Turning Seashell Waste into Electrically Conductive Particles
Abstract
:1. Introduction
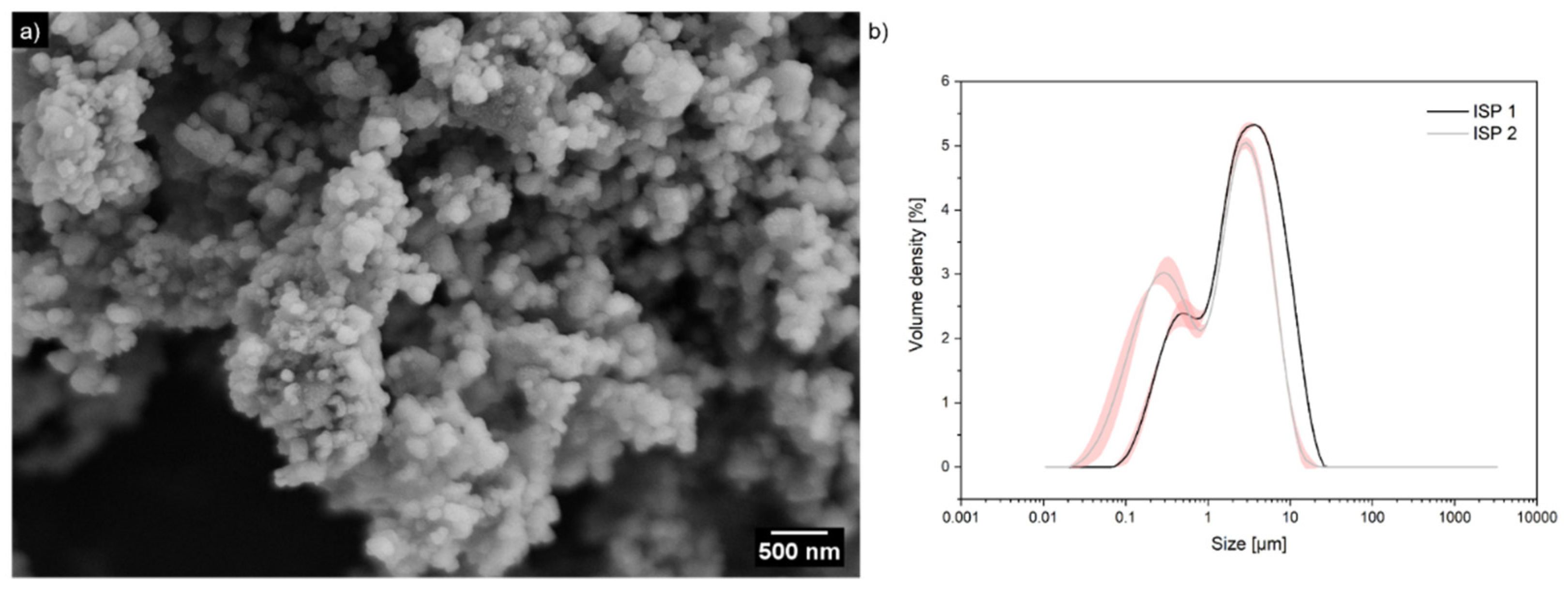
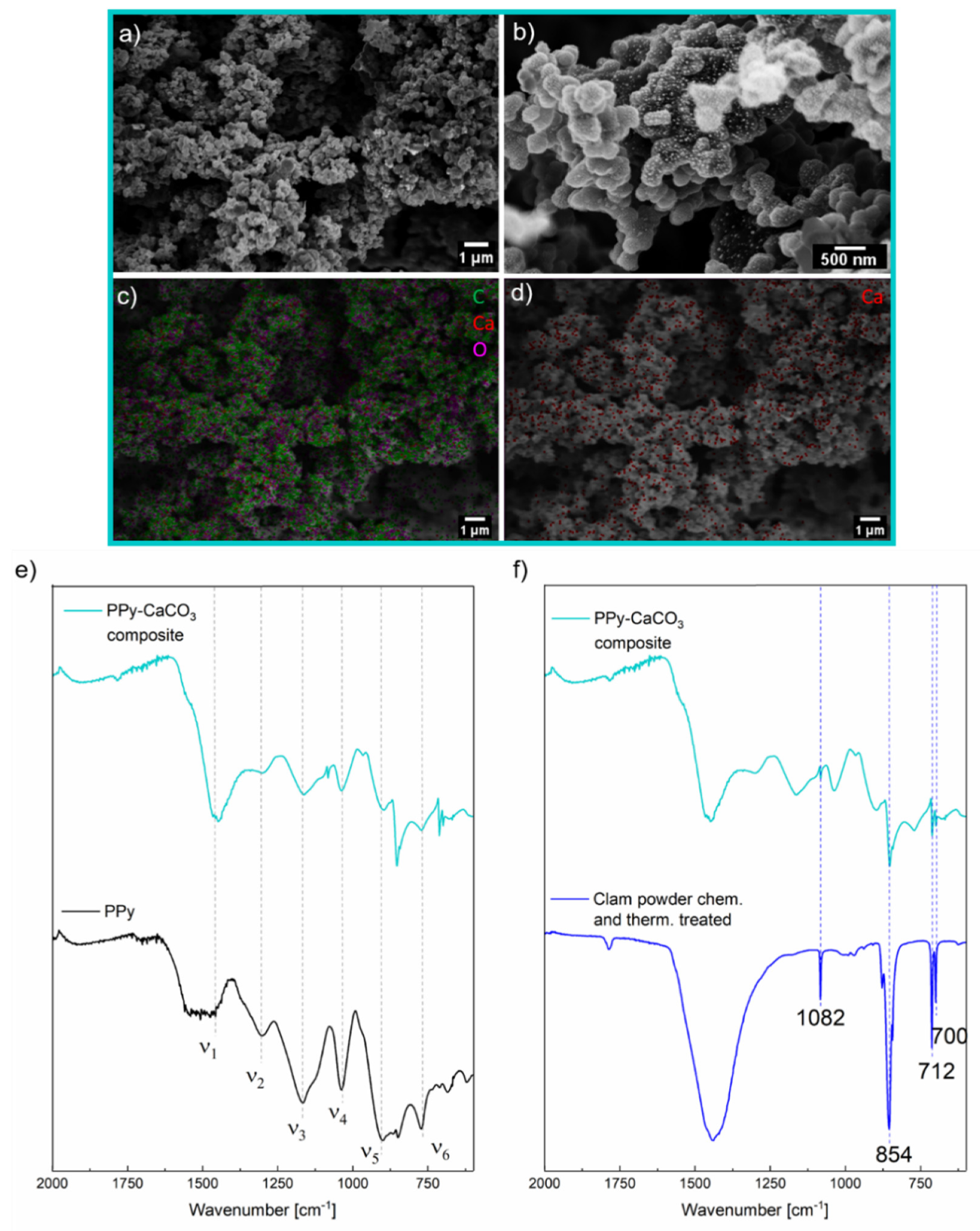
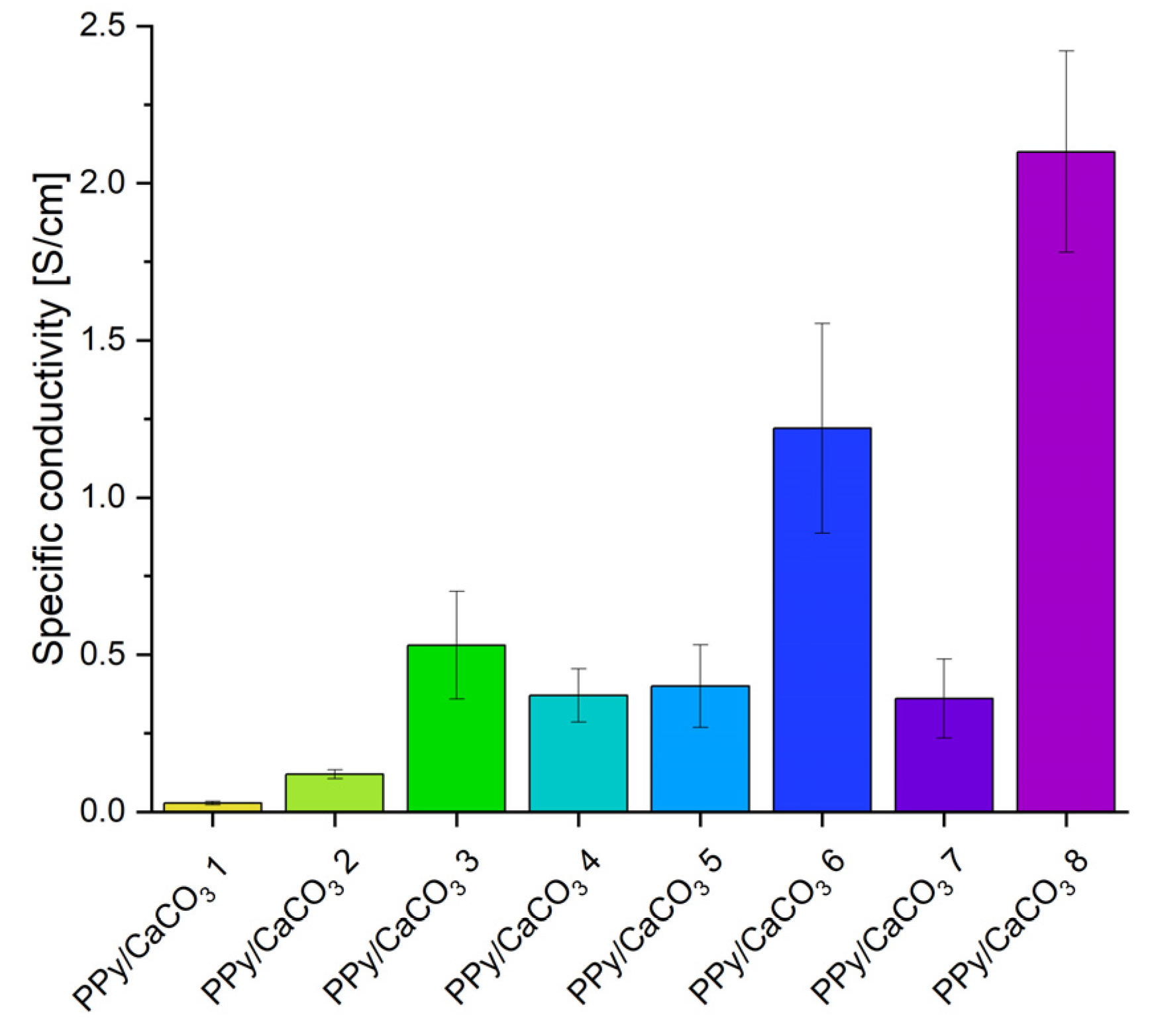
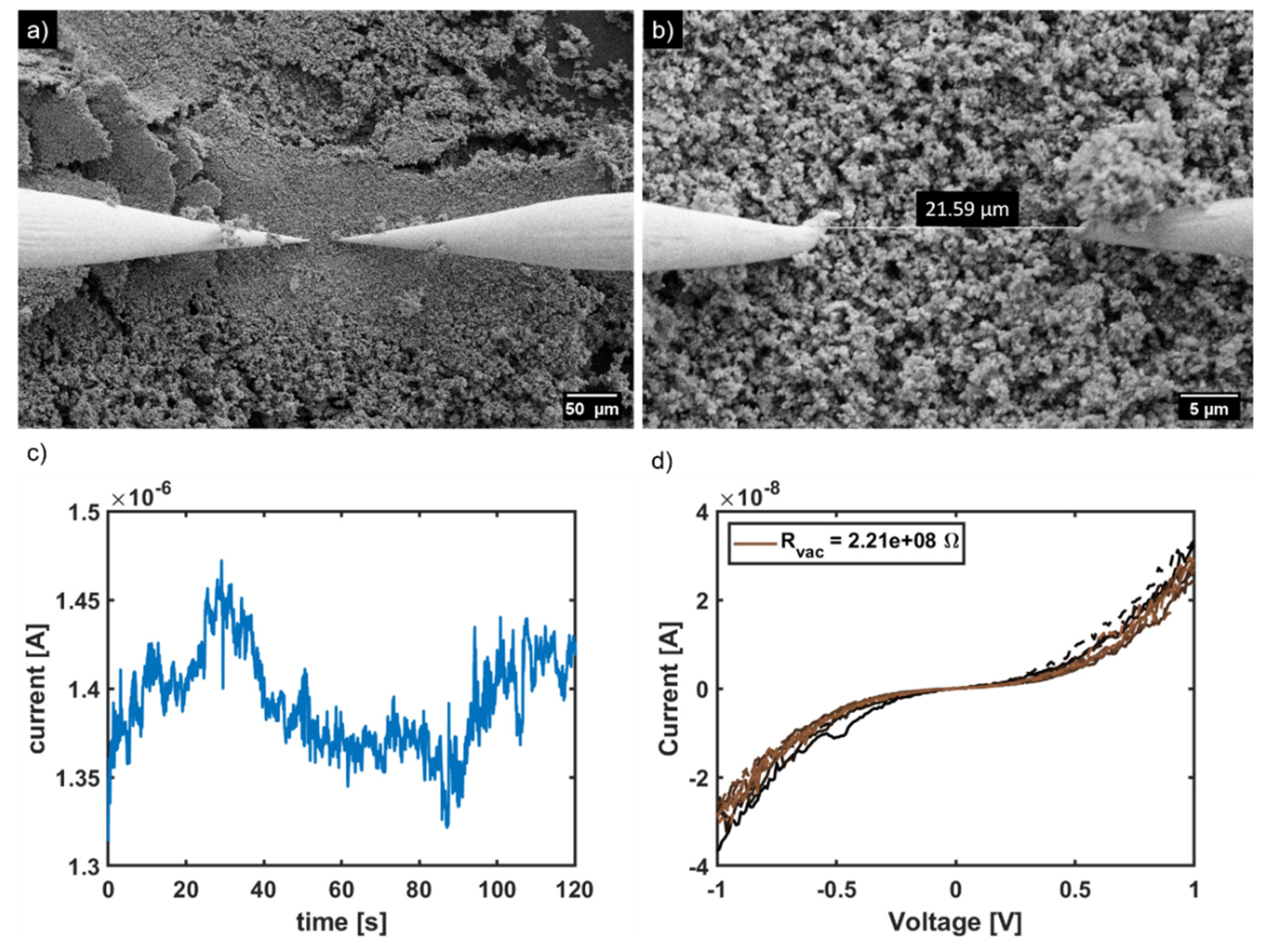
2. Results and Discussion
2.1. Synthesis of Electrically Conductive PPy/CaCO3 Particles
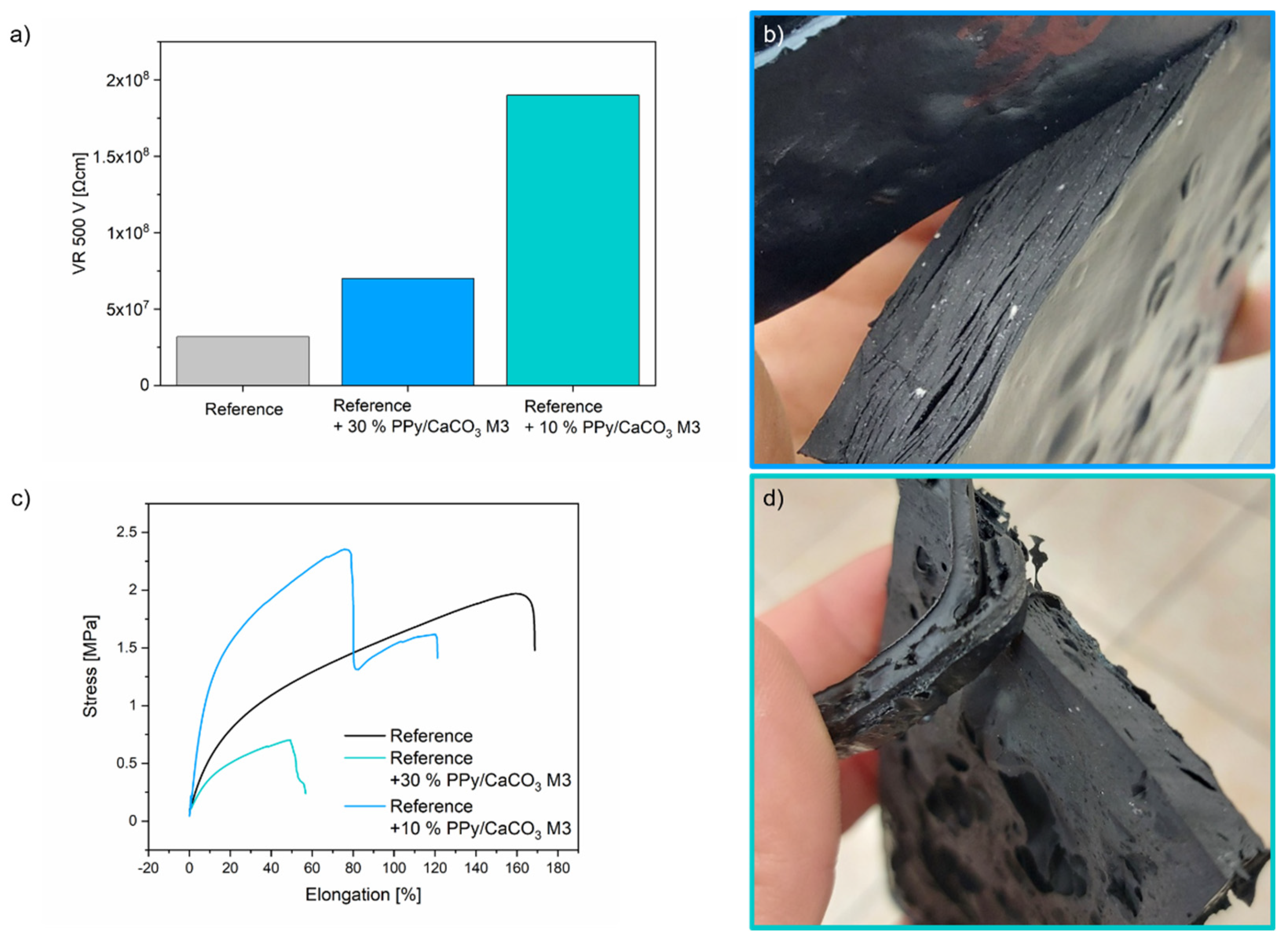
2.2. Application of PPy/CaCO3 Particles as an Antistatic Agent
3. Materials and Methods
3.1. Preparation of Clam Shell Particles
3.2. Synthesis of Polypyrrole: Adding Py First
3.3. Synthesis of Polypyrrole: Adding CuCl2 First
3.3.1. Polymerization under Nitrogen
3.3.2. Polymerization in the Desiccator
3.4. Doping
3.5. Compression Molding
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press on Demand: Oxford, UK, 1989. [Google Scholar]
- Jackson, A.; Vincent, J.F.; Turner, R. The mechanical design of nacre. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 1988, 234, 415–440. [Google Scholar]
- Rubner, M. Synthetic sea shell. Nature 2003, 423, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Yourdkhani, M.; Pasini, D.; Barthelat, F. The hierarchical structure of seashells optimized to resist mechanical threats. Des. Nat. V Comp. Des. Nat. Sci. Eng. Trans. Ecol. Environ. 2010, 138, 141–153. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.-d.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Hasaniyah, M.W.; Zeyad, A.; Yusuf, M.O. Properties of concrete containing recycled seashells as cement partial replacement: A review. J. Clean. Prod. 2019, 237, 117723. [Google Scholar] [CrossRef]
- Balan, G.S.; Kumar, V.S.; Rajaram, S.; Ravichandran, M. Investigation on water absorption and wear characteristics of waste plastics and seashell powder reinforced polymer composite. J. Tribol. 2020, 27, 57–70. [Google Scholar]
- Owuamanam, S.; Cree, D. Progress of bio-calcium carbonate waste eggshell and seashell fillers in polymer composites: A review. J. Compos. Sci. 2020, 4, 70. [Google Scholar] [CrossRef]
- Cangiotti, J.; Scatto, M.; Araya-Hermosilla, E.; Micheletti, C.; Crivellari, D.; Balloni, A.; Pucci, A.; Benedetti, A. Valorization of seashell waste in polypropylene composites: An accessible solution to overcome marine landfilling. Eur. Polym. J. 2022, 162, 110877. [Google Scholar] [CrossRef]
- Krishna, U.G.; Srinivasa, C.; Amara, N.; Gudoor, S. Processing, characterization and property evaluation of seashell and glass fibre added epoxy based polymer matrix composite. Mater. Today Proc. 2021, 35, 417–422. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C., Jr.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Mammone, R.; Kaner, R.; Porter, L. The concept of ‘doping’ of conducting polymers: The role of reduction potentials. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1985, 314, 3–15. [Google Scholar]
- Wang, Q.X.; Zhang, C.Y. Oriented Synthesis of One-Dimensional Polypyrrole Molecule Chains in a Metal-Organic Framework. Macromol. Rapid Commun. 2011, 32, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S. Conducting Polymers—Materials of Commerce; Wiley Online Library: Hoboken, NJ, USA, 1993. [Google Scholar]
- Otero, T.F.n.; Cortes, M.T. Artificial muscles with tactile sensitivity. Adv. Mater. 2003, 15, 279–282. [Google Scholar] [CrossRef]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Polypyrrole/TiO2 composites for the application of photocatalysis. Sens. Actuators B Chem. 2017, 241, 1161–1169. [Google Scholar] [CrossRef]
- Göppert, A.; Cölfen, H. Infiltration of biomineral templates for nanostructured polypyrrole. RSC Adv. 2018, 8, 33748–33752. [Google Scholar] [CrossRef] [Green Version]
- Oaki, Y.; Kijima, M.; Imai, H. Synthesis and morphogenesis of organic polymer materials with hierarchical structures in biominerals. J. Am. Chem. Soc. 2011, 133, 8594–8599. [Google Scholar] [CrossRef]
- Hellström, S. Protection methods—Antistatic materials. In ESD—The Scourge of Electronics; Springer: Berlin/Heidelberg, Germany, 1998; pp. 136–170. [Google Scholar]
- Mancuso, A.; Stagioni, M.; Prada, F.; Scarponi, D.; Piccinetti, C.; Goffredo, S. Environmental influence on calcification of the bivalve Chamelea gallina along a latitudinal gradient in the Adriatic Sea. Sci. Rep. 2019, 9, 11198. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chem. A Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Li, J.; Hu, M.; Yu, R. Pressed-pellet solid potentiometric sensor for ascorbic acid based on derivatives of cobalt (II) phthalocyanine doped with iodine. Sens. Actuators B Chem. 1996, 30, 65–69. [Google Scholar]
- Lee, J.; Kim, D.; Kim, C. Synthesis of soluble polypyrrole of the doped state in organic solvents. Synth. Met. 1995, 74, 103–106. [Google Scholar] [CrossRef]
- Nayak, J.; Mahadeva, S.K.; Kim, J. Characteristics of flexible electrode made on cellulose by soluble polypyrrole coating. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2012, 226, 2605–2609. [Google Scholar] [CrossRef]
- Tian, B.; Zerbi, G. Lattice dynamics and vibrational spectra of pristine and doped polypyrrole: Effective conjugation coordinate. J. Chem. Phys. 1990, 92, 3892–3898. [Google Scholar] [CrossRef]
- Tian, B.; Zerbi, G. Lattice dynamics and vibrational spectra of polypyrrole. J. Chem. Phys. 1990, 92, 3886–3891. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Mahapatra, S. Aragonite crystals with unconventional morphologies. J. Mater. Chem. 1999, 9, 2953–2957. [Google Scholar] [CrossRef]
- Gvozdenović, M.M.; Jugović, B.; Stevanović, J.; Grgur, B.N. Electrochemical synthesis of electroconducting polymers. Hem. Ind. 2014, 68, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Kopecký, D.; Vrňata, M.; Prokeš, J.; Varga, M.; Watzlová, E. Polypyrrole salts and bases: Superior conductivity of nanotubes and their stability towards the loss of conductivity by deprotonation. RSC Adv. 2016, 6, 88382–88391. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, R.; Oaki, Y.; Kuwabara, K.; Hayashi, K.; Imai, H. Solvent-free synthesis, coating and morphogenesis of conductive polymer materials through spontaneous generation of activated monomers. Chem. Commun. 2014, 50, 11840–11843. [Google Scholar] [CrossRef] [Green Version]
| Material | Nitrogen Atmosphere | Solvent | CuCl2 Infiltrations | Porosity [cm3/g] | Size [µm] | Py or CuCl2 First |
|---|---|---|---|---|---|---|
| PPy/CaCO3 M1 | No | Isopropanol | 1× | 0.014 | 0.101 | CuCl2 |
| PPy/CaCO3 M2 | No | Isopropanol | 1× | 0.014 | 0.101 | Py |
| PPy/CaCO3 M3 | No | Methanol | 1× | 0.014 | 0.101 | Py |
| PPy/CaCO3 M4 | Yes | Isopropanol | 1× | 0.014 | 0.101 | CuCl2 |
| PPy/CaCO3 M5 | Yes | Isopropanol | 2× | 0.014 | 0.101 | CuCl2 |
| PPy/CaCO3 M6 | Yes | Methanol | 2× | 0.014 | 0.101 | CuCl2 |
| PPy/CaCO3 M7 | Yes | Methanol | 2× | 0.016 | 3.77 and 0.105 | CuCl2 |
| PPy/CaCO3 M8 | Yes | Methanol | 2× | 0.016 | 5.26 and 0.531 | CuCl2 |
| Material | TG [°C] | TM [°C] | Tensile Strength [MPa] |
|---|---|---|---|
| Reference | −12 to −18 | 87.2 | 1.97 |
| Reference +10% PPy/CaCO3 M3 | −12 to −18 | 87.6 | 0.701 |
| Reference + 30% PPy/CaCO3 M3 | −12 to −18 | 88.1 | 2.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gärtner, S.; Graf, A.; Triunfo, C.; Laurenzi, D.; Schupp, S.M.; Maoloni, G.; Falini, G.; Cölfen, H. Turning Seashell Waste into Electrically Conductive Particles. Int. J. Mol. Sci. 2022, 23, 7256. https://doi.org/10.3390/ijms23137256
Gärtner S, Graf A, Triunfo C, Laurenzi D, Schupp SM, Maoloni G, Falini G, Cölfen H. Turning Seashell Waste into Electrically Conductive Particles. International Journal of Molecular Sciences. 2022; 23(13):7256. https://doi.org/10.3390/ijms23137256
Chicago/Turabian StyleGärtner, Stefanie, Angelina Graf, Carla Triunfo, Davide Laurenzi, Stefan M. Schupp, Gabriele Maoloni, Giuseppe Falini, and Helmut Cölfen. 2022. "Turning Seashell Waste into Electrically Conductive Particles" International Journal of Molecular Sciences 23, no. 13: 7256. https://doi.org/10.3390/ijms23137256
APA StyleGärtner, S., Graf, A., Triunfo, C., Laurenzi, D., Schupp, S. M., Maoloni, G., Falini, G., & Cölfen, H. (2022). Turning Seashell Waste into Electrically Conductive Particles. International Journal of Molecular Sciences, 23(13), 7256. https://doi.org/10.3390/ijms23137256







