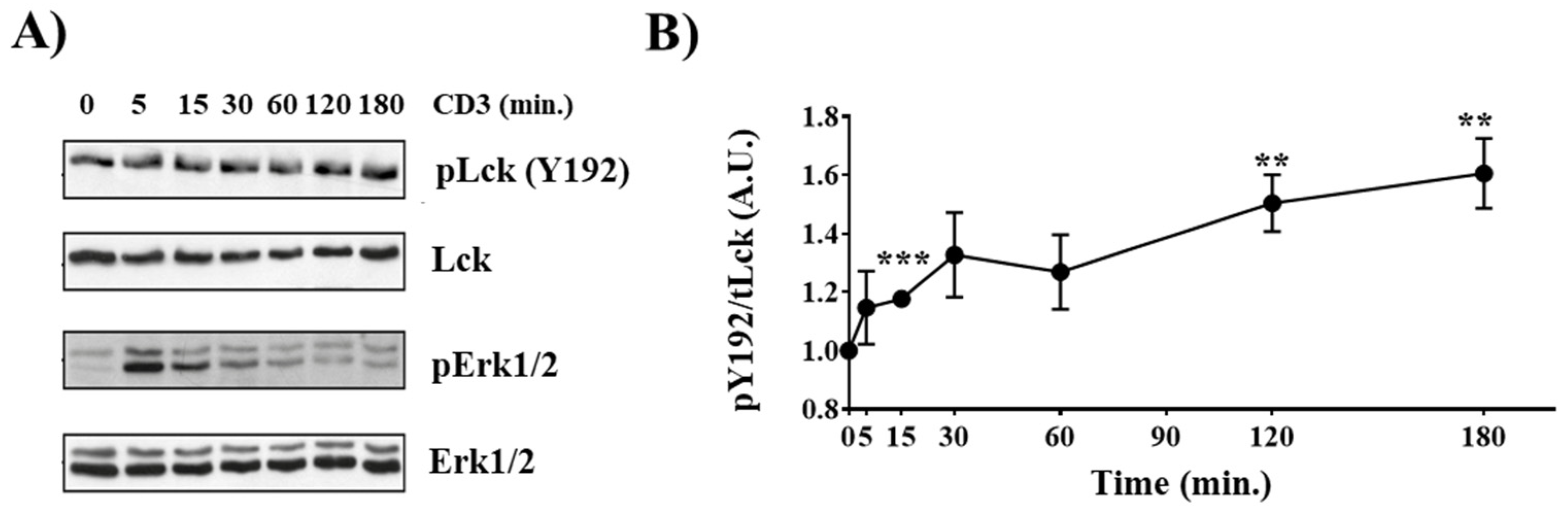
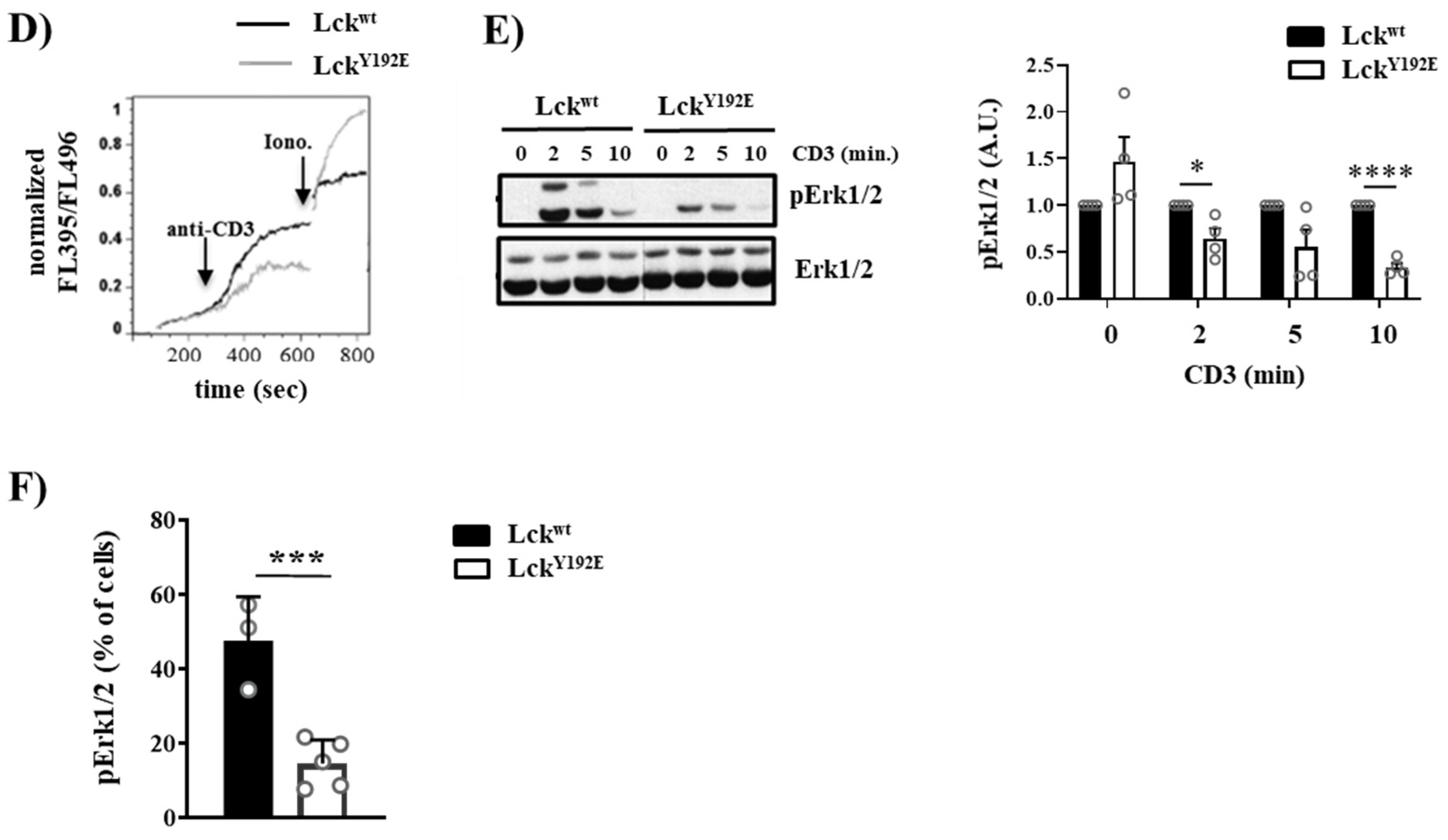
2.2. LckY192E Initiates TCR Signaling in Thymocytes
2.3. The TCR Is Uncoupled from the Activation of Pathways Downstream of PLC-γ1 in LckY192E Thymocytes
3. Discussion
4. Materials and Methods
4.1. Antibodies
4.2. Mice
4.3. Stimulation and Lysis of Cells
4.4. Immunoblotting
4.5. Flow Cytometry Measurements
4.6. Calcium Flux
4.7. Intracellular Staining
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef]
- Morris, G.P.; Allen, P.M. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat. Immunol. 2012, 13, 121–128. [Google Scholar] [CrossRef]
- Dutta, A.; Zhao, B.; Love, P.E. New insights into TCR beta-selection. Trends Immunol. 2021, 42, 735–750. [Google Scholar] [CrossRef]
- Gascoigne, N.R.; Palmer, E. Signaling in thymic selection. Curr. Opin. Immunol. 2011, 23, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Palacios, E.H.; Weiss, A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 2004, 23, 7990–8000. [Google Scholar] [CrossRef] [Green Version]
- Zamoyska, R.; Basson, A.; Filby, A.; Legname, G.; Lovatt, M.; Seddon, B. The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol. Rev. 2003, 191, 107–118. [Google Scholar] [CrossRef]
- Molina, T.J.; Kishihara, K.; Siderovski, D.P.; van Ewijk, W.; Narendran, A.; Timms, E.; Wakeham, A.; Paige, C.J.; Hartmann, K.U.; Veillette, A.; et al. Profound block in thymocyte development in mice lacking p56lck. Nature 1992, 357, 161–164. [Google Scholar] [CrossRef]
- Salmond, R.J.; Filby, A.; Pirinen, N.; Magee, A.I.; Zamoyska, R. Mislocalization of Lck impairs thymocyte differentiation and can promote development of thymomas. Blood 2011, 117, 108–117. [Google Scholar] [CrossRef] [Green Version]
- van Oers, N.S.; Lowin-Kropf, B.; Finlay, D.; Connolly, K.; Weiss, A. alpha beta T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity 1996, 5, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Groves, T.; Smiley, P.; Cooke, M.P.; Forbush, K.; Perlmutter, R.M.; Guidos, C.J. Fyn can partially substitute for Lck in T lymphocyte development. Immunity 1996, 5, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Legname, G.; Seddon, B.; Lovatt, M.; Tomlinson, P.; Sarner, N.; Tolaini, M.; Williams, K.; Norton, T.; Kioussis, D.; Zamoyska, R. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity 2000, 12, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.J.; Hodes, R.J. Regulation of T cell development by c-Cbl: Essential role of Lck. Int Immunol. 2015, 27, 245–251. [Google Scholar] [CrossRef] [Green Version]
- van Oers, N.S.; Killeen, N.; Weiss, A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 1996, 183, 1053–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.J.; Hodes, R.J. T-cell development is regulated by the coordinated function of proximal and distal Lck promoters active at different developmental stages. Eur. J. Immunol. 2016, 46, 2401–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, K.M.; Levin, S.D.; Marth, J.D.; Forbush, K.A.; Perlmutter, R.M. Delayed thymocyte development induced by augmented expression of p56lck. J. Exp. Med. 1991, 173, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Levin, S.D.; Anderson, S.J.; Forbush, K.A.; Perlmutter, R.M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993, 12, 1671–1680. [Google Scholar] [CrossRef]
- Sohn, S.J.; Forbush, K.A.; Pan, X.C.; Perlmutter, R.M. Activated p56lck directs maturation of both CD4 and CD8 single-positive thymocytes. J. Immunol. 2001, 166, 2209–2217. [Google Scholar] [CrossRef] [Green Version]
- Courtney, A.H.; Amacher, J.F.; Kadlecek, T.A.; Mollenauer, M.N.; Au-Yeung, B.B.; Kuriyan, J.; Weiss, A. A Phosphosite within the SH2 Domain of Lck Regulates Its Activation by CD45. Mol. Cell 2017, 67, 498–511.e6. [Google Scholar] [CrossRef]
- Kastle, M.; Merten, C.; Hartig, R.; Kaehne, T.; Liaunardy-Jopeace, A.; Woessner, N.M.; Schamel, W.W.; James, J.; Minguet, S.; Simeoni, L.; et al. Tyrosine 192 within the SH2 domain of the Src-protein tyrosine kinase p56(Lck) regulates T-cell activation independently of Lck/CD45 interactions. Cell Commun. Signal. 2020, 18, 183. [Google Scholar] [CrossRef]
- Granum, S.; Sundvold-Gjerstad, V.; Gopalakrishnan, R.P.; Berge, T.; Koll, L.; Abrahamsen, G.; Sorlie, M.; Spurkland, A. The kinase Itk and the adaptor TSAd change the specificity of the kinase Lck in T cells by promoting the phosphorylation of Tyr192. Sci. Signal. 2014, 7, ra118. [Google Scholar] [CrossRef]
- Couture, C.; Songyang, Z.; Jascur, T.; Williams, S.; Tailor, P.; Cantley, L.C.; Mustelin, T. Regulation of the Lck SH2 domain by tyrosine phosphorylation. J. Biol. Chem. 1996, 271, 24880–24884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustelin, T.; Coggeshall, K.M.; Altman, A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc. Natl. Acad. Sci. USA 1989, 86, 6302–6306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostergaard, H.L.; Shackelford, D.A.; Hurley, T.R.; Johnson, P.; Hyman, R.; Sefton, B.M.; Trowbridge, I.S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc. Natl. Acad. Sci. USA 1989, 86, 8959–8963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieh, M.; Bolen, J.B.; Weiss, A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993, 12, 315–321. [Google Scholar] [CrossRef]
- Kydd, R.; Lundberg, K.; Vremec, D.; Harris, A.W.; Shortman, K. Intermediate steps in thymic positive selection. Generation of CD4-8+ T cells in culture from CD4+8+, CD4int8+, and CD4+8int thymocytes with up-regulated levels of TCR-CD3. J. Immunol. 1995, 155, 3806–3814. [Google Scholar]
- Lundberg, K.; Heath, W.; Kontgen, F.; Carbone, F.R.; Shortman, K. Intermediate steps in positive selection: Differentiation of CD4+8int TCRint thymocytes into CD4-8+TCRhi thymocytes. J. Exp. Med. 1995, 181, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Swat, W.; Dessing, M.; von Boehmer, H.; Kisielow, P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur. J. Immunol. 1993, 23, 739–746. [Google Scholar] [CrossRef]
- Yamashita, I.; Nagata, T.; Tada, T.; Nakayama, T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection. Int. Immunol. 1993, 5, 1139–1150. [Google Scholar] [CrossRef]
- Hu, Q.; Nicol, S.A.; Suen, A.Y.; Baldwin, T.A. Examination of thymic positive and negative selection by flow cytometry. J. Vis. Exp. 2012, 68, e4269. [Google Scholar] [CrossRef] [Green Version]
- Azzam, H.S.; Grinberg, A.; Lui, K.; Shen, H.; Shores, E.W.; Love, P.E. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 1998, 188, 2301–2311. [Google Scholar] [CrossRef] [Green Version]
- von Boehmer, H. Developmental biology of T cells in T cell-receptor transgenic mice. Ann. Rev. Immunol. 1990, 8, 531–556. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Ho, S.S.; Zakarian, A.; Ohashi, P.S. Degree of ERK activation influences both positive and negative thymocyte selection. Eur. J. Immunol. 2000, 30, 1060–1068. [Google Scholar] [CrossRef]
- Bommhardt, U.; Basson, M.A.; Krummrei, U.; Zamoyska, R. Activation of the extracellular signal-related kinase/mitogen-activated protein kinase pathway discriminates CD4 versus CD8 lineage commitment in the thymus. J. Immunol. 1999, 163, 715–722. [Google Scholar]
- McNeil, L.K.; Starr, T.K.; Hogquist, K.A. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 13574–13579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, M.A.; Teixeiro, E.; Gill, J.; Hausmann, B.; Roubaty, D.; Holmberg, K.; Werlen, G.; Hollander, G.A.; Gascoigne, N.R.; Palmer, E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 2006, 444, 724–729. [Google Scholar] [CrossRef]
- Melichar, H.J.; Ross, J.O.; Herzmark, P.; Hogquist, K.A.; Robey, E.A. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Sci. Signal. 2013, 6, ra92. [Google Scholar] [CrossRef] [Green Version]
- Cante-Barrett, K.; Gallo, E.M.; Winslow, M.M.; Crabtree, G.R. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of bim [corrected]. J. Immunol. 2006, 176, 2299–2306. [Google Scholar] [CrossRef] [Green Version]
- Kane, L.P.; Hedrick, S.M. A role for calcium influx in setting the threshold for CD4+CD8+ thymocyte negative selection. J. Immunol. 1996, 156, 4594–4601. [Google Scholar]
- Seavitt, J.R.; White, L.S.; Murphy, K.M.; Loh, D.Y.; Perlmutter, R.M.; Thomas, M.L. Expression of the p56(Lck) Y505F mutation in CD45-deficient mice rescues thymocyte development. Mol. Cell Biol. 1999, 19, 4200–4208. [Google Scholar] [CrossRef] [Green Version]
- Falahati, R.; Leitenberg, D. Selective regulation of TCR signaling pathways by the CD45 protein tyrosine phosphatase during thymocyte development. J. Immunol. 2008, 181, 6082–6091. [Google Scholar] [CrossRef] [Green Version]
- Rudd, M.L.; Tua-Smith, A.; Straus, D.B. Lck SH3 domain function is required for T-cell receptor signals regulating thymocyte development. Mol. Cell Biol. 2006, 26, 7892–7900. [Google Scholar] [CrossRef] [Green Version]
- Denny, M.F.; Kaufman, H.C.; Chan, A.C.; Straus, D.B. The lck SH3 domain is required for activation of the mitogen-activated protein kinase pathway but not the initiation of T-cell antigen receptor signaling. J. Biol. Chem. 1999, 274, 5146–5152. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Ong, S.S.; Rajwa, B.; Thieu, V.T.; Geahlen, R.L.; Harrison, M.L. The SH3 domain of Lck modulates T-cell receptor-dependent activation of extracellular signal-regulated kinase through activation of Raf-1. Mol. Cell Biol. 2008, 28, 630–641. [Google Scholar] [CrossRef] [Green Version]
- Harr, M.W.; Rong, Y.; Bootman, M.D.; Roderick, H.L.; Distelhorst, C.W. Glucocorticoid-mediated inhibition of Lck modulates the pattern of T cell receptor-induced calcium signals by down-regulating inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 2009, 284, 31860–31871. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Cen, H.; Gong, B.N.; Mai, S.; Wang, Q.L.; Mou, S.; Li, Y. TCR-Induced Tyrosine Phosphorylation at Tyr270 of SUMO Protease SENP1 by Lck Modulates SENP1 Enzyme Activity and Specificity. Front. Cell Dev. Biol. 2021, 9, 789348. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Zhao, C.S.; Xiao, Z.; Gong, Y.; Li, Y.Y.; Chen, Y.; Du, Y.; Feng, D.; Altman, A.; et al. T-cell receptor (TCR) signaling promotes the assembly of RanBP2/RanGAP1-SUMO1/Ubc9 nuclear pore subcomplex via PKC-theta-mediated phosphorylation of RanGAP1. eLife 2021, 10, 67123. [Google Scholar] [CrossRef]
- Wang, X.D.; Gong, Y.; Chen, Z.L.; Gong, B.N.; Xie, J.J.; Zhong, C.Q.; Wang, Q.L.; Diao, L.H.; Xu, A.; Han, J.; et al. TCR-induced sumoylation of the kinase PKC-theta controls T cell synapse organization and T cell activation. Nat. Immunol. 2015, 16, 1195–1203. [Google Scholar] [CrossRef]
- Xiong, Y.; Yi, Y.; Wang, Y.; Yang, N.; Rudd, C.E.; Liu, H. Ubc9 Interacts with and SUMOylates the TCR Adaptor SLP-76 for NFAT Transcription in T Cells. J. Immunol. 2019, 203, 3023–3036. [Google Scholar] [CrossRef]
- Wang, Q.L.; Liang, J.Q.; Gong, B.N.; Xie, J.J.; Yi, Y.T.; Lan, X.; Li, Y. T Cell Receptor (TCR)-Induced PLC-gamma1 Sumoylation via PIASxbeta and PIAS3 SUMO E3 Ligases Regulates the Microcluster Assembly and Physiological Function of PLC-gamma1. Front. Immunol. 2019, 10, 314. [Google Scholar] [CrossRef]
- Barnden, M.J.; Allison, J.; Heath, W.R.; Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998, 76, 34–40. [Google Scholar] [CrossRef]
- Kisielow, P.; Bluthmann, H.; Staerz, U.D.; Steinmetz, M.; von Boehmer, H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 1988, 333, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Arndt, B.; Poltorak, M.; Kowtharapu, B.S.; Reichardt, P.; Philipsen, L.; Lindquist, J.A.; Schraven, B.; Simeoni, L. Analysis of TCR activation kinetics in primary human T cells upon focal or soluble stimulation. J. Immunol. Methods 2013, 387, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kästle, M.; Merten, C.; Hartig, R.; Plaza-Sirvent, C.; Schmitz, I.; Bommhardt, U.; Schraven, B.; Simeoni, L. Y192 within the SH2 Domain of Lck Regulates TCR Signaling Downstream of PLC-γ1 and Thymic Selection. Int. J. Mol. Sci. 2022, 23, 7271. https://doi.org/10.3390/ijms23137271
Kästle M, Merten C, Hartig R, Plaza-Sirvent C, Schmitz I, Bommhardt U, Schraven B, Simeoni L. Y192 within the SH2 Domain of Lck Regulates TCR Signaling Downstream of PLC-γ1 and Thymic Selection. International Journal of Molecular Sciences. 2022; 23(13):7271. https://doi.org/10.3390/ijms23137271
Chicago/Turabian StyleKästle, Matthias, Camilla Merten, Roland Hartig, Carlos Plaza-Sirvent, Ingo Schmitz, Ursula Bommhardt, Burkhart Schraven, and Luca Simeoni. 2022. "Y192 within the SH2 Domain of Lck Regulates TCR Signaling Downstream of PLC-γ1 and Thymic Selection" International Journal of Molecular Sciences 23, no. 13: 7271. https://doi.org/10.3390/ijms23137271
APA StyleKästle, M., Merten, C., Hartig, R., Plaza-Sirvent, C., Schmitz, I., Bommhardt, U., Schraven, B., & Simeoni, L. (2022). Y192 within the SH2 Domain of Lck Regulates TCR Signaling Downstream of PLC-γ1 and Thymic Selection. International Journal of Molecular Sciences, 23(13), 7271. https://doi.org/10.3390/ijms23137271





