Biocompatibility Analyses of HF-Passivated Magnesium Screws for Guided Bone Regeneration (GBR)
Abstract
:1. Introduction
2. Results
2.1. Cytocompatibility Analysis
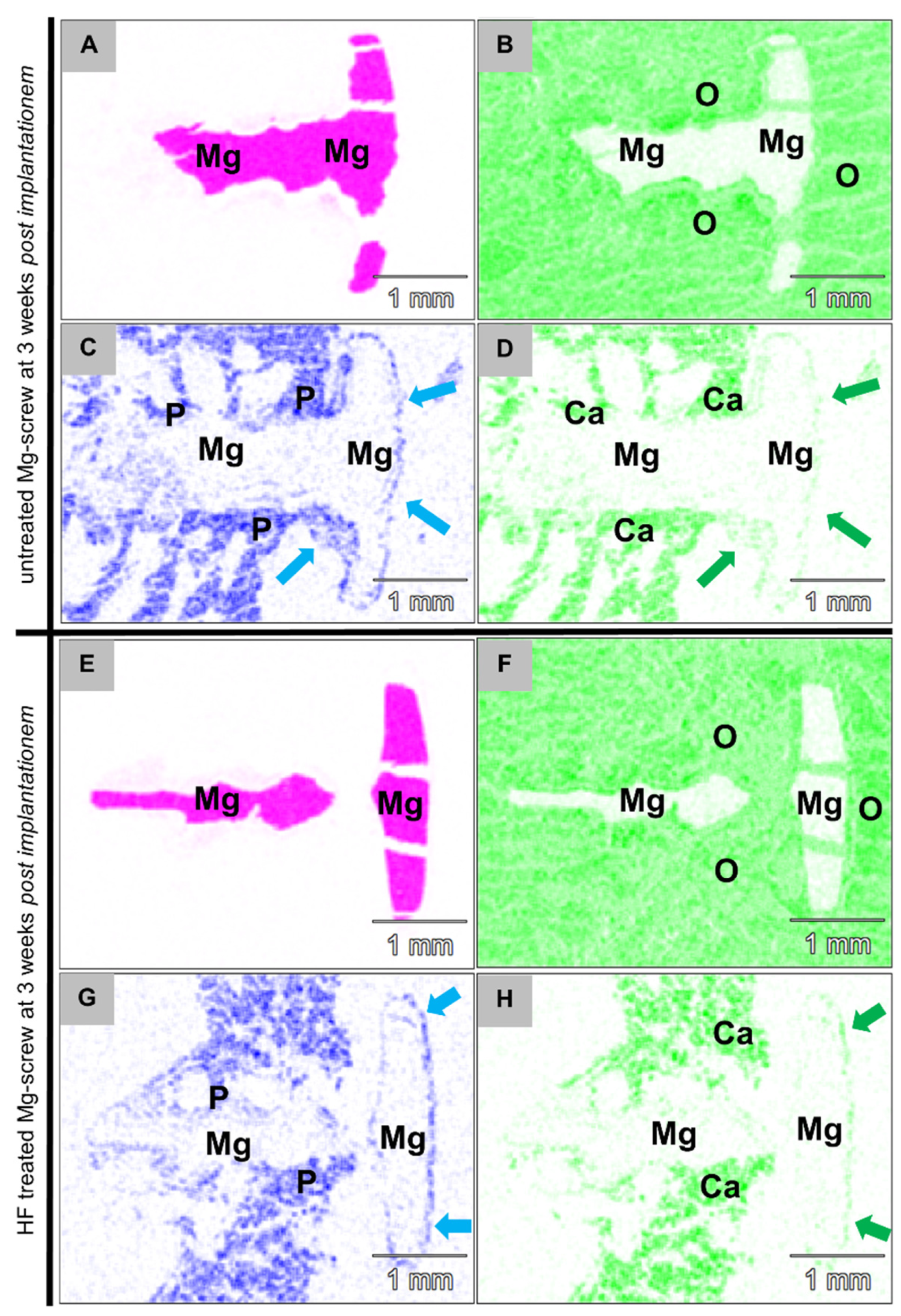
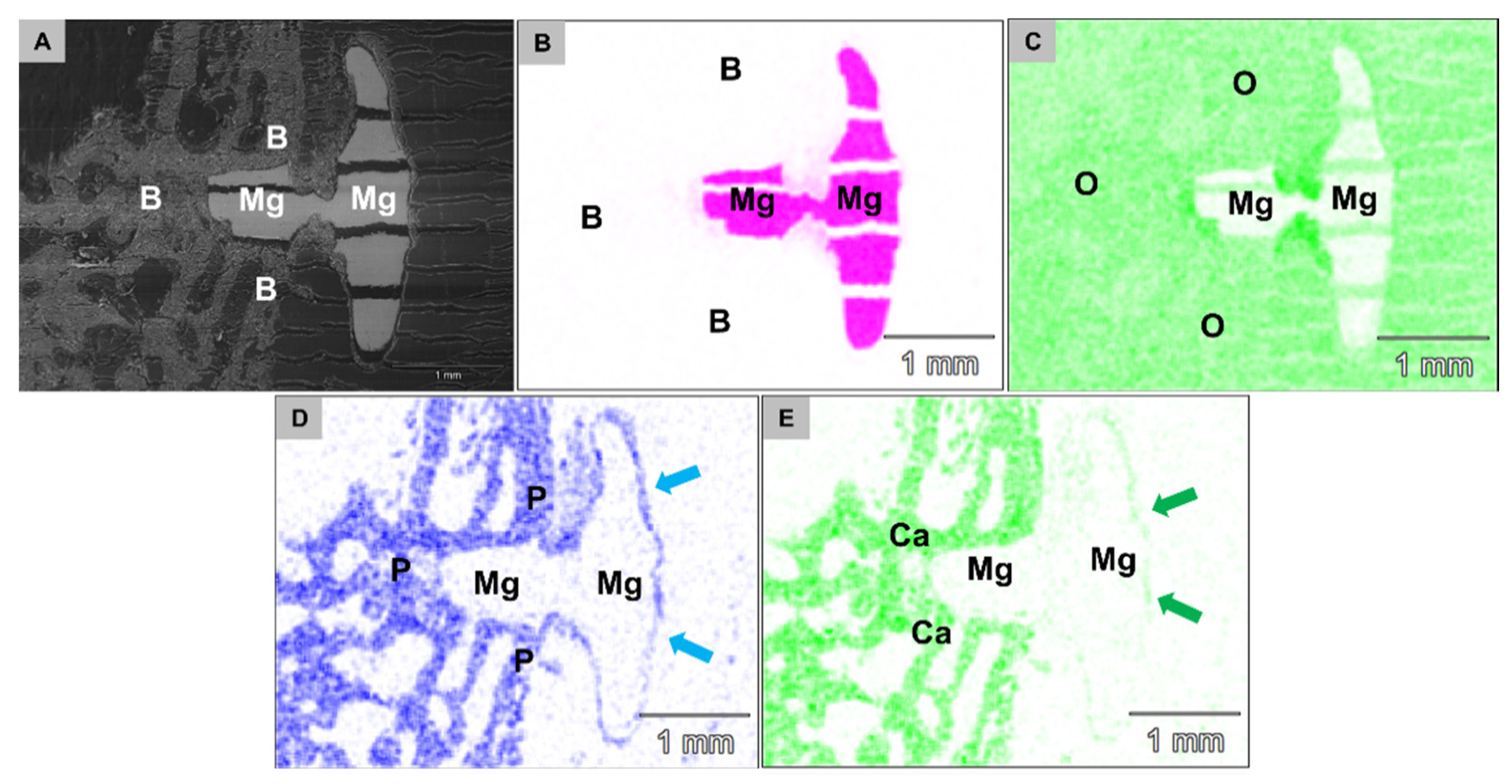
2.2. Synchrotron µCT Analysis
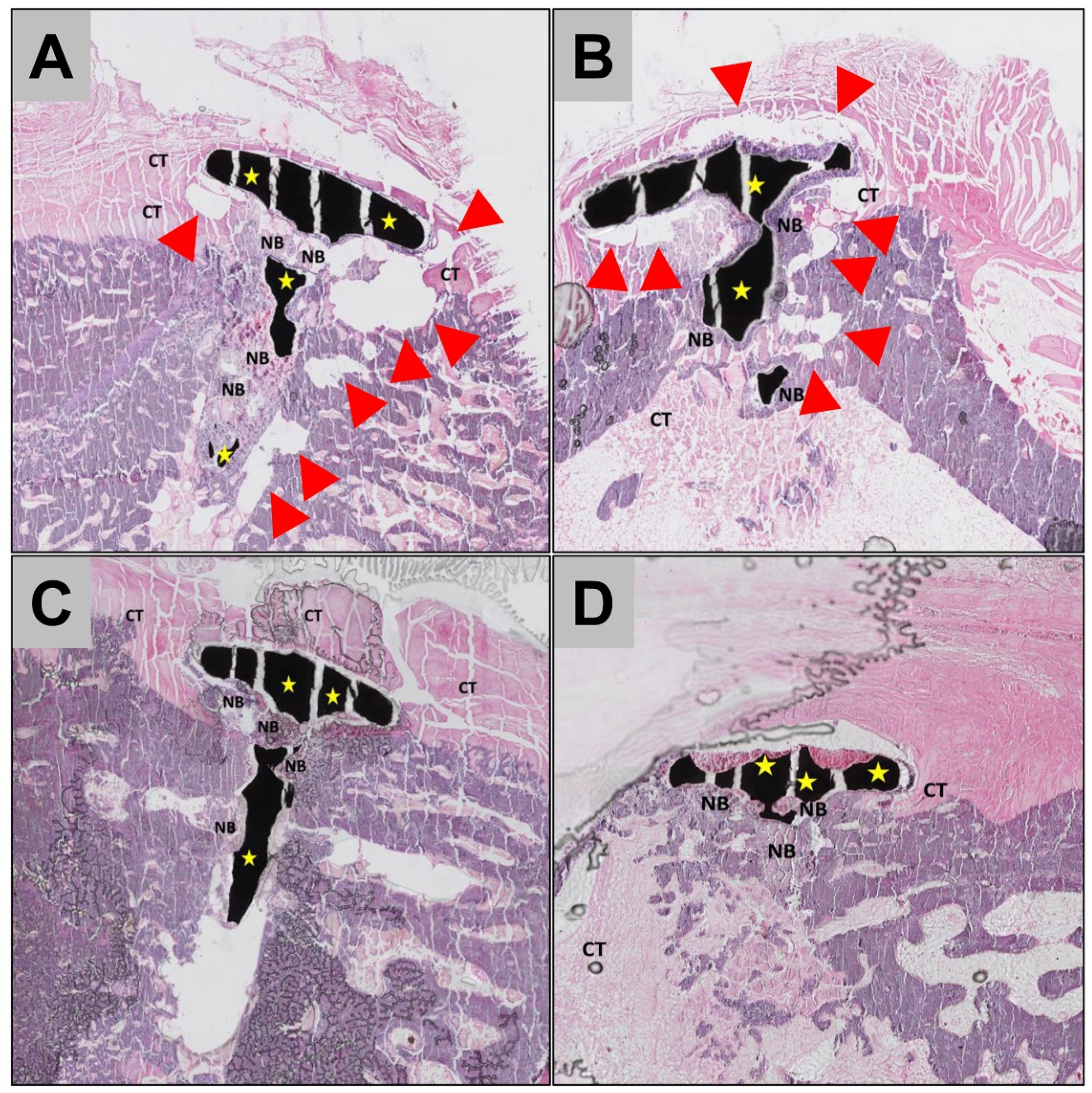
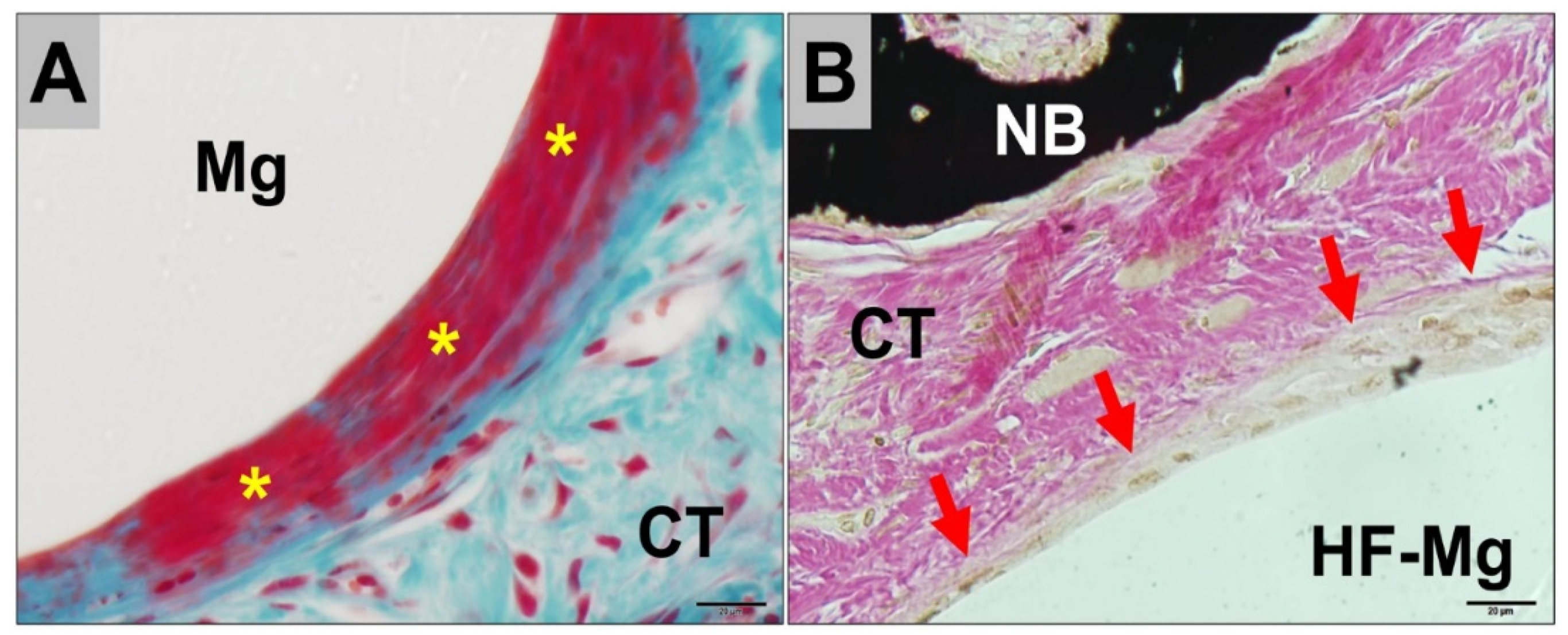
2.3. Histopathological Results
2.4. Histomorphometrical Results
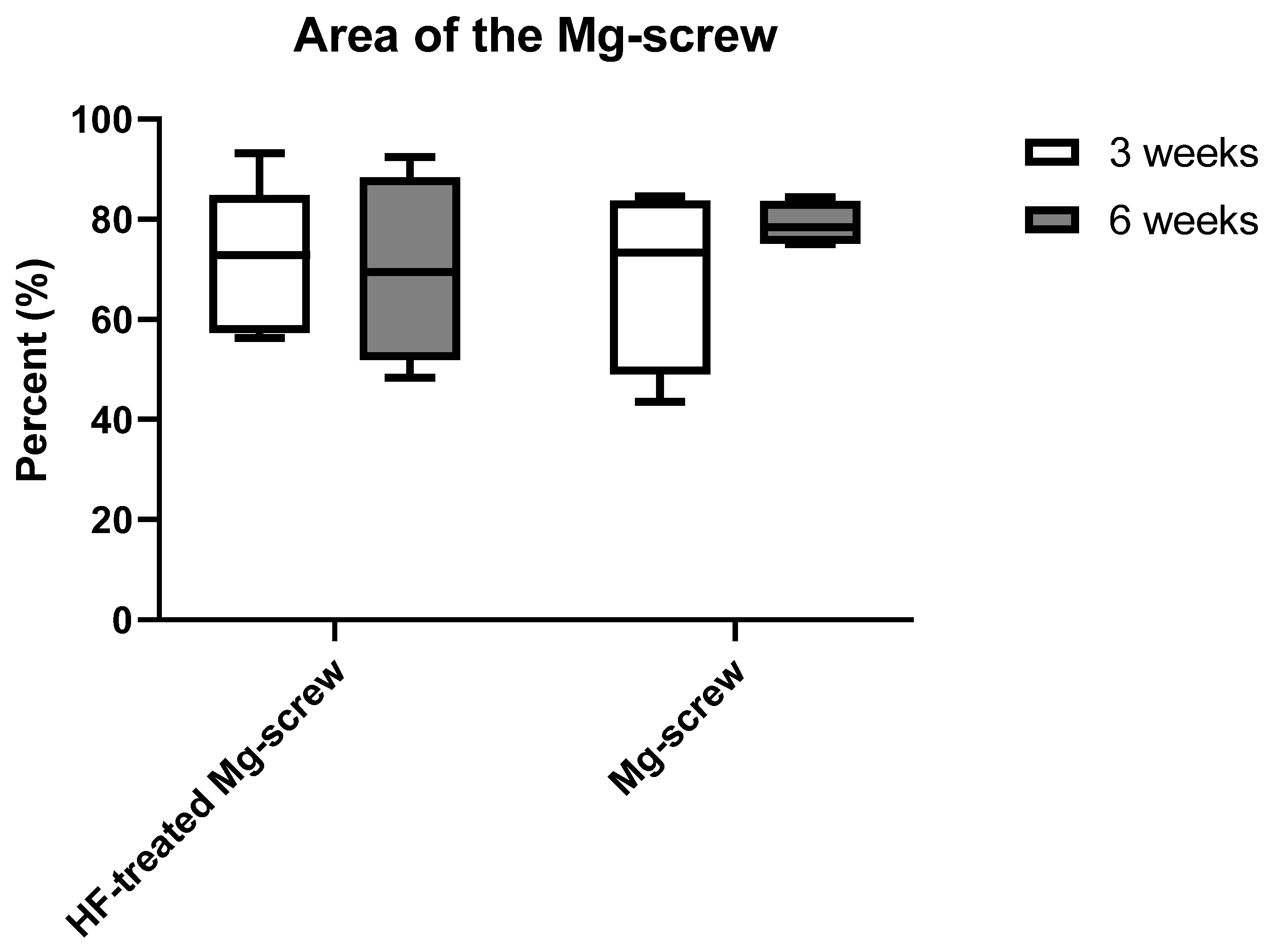
2.4.1. Measurement of the Remaining Mg screw Areas
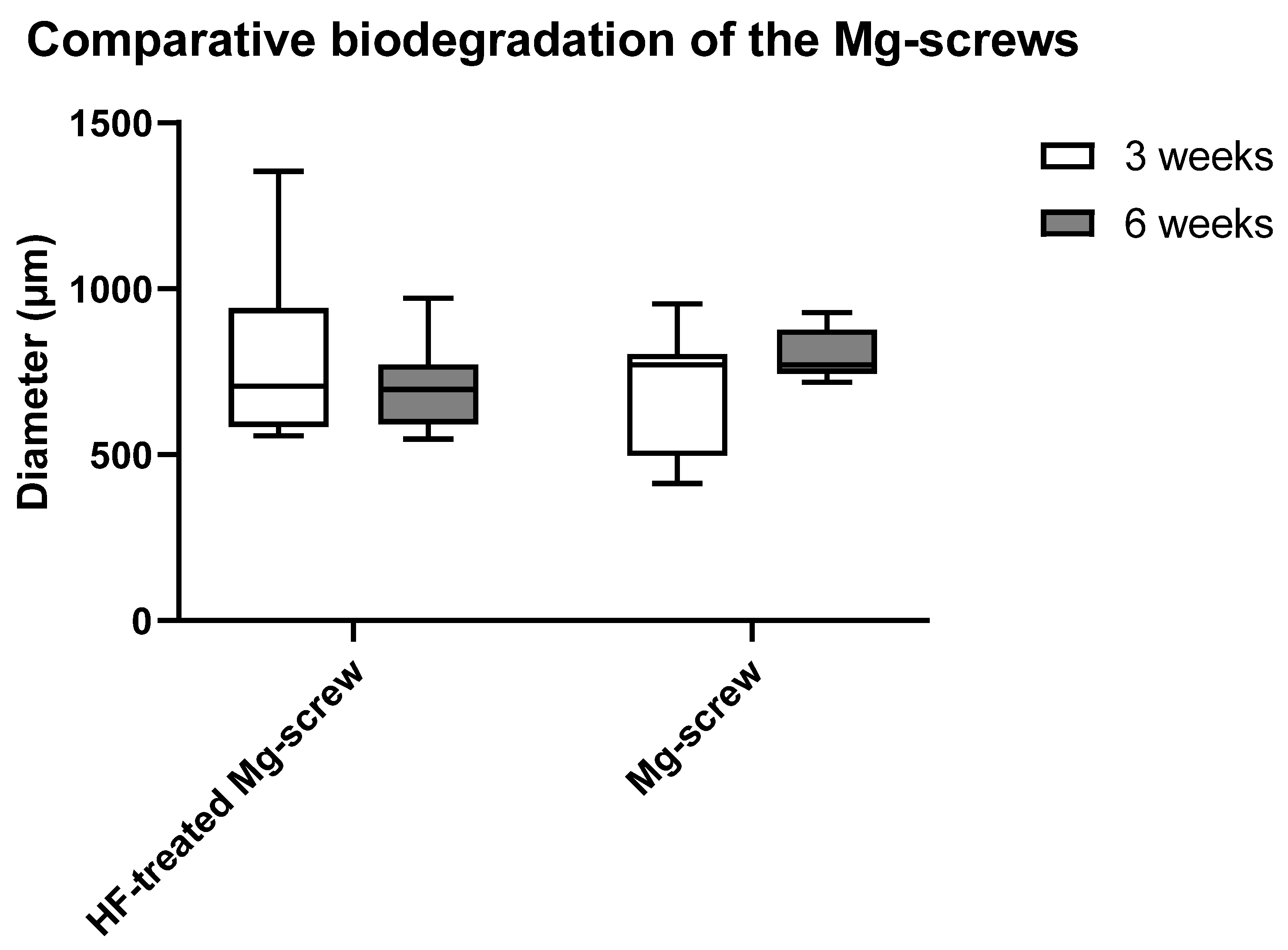
2.4.2. Measurements of the Mg screw Diameters
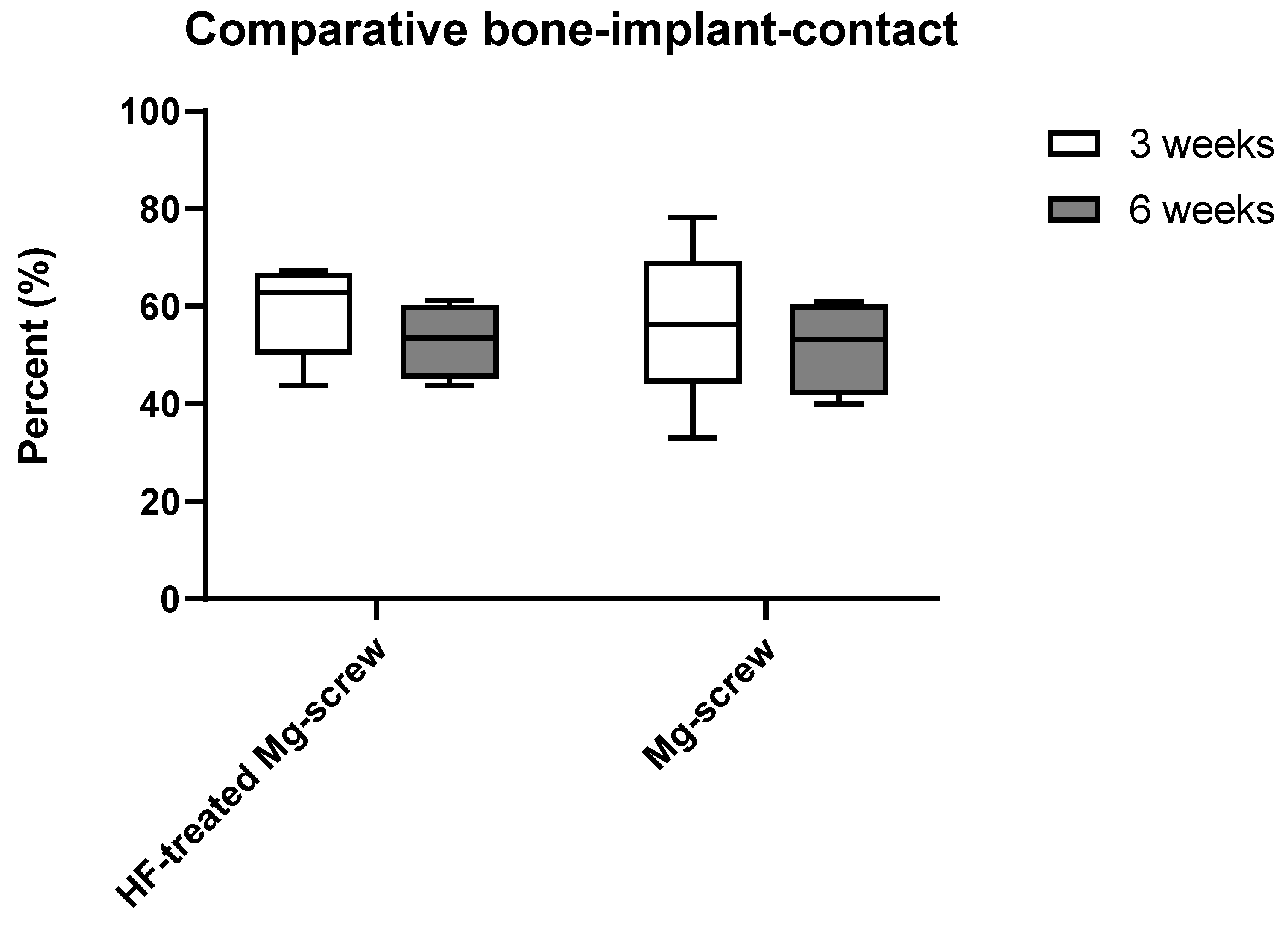
2.4.3. Measurements of the Implant-Bone-Contact
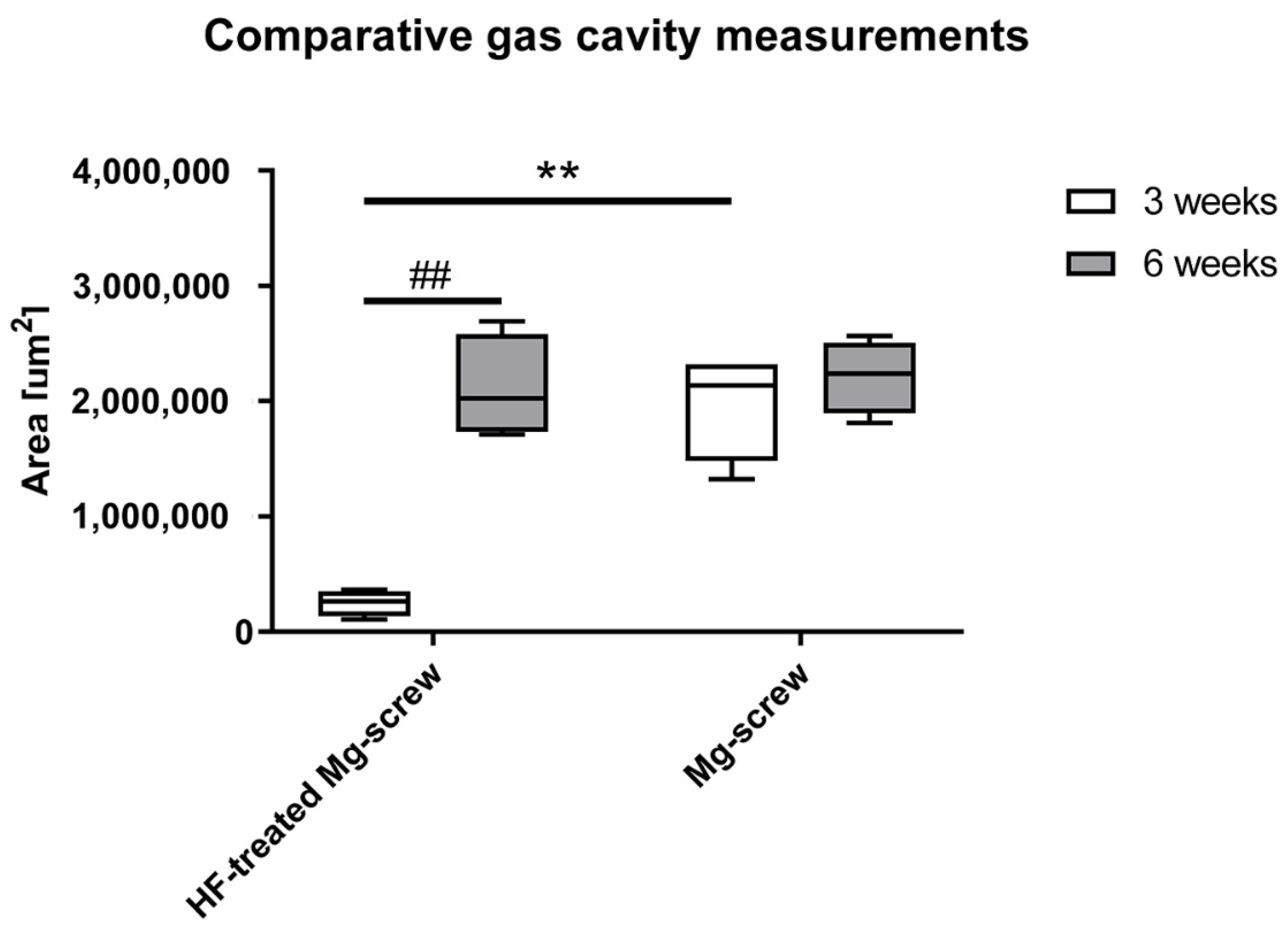
2.4.4. Material-Related Gas Cavity Measurements
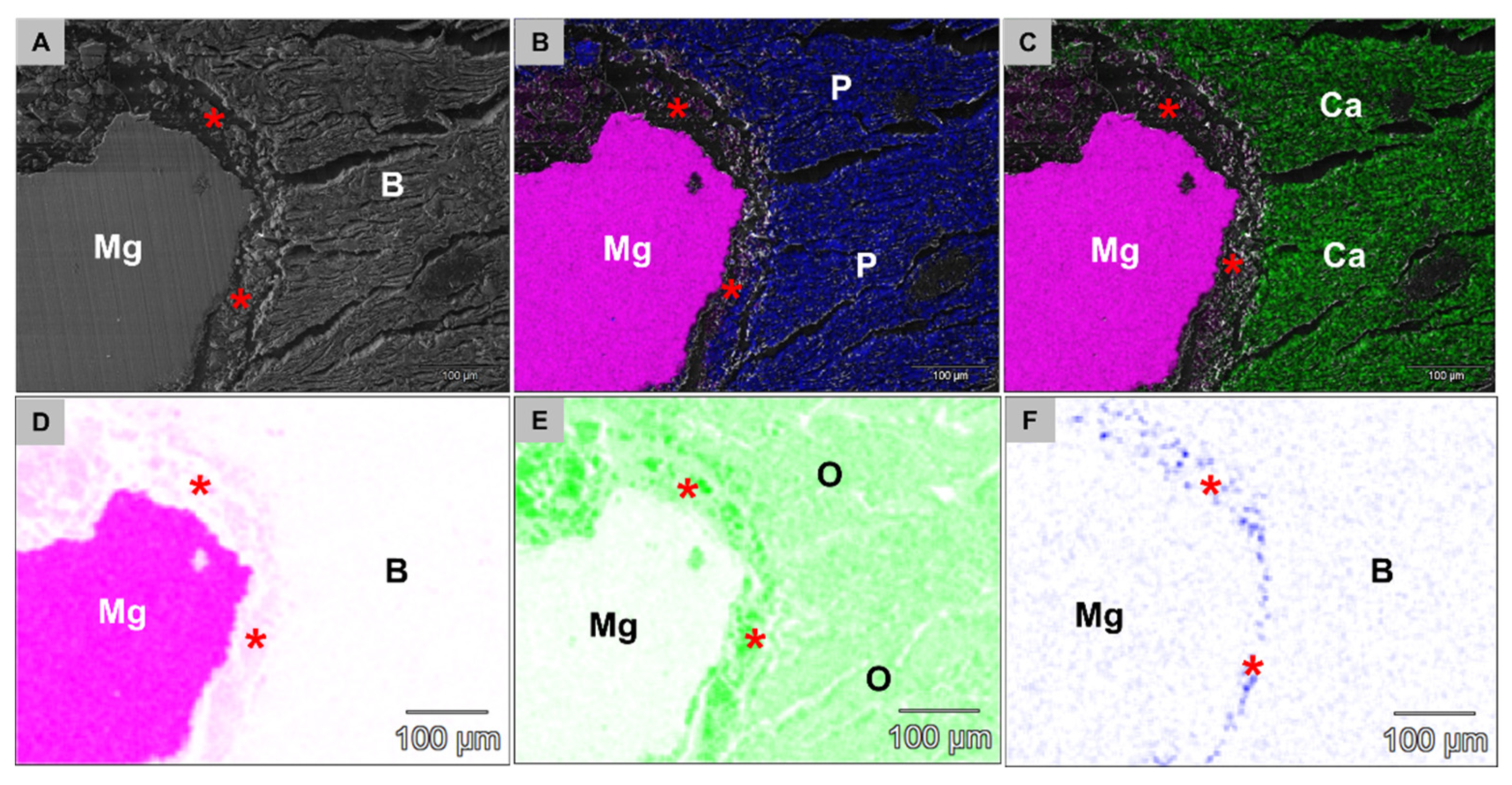
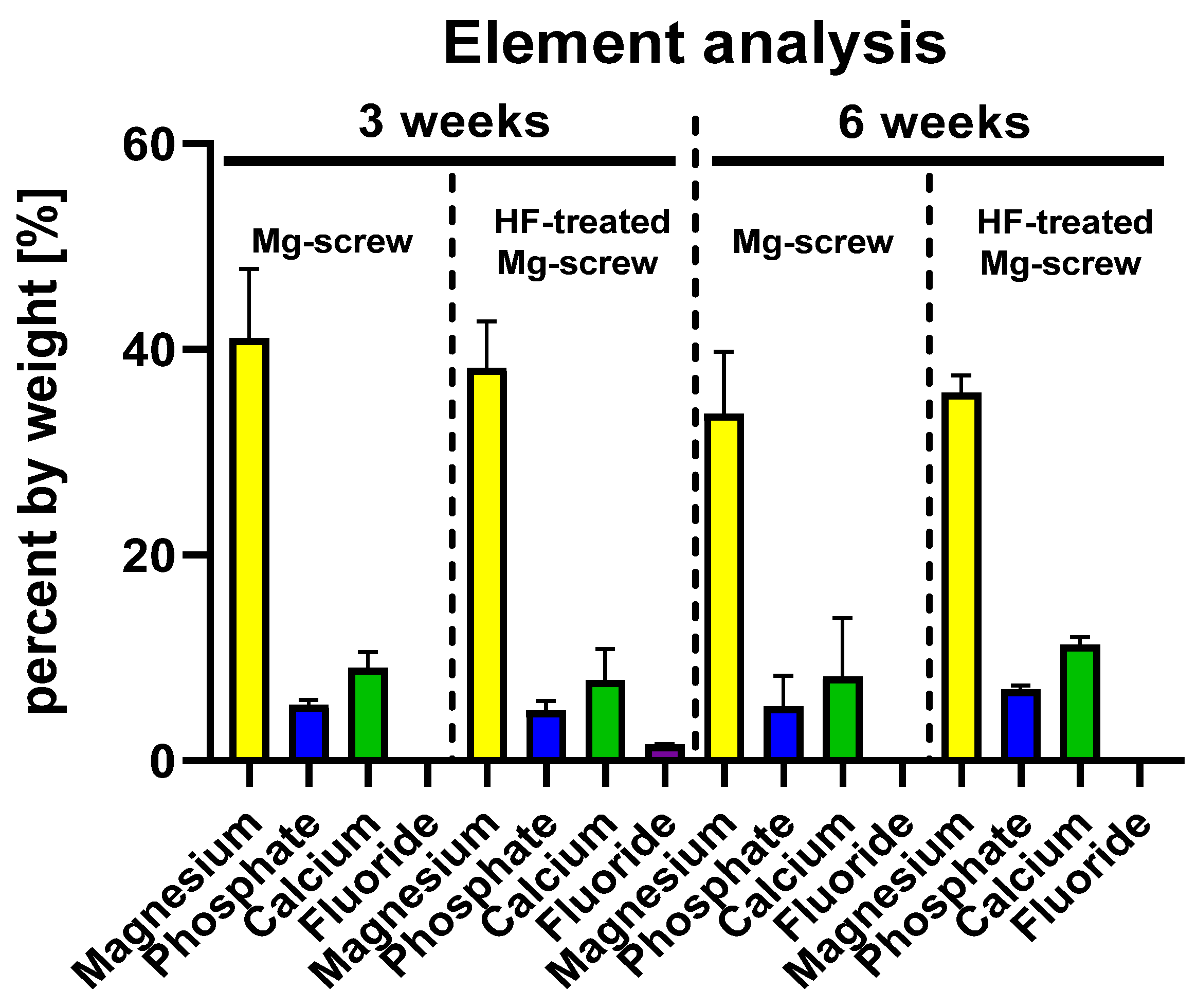
2.4.5. Element Analysis of the Implantation Beds
3. Discussion
4. Materials and Methods
4.1. Biomaterial Preparation
4.2. Cytocompatibility Analysis
4.2.1. Reference Material
4.2.2. Cells and Cell Culture
4.2.3. Extract Analysis
Extraction
Assay Procedure
Bromodeoxyuridine/5-Bromo-2′-Deoxyuridine (BrdU)-Assay
Sodium 3,3′-[1(Phenylamino)carbonyl]-3,4-tetrazolium]-3is(4-methoxy-6-nitro) Benzene Sulfonic Acid Hydrate (XTT)-Assay
Lactate Dehydrogenase (LDH)-Assay
4.3. Pre- and Post-Implantation Procedure, Surgical Procedure
4.4. Synchrotron Micro-CT
4.5. Histological Workup
4.6. Histopathological Analysis
4.7. Histomorphometrical Analysis
4.8. Elemental Analysis within the Implantation Beds
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sanchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef] [Green Version]
- Hämmerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [Green Version]
- Zitzmann, N.U.; Schärer, P.; Marinello, C.P. Long-term results of implants treated with guided bone regeneration: A 5-year prospective study. Int. J. Oral Maxillofac. Implant. 2001, 16, 355–366. [Google Scholar]
- Urban, I.A.; Monje, A.; Lozada, J.L.; Wang, H.-L. Long-term Evaluation of Peri-implant Bone Level after Reconstruction of Severely Atrophic Edentulous Maxilla via Vertical and Horizontal Guided Bone Regeneration in Combination with Sinus Augmentation: A Case Series with 1 to 15 Years of Loading. Clin. Implant. Dent. Relat. Res. 2017, 19, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Chappuis, V.; Rahman, L.; Buser, R.; Janner, S.; Belser, U.; Buser, D. Effectiveness of Contour Augmentation with Guided Bone Regeneration: 10-Year Results. J. Dent. Res. 2017, 97, 266–274. [Google Scholar] [CrossRef]
- Wang, H.-L.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- De Santis, D.; Gelpi, F.; Verlato, G.; Luciano, U.; Torroni, L.; Antonucci, N.; Bernardello, F.; Zarantonello, M.; Nocini, P. Digital Customized Titanium Mesh for Bone Regeneration of Vertical, Horizontal and Combined Defects: A Case Series. Medicina 2021, 57, 60. [Google Scholar] [CrossRef]
- Steigmann, L.; Jung, O.; Kieferle, W.; Stojanovic, S.; Proehl, A.; Görke, O.; Emmert, S.; Najman, S.; Barbeck, M.; Rothamel, D. Biocompatibility and Immune Response of a Newly Developed Volume-Stable Magnesium-Based Barrier Membrane in Combination with a PVD Coating for Guided Bone Regeneration (GBR). Biomedicines 2020, 8, 636. [Google Scholar] [CrossRef]
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2020, 28, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Shi, B.; Miron, R. Membranes for guided tissue and bone regeneration. Ann. Oral Maxillofac. Surg. 2013, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Bunyaratavej, P.; Wang, H.-L. Collagen Membranes: A Review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Caballe-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Penarrocha, M.; Hernandez-Alfaro, F. On the search of the ideal barrier membrane for guided bone regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef]
- Carbonell, J.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.; Nart, J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral Maxillofac. Surg. 2013, 43, 75–84. [Google Scholar] [CrossRef]
- Barbeck, M.; Kühnel, L.; Witte, F.; Pissarek, J.; Precht, C.; Xiong, X.; Krastev, R.; Wegner, N.; Walther, F.; Jung, O. Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane. Int. J. Mol. Sci. 2020, 21, 3098. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Porchetta, D.; Kopp, A.; Ptock, C.; Müller, U.; Heiland, M.; Schwade, M.; Behr, B.; Kröger, N.; et al. Optimized in vitro procedure for assessing the cytocompatibility of magnesium-based biomaterials. Acta Biomater. 2015, 23, 354–363. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Hartjen, P.; Schnettler, R.; Feyerabend, F.; Klein, M.; Wegner, N.; Walther, F.; Stangier, D.; Henningsen, A.; et al. Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12. Int. J. Mol. Sci. 2019, 20, 255. [Google Scholar] [CrossRef] [Green Version]
- Jung, O.; Porchetta, D.; Schroeder, M.-L.; Klein, M.; Wegner, N.; Walther, F.; Feyerabend, F.; Barbeck, M.; Kopp, A. In Vivo Simulation of Magnesium Degradability Using a New Fluid Dynamic Bench Testing Approach. Int. J. Mol. Sci. 2019, 20, 4859. [Google Scholar] [CrossRef] [Green Version]
- Cestari, T.M.; De Oliveira, R.C.; Sanada, J.T.; Garlet, G.P.; Taga, R.; Granjeiro, J.M. Biocompatibility evaluation of a new bioresorbable pin for membrane fixation. Braz. Dent. J. 2010, 21, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, Y.; Ota, M.; Sekiguchi, K.; Shibukawa, Y.; Yamada, S. Evaluation of a poly-l-lactic acid membrane and membrane fixing pin for guided tissue regeneration on bone defects in dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Augat, P.; Robioneck, P.B.; Abdulazim, A.; Wipf, F.; Lips, K.S.; Alt, V.; Schnettler, R.; Heiss, C. Fixation performance of an ultrasonically fused, bioresorbable osteosynthesis implant: A biomechanical and biocompatibility study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 170–179. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Tian, N.; Dong, X.; Cheng, C.-K. Polylactide Composite Pins Reinforced with Bioresorbable Continuous Glass Fibers Demonstrating Bone-like Apatite Formation and Spiral Delamination Degradation. Polymers 2019, 11, 812. [Google Scholar] [CrossRef] [Green Version]
- Rocchio, T.M. Resorbable Polymer Pin Inserted with Ultrasound Activated BoneWelding Technique Compared with a Screw for Osteotomy Fixation in the Reverse L Bunion Correction. Clin. Podiatr. Med. Surg. 2018, 35, 373–385. [Google Scholar] [CrossRef]
- Neumann, H.; Schulz, A.P.; Gille, J.; Klinger, M.; Jurgens, C.; Reimers, N.; Kienast, B. Refixation of osteochondral fractures by ultrasound-activated, resorbable pins. Bone Jt. Res. 2013, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kienast, B.; Mohsen, H.; Wendlandt, R.; Reimers, N.; Schulz, A.P.; Heuer, H.; Gille, J.; Neumann, H. Biomechanical evaluation of novel ultrasound-activated bioresorbable pins for the treatment of osteochondral fractures compared to established methods. Biomed. Tech. Eng. 2017, 62, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Hu, N.; Ma, Y.; Li, Y.; Liu, J.; Zhang, X.; Pan, H. The interfacial pH of acidic degradable polymeric biomaterials and its effects on osteoblast behavior. Sci. Rep. 2017, 7, 6794. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly (lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2020, 6, 346–360. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Zhu, H.; Yan, L.; Zhu, S.; Yan, Y. Preparation, characterization, and in vitro and in vivo biocompatibility evaluation of polymer (amino acid and glycolic acid)/hydroxyapatite composite for bone repair. Biomed. Mater. 2021, 16, 025004. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Staiger, M.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1691–1701. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen. Biomater. 2014, 2, 135–151. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [Green Version]
- Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W.; et al. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2014, 11, 554–562. [Google Scholar] [CrossRef]
- Thomann, M.; Krause, C.; Angrisani, N.; Bormann, D.; Hassel, T.; Windhagen, H.; Meyer-Lindenberg, A. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0.8) on degradation in a rabbit model. J. Biomed. Mater. Res. A 2010, 93, 1609–1619. [Google Scholar] [CrossRef]
- Carboneras, M.; García-Alonso, M.; Escudero, M. Biodegradation kinetics of modified magnesium-based materials in cell culture medium. Corros. Sci. 2011, 53, 1433–1439. [Google Scholar] [CrossRef]
- Yan, T.; Tan, L.; Xiong, D.; Liu, X.; Zhang, B.; Yang, K. Fluoride treatment and in vitro corrosion behavior of an AZ31B magnesium alloy. Mater. Sci. Eng. C 2010, 30, 740–748. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef]
- Kang, M.-H.; Jang, T.-S.; Kim, S.W.; Park, H.-S.; Song, J.; Kim, H.-E.; Jung, K.-H.; Jung, H.-D. MgF2-coated porous magnesium/alumina scaffolds with improved strength, corrosion resistance, and biological performance for biomedical applications. Mater. Sci. Eng. C 2016, 62, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Da Conceicao, T.; Scharnagl, N.; Blawert, C.; Dietzel, W.; Kainer, K. Surface modification of magnesium alloy AZ31 by hydrofluoric acid treatment and its effect on the corrosion behaviour. Thin Solid Films 2010, 518, 5209–5218. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implant. 2015, 41, e212–e222. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Hoffmann, C.; Sader, R.; Peters, F.; Hübner, W.-D.; Kirkpatrick, C.J.; Ghanaati, S. Injectable Bone Substitute Based on β-TCP Combined with a Hyaluronan-Containing Hydrogel Contributes to Regeneration of a Critical Bone Size Defect Towards Restitutio ad Integrum. J. Oral Implant. 2016, 42, 127–137. [Google Scholar] [CrossRef]
- Barbeck, M.; Jung, O.; Smeets, R.; Gosau, M.; Schnettler, R.; Rider, P.; Houshmand, A.; Korzinskas, T. Implantation of an Injectable Bone Substitute Material Enables Integration Following the Principles of Guided Bone Regeneration. In Vivo 2020, 34, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Gueldenpfennig, T.; Houshmand, A.; Najman, S.; Stojanovic, S.; Korzinskas, T.; Smeets, R.; Gosau, M.; Pissarek, J.; Emmert, S.; Jung, O.; et al. The Condensation of Collagen Leads to an Extended Standing Time and a Decreased Pro-inflammatory Tissue Response to a Newly Developed Pericardium-based Barrier Membrane for Guided Bone Regeneration. In Vivo 2020, 34, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef] [Green Version]
- Sieger, D.; Korzinskas, T.; Jung, O.; Stojanovic, S.; Wenisch, S.; Smeets, R.; Gosau, M.; Schnettler, R.; Najman, S.; Barbeck, M. The Addition of High Doses of Hyaluronic Acid to a Biphasic Bone Substitute Decreases the Proinflammatory Tissue Response. Int. J. Mol. Sci. 2019, 20, 1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Lorenz, J.; Kubesch, A.; Böhm, N.; Booms, P.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis-Derived Collagen Membranes Induce Implantation Bed Vascularization Via Multinucleated Giant Cells: A Physiological Reaction? J. Oral Implant. 2015, 41, e238–e251. [Google Scholar] [CrossRef]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef]
- Berglund, I.S.; Jacobs, B.Y.; Allen, K.D.; Kim, S.E.; Pozzi, A.; Allen, J.B.; Manuel, M.V. Peri-implant tissue response and biodegradation performance of a Mg–1.0Ca–0.5Sr alloy in rat tibia. Mater. Sci. Eng. C 2016, 62, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lindtner, R.A.; Castellani, C.; Tangl, S.; Zanoni, G.; Hausbrandt, P.; Tschegg, E.K.; Stanzl-Tschegg, S.E.; Weinberg, A.-M. Comparative biomechanical and radiological characterization of osseointegration of a biodegradable magnesium alloy pin and a copolymeric control for osteosynthesis. J. Mech. Behav. Biomed. Mater. 2013, 28, 232–243. [Google Scholar] [CrossRef]
- Matthews, L.S.; Green, C.A.; Goldstein, S.A. The thermal effects of skeletal fixation-pin insertion in bone. J. Bone Jt. Surg. Am. 1984, 66, 1077–1083. [Google Scholar] [CrossRef]
- Tang, T.; Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar] [CrossRef] [Green Version]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.; Hesse, B.; Addison, O.; Morrell, A.P.; Gross, C.; Lagrange, A.; Suárez, V.I.; Kohal, R.; Fretwurst, T. Distribution and Chemical Speciation of Exogenous Micro- and Nanoparticles in Inflamed Soft Tissue Adjacent to Titanium and Ceramic Dental Implants. Anal. Chem. 2020, 92, 14432–14443. [Google Scholar] [CrossRef] [PubMed]
- Schoon, J.; Hesse, B.; Rakow, A.; Ort, M.J.; Lagrange, A.; Jacobi, D.; Winter, A.; Huesker, K.; Reinke, S.; Cotte, M.; et al. Metal-Specific Biomaterial Accumulation in Human Peri-Implant Bone and Bone Marrow. Adv. Sci. 2020, 7, 2000412. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. The in vitro and in vivo evaluation of the biocompatibility of Mg alloys. Biomed. Mater. 2013, 9, 15006. [Google Scholar] [CrossRef]
- Perale, G.; Hilborn, J. Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Pillai, C.K.S.; Sharma, C. Review Paper: Absorbable Polymeric Surgical Sutures: Chemistry, Production, Properties, Biodegradability, and Performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef]
- Durisin, M.; Reifenrath, J.; Weber, C.M.; Eifler, R.; Maier, H.J.; Lenarz, T.; Seitz, J.-M. Biodegradable nasal stents (MgF2-coated Mg-2 wt %Nd alloy)-A long-termin vivostudy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 105, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.-M.; Eifler, R.; Weber, C.; Lenarz, T.H.; Maier, H.J.; Durisin, M. In vivodegradation effects of alloy MgNd2 in contact with mucous tissue. J. Biomed. Mater. Res. Part A 2014, 103, 2427–2440. [Google Scholar] [CrossRef]
- Weber, C.M.; Eifler, R.; Seitz, J.-M.; Maier, H.J.; Reifenrath, J.; Lenarz, T.; Durisin, M. Biocompatibility of MgF2-coated MgNd2 specimens in contact with mucosa of the nasal sinus—A long term study. Acta Biomater. 2015, 18, 249–261. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Jiang, H.; Chen, M.; Bi, Y.; Liu, D. In vivo comparative property study of the bioactivity of coated Mg–3Zn–0.8Zr alloy. Mater. Sci. Eng. C 2013, 33, 3263–3272. [Google Scholar] [CrossRef]
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater. 2010, 6, 1813–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Zhao, H.; Ding, Z.; Zhang, Z.; Sun, B.; Shen, J.; Chen, S.; Zhang, B.; Yang, K.; Liu, M.; et al. In vitro and in vivo evaluation of MgF2 coated AZ31 magnesium alloy porous scaffolds for bone regeneration. Colloids Surfaces B Biointerfaces 2016, 149, 330–340. [Google Scholar] [CrossRef]
- Li, Z.; Shizhao, S.; Chen, M.; Fahlman, B.D.; Liu, D.; Bi, H. In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF 2 -coated Mg-Zn-Zr alloy as cancellous screws. Mater. Sci. Eng. C 2017, 75, 1268–1280. [Google Scholar] [CrossRef]
- Poinern, G.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef] [Green Version]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.; Chen, M.; Liu, D. Biological activity evaluation of magnesium fluoride coated Mg-Zn-Zr alloy in vivo. Mater. Sci. Eng. C 2017, 75, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Park, J.-I.; Chae, J.S.; Yeo, I.-S.L. Comparison of micro-computed tomography and histomorphometry in the measurement of bone–implant contact ratios. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 87–95. [Google Scholar] [CrossRef]
- Lyu, H.-Z.; Lee, J.H. Correlation between two-dimensional micro-CT and histomorphometry for assessment of the implant osseointegration in rabbit tibia model. Biomater. Res. 2021, 25, 11. [Google Scholar] [CrossRef]
- Kapogianni, E.; Barbeck, M.; Jung, O.; Arslan, A.; Kuhnel, L.; Xiong, X.; Krastev, R.; Friedrich, R.E.; Schnettler, R.; Fienitz, T.; et al. Comparison of Material-mediated Bone Regeneration Capacities of Sintered and Non-sintered Xenogeneic Bone Substitutes via 2D and 3D Data. In Vivo 2019, 33, 2169–2179. [Google Scholar] [CrossRef] [Green Version]
- Marco, I.; Feyerabend, F.; Willumeit, R.; Van der Biest, O. Degradation testing of Mg alloys in Dulbecco’s modified eagle medium: Influence of medium sterilization. Mater. Sci. Eng. C 2016, 62, 68–78. [Google Scholar] [CrossRef]
- Marco, I.; Myrissa, A.; Martinelli, E.; Feyerabend, F.; Willumeit-Römer, R.; Weinberg, A.M.; Van Der Biest, O. In vivo and in vitro degradation comparison of pure Mg, Mg-10Gd and Mg-2Ag: A short term study. Eur. Cells Mater. 2017, 33, 90–104. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Kopp, A.; Porchetta, D.; Hiester, P.; Heiland, M.; E Friedrich, R.; Precht, C.; Hanken, H.; Gröbe, A.; et al. PEO-generated Surfaces Support Attachment and Growth of Cells In Vitro with No Additional Benefit for Micro-roughness in Sa (0.2–4 μm). In Vivo 2016, 30, 27–33. [Google Scholar]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Barbeck, M.; Najman, S.; Stojanovic, S.; Mitić, Z.; Živković, J.M.; Choukroun, J.; Kovačević, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Addition of blood to a phycogenic bone substitute leads to increased in vivo vascularization. Biomed. Mater. 2015, 10, 055007. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Unger, R.E.; Booms, P.; Dohle, E.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Monocyte preseeding leads to an increased implant bed vascularization of biphasic calcium phosphate bone substitutes via vessel maturation. J. Biomed. Mater. Res. Part A 2016, 104, 2928–2935. [Google Scholar] [CrossRef]
- Weitkamp, T.; Scheel, M.; Perrin, J.; Daniel, G.; King, A.; Roux, L.; Giorgetta, J.L.; Carcy, A.; Langlois, F.; Desjardins, K.; et al. Microtomography developments on the ANATOMIX beamline at Synchrotron SOLEIL. arXiv 2020, arXiv:2002.03242. [Google Scholar]
- Weitkamp, T.; Scheel, M.; Giorgetta, J.L.; Joyet, V.; Le Roux, V.; Cauchon, C.; Moreno, T.; Polack, F.; Thompson, A.; Samama, J.P. The tomography beamline ANATOMIX at Synchrotron SOLEIL. J. Phys. Conf. Ser. 2017, 849, 012037. [Google Scholar] [CrossRef] [Green Version]
- Mirone, A.; Brun, E.; Gouillart, E.; Tafforeau, P.; Kieffer, J. The PyHST2 hybrid distributed code for high speed tomographic reconstruction with iterative reconstruction and a priori knowledge capabilities. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2014, 324, 41–48. [Google Scholar] [CrossRef] [Green Version]



| Assay | Medium Control | Titanium Grade 4 | Titanium Grade 5 | Positive Control | HF-Treated Screw | Untreated Screw |
|---|---|---|---|---|---|---|
| Proliferation (BrdU) | 100 ± 1.64 | 96.06 ± 1.66 | 96.4 ± 3.04 | 1.55 ± 0.53 | 90.32 ± 2.13 | 0.06 ± 0.18 |
| Viability (XTT) | 100 ± 1.12 | 100.37 ± 3.55 | 100.13 ± 2.05 | −0.51 ± 0.12 | 89.79 ± 0.64 | 1.70 ± 0.23 |
| Toxicity (LDH) | 100 ± 43.0 | 66.8 ± 11.9 | 58.7 ± 39.2 | 3886.4 ± 169.7 | −7.6 ± 36.8 | 2832.0 ± 97.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, O.; Hesse, B.; Stojanovic, S.; Seim, C.; Weitkamp, T.; Batinic, M.; Goerke, O.; Kačarević, Ž.P.; Rider, P.; Najman, S.; et al. Biocompatibility Analyses of HF-Passivated Magnesium Screws for Guided Bone Regeneration (GBR). Int. J. Mol. Sci. 2021, 22, 12567. https://doi.org/10.3390/ijms222212567
Jung O, Hesse B, Stojanovic S, Seim C, Weitkamp T, Batinic M, Goerke O, Kačarević ŽP, Rider P, Najman S, et al. Biocompatibility Analyses of HF-Passivated Magnesium Screws for Guided Bone Regeneration (GBR). International Journal of Molecular Sciences. 2021; 22(22):12567. https://doi.org/10.3390/ijms222212567
Chicago/Turabian StyleJung, Ole, Bernhard Hesse, Sanja Stojanovic, Christian Seim, Timm Weitkamp, Milijana Batinic, Oliver Goerke, Željka Perić Kačarević, Patrick Rider, Stevo Najman, and et al. 2021. "Biocompatibility Analyses of HF-Passivated Magnesium Screws for Guided Bone Regeneration (GBR)" International Journal of Molecular Sciences 22, no. 22: 12567. https://doi.org/10.3390/ijms222212567











