Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia coli and Staphylococcus aureus
Abstract
:1. Introduction
2. Results
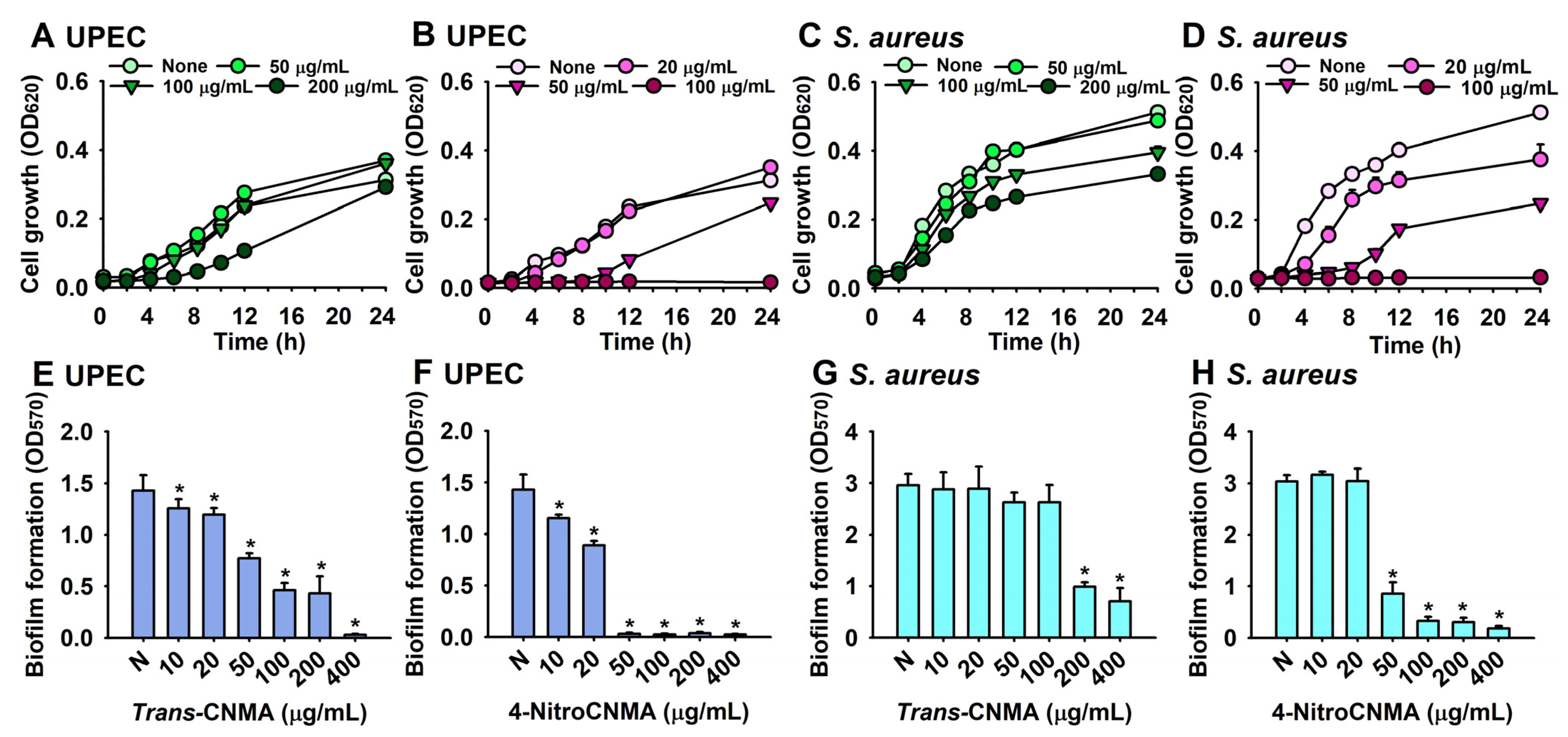
2.1. Antibiofilm and Antimicrobial Activities of Trans-CNMA and Its Derivatives against UPEC and S. aureus
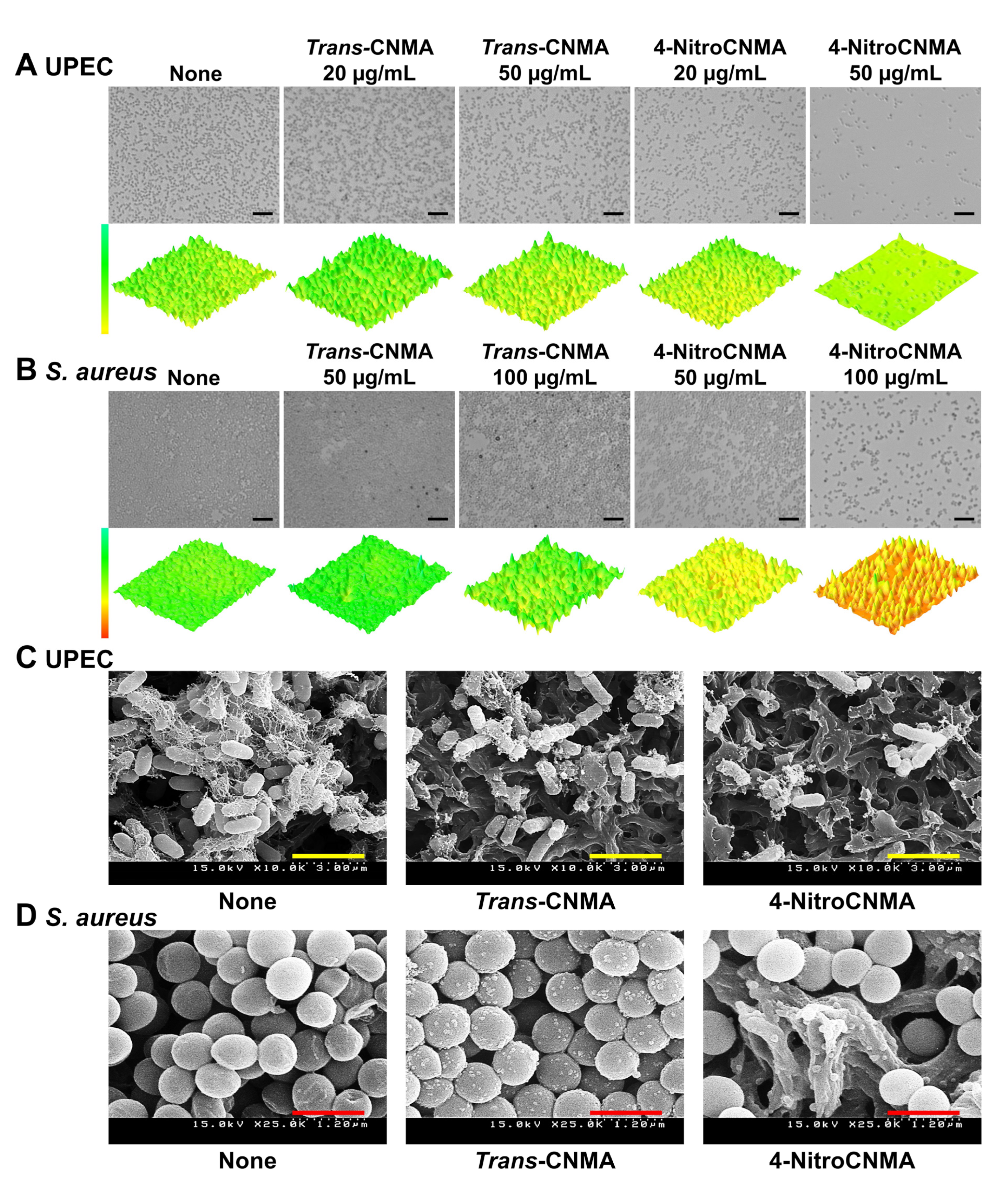
2.2. Microscopic Observations of Biofilm Formation by UPEC and S. aureus
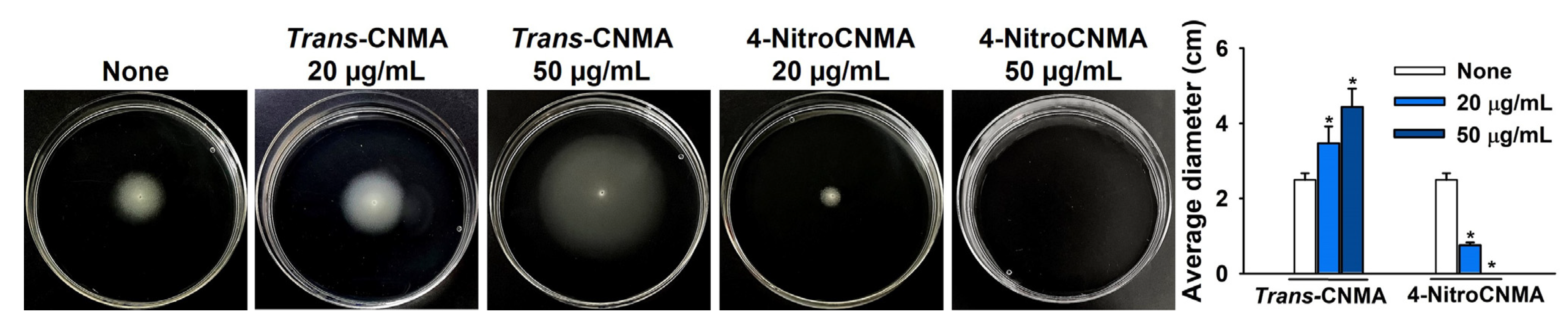
2.3. Trans-CNMA and 4-nitroCNMA Affected the Swimming Motility of UPEC
2.4. Effects of Trans-CNMA and 4-nitroCNMA on Cell Hydrophobicity and Hemolysis
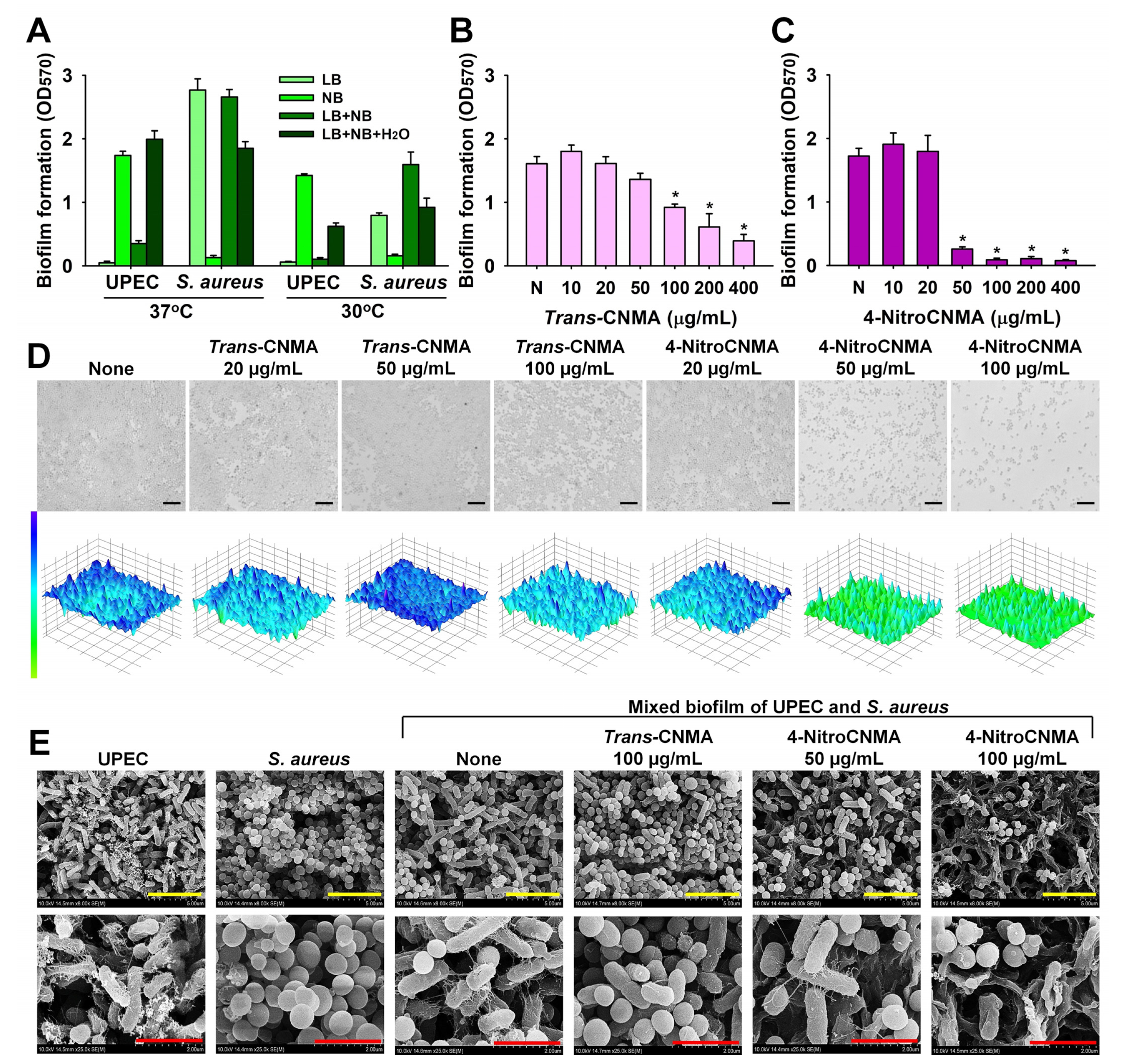
2.5. Antibiofilm Activities of CNMAs against Mixed UPEC and S. aureus Biofilms
3. Discussion
4. Materials and Methods
4.1. Reagents and Culture Strains
4.2. Crystal Violet Biofilm Assay
4.3. Microscopic Observations of Biofilms
4.4. Swimming Motilities of UPEC
4.5. Cell Surface Hydrophobicities
4.6. Staphyloxanthin Assay
4.7. Sheep Red Blood Cell Hemolysis Assay
4.8. The Mixed UPEC/S. aureus Biofilm Model
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. Inhibitors of antibiotic resistance mechanisms: Clinical applications and future perspectives. Future Med. Chem. 2020, 12, 357–359. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Dongmei, D.; Cascioferro, S.M.; Diana, P.; Giovannetti, E. Biofilm formation as valuable target to fight severe chronic infections. Curr. Med. Chem. 2022, 29, 4307–4310. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, W.; Hu, L.; Qi, Y.; Ning, H.; Chen, J.; Mo, H. Bactericidal activity of alpha-bromocinnamaldehyde against persisters in Escherichia coli. PLoS ONE 2017, 12, e0182122. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-G.; Lee, J.-H.; Kim, S.-I.; Baek, K.-H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef]
- Kot, B.; Wicha, J.; Piechota, M.; Wolska, K.; Grużewska, A. Antibiofilm activity of trans-cinnamaldehyde, p-coumaric, and ferulic acids on uropathogenic Escherichia coli. Turk. J. Med. Sci. 2015, 45, 919–924. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef] [Green Version]
- Kot, B.; Wierzchowska, K.; Grużewska, A.; Lohinau, D. The effects of selected phytochemicals on biofilm formed by five methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 1299–1302. [Google Scholar] [CrossRef]
- Beema Shafreen, R.M.; Selvaraj, C.; Singh, S.K.; Karutha Pandian, S. In silico and in vitro studies of cinnamaldehyde and their derivatives against LuxS in Streptococcus pyogenes: Effects on biofilm and virulence genes. J. Mol. Recognit. 2014, 27, 106–116. [Google Scholar] [CrossRef]
- Faleye, O.S.; Sathiyamoorthi, E.; Lee, J.-H.; Lee, J. Inhibitory effects of cinnamaldehyde derivatives on biofilm formation and virulence factors in Vibrio species. Pharmaceutics 2021, 13, 2176. [Google Scholar] [CrossRef]
- Khadke, S.K.; Lee, J.-H.; Kim, Y.-G.; Raj, V.; Lee, J. Appraisal of cinnamaldehyde analogs as dual-acting antibiofilm and anthelmintic agents. Front. Microbiol. 2022, 13, 818165. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. Uropathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef]
- Wood, T.K. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ. Microbiol. 2009, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Giaouris, E.; Chapot-Chartier, M.P.; Briandet, R. Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties. Int. J. Food Microbiol. 2009, 131, 2–9. [Google Scholar] [CrossRef]
- Bhakdi, S.; Muhly, M.; Füssle, R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal α-toxin. Infect. Immun. 1984, 46, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef]
- Caiazza, N.C.; O’Toole, G.A. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 2003, 185, 3214–3217. [Google Scholar] [CrossRef] [Green Version]
- Ferro, T.A.F.; Araújo, J.M.M.; Dos Santos Pinto, B.L.; Dos Santos, J.S.; Souza, E.B.; da Silva, B.L.R.; Colares, V.L.P.; Novais, T.M.G.; Filho, C.M.B.; Struve, C.; et al. Cinnamaldehyde inhibits Staphylococcus aureus virulence factors and protects against infection in a Galleria mellonella model. Front. Microbiol. 2016, 7, 2052. [Google Scholar] [CrossRef] [Green Version]
- Carlson, E.; Johnson, G. Protection by Candida albicans of Staphylococcus aureus in the establishment of dual infection in mice. Infect. Immun. 1985, 50, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Harriott, M.M.; Noverr, M.C. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011, 19, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Stoodley, P.; Sidhu, S.; Nistico, L.; Mather, M.; Boucek, A.; Hall-Stoodley, L.; Kathju, S. Kinetics and morphology of polymicrobial biofilm formation on polypropylene mesh. FEMS Immunol. Med. Microbiol. 2012, 65, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Alves, P.M.; Al-Badi, E.; Withycombe, C.; Jones, P.M.; Purdy, K.J.; Maddocks, S.E. Interaction between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathog. Dis. 2018, 76, fty003. [Google Scholar] [CrossRef]
- Boya, B.R.; Lee, J.-H.; Lee, J. Antibiofilm and antimicrobial activities of chloroindoles against uropathogenic Escherichia coli. Front. Microbiol. 2022, 13, 872943. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, antimicrobial mechanisms, and antibiotic activities of cinnamaldehyde against pathogenic bacteria in animal feeds and human foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Van Calenbergh, S.; Nelis, H.; Coenye, T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.; Narayanan, A.; Venkitanarayanan, K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011, 185, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.A.; Pereira, C.D.S.; Assunção, R.G.; da Silva, I.S.C.; Rego, F.S.; Alves, L.S.R.; Santos, J.S.; Nogueira, F.J.R.; Zagmignan, A.; Thomsen, T.T.; et al. New insights into the antimicrobial action of cinnamaldehyde towards Escherichia coli and its effects on intestinal colonization of mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef]
- Jia, P.; Xue, Y.J.; Duan, X.J.; Shao, S.H. Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 409–416. [Google Scholar] [CrossRef]
- Kot, B.; Sytykiewicz, H.; Sprawka, I.; Witeska, M. Effect of trans-cinnamaldehyde on methicillin-resistant Staphylococcus aureus biofilm formation: Metabolic activity assessment and analysis of the biofilm-associated genes expression. Int. J. Mol. Sci. 2019, 21, 102. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Kang, O.-H.; Kwon, D.-Y. Trans-cinnamaldehyde exhibits synergy with conventional antibiotic against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 2752. [Google Scholar] [CrossRef]
- Doyle, A.A.; Krämer, T.; Kavanagh, K.; Stephens, J.C. Cinnamaldehydes: Synthesis, antibacterial evaluation, and the effect of molecular structure on antibacterial activity. Results Chem. 2019, 1, 100013. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Marin, L.; Moraru, S.; Popescu, M.C.; Nicolescu, A.; Zgardan, C.; Simionescu, B.C.; Barboiu, M. Out-of-water constitutional self-organization of chitosan-cinnamaldehyde dynagels. Chem. Eur. J. 2014, 20, 4814–4821. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomedicine 2017, 12, 2813–2828. [Google Scholar] [CrossRef] [Green Version]
- Faikoh, E.N.; Hong, Y.-H.; Hu, S.-Y. Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish Shellfish Immunol. 2014, 38, 15–24. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Khadke, S.K.; Lee, J. Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol. Microb. Biotechnol. 2021, 14, 1353–1366. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Daum, R.S. Removing the golden coat of Staphylococcus aureus. N. Engl. J. Med. 2008, 359, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Rajmuthiah, R.; Kim, W.; Fuchs, B.B.; Jeyamani, E.; Kelso, M.J.; Mylonakis, E. Antibacterial properties of four novel hit compounds from a methicillin-resistant Staphylococcus aureus-Caenorhabditis elegans high-throughput screen. Microb. Drug Resist. 2018, 24, 666–674. [Google Scholar] [CrossRef] [Green Version]


| Test Compound | Structure | MIC (µg/mL) against UPEC | MIC (µg/mL) against S. aureus |
|---|---|---|---|
| Trans-CNMA |  | 400 | >400 |
| 4-BromoCNMA |  | 400 | >400 |
| 4-ChloroCNMA |  | 200 | 400 |
| CNMA oxime |  | >400 | >400 |
| 4-DimethylaminoCNMA | 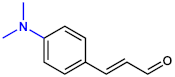 | >400 | >400 |
| 4-FluoroCNMA | 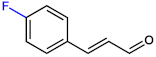 | 200 | >400 |
| 2-MethoxyCNMA | 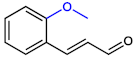 | >400 | >400 |
| 4-MethoxyCNMA | 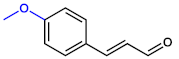 | >400 | >400 |
| α-MethylCNMA |  | >400 | >400 |
| 2-NitroCNMA | 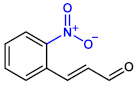 | >400 | >400 |
| 4-NitroCNMA | 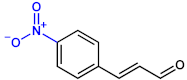 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, S.; Cho, K.-H.; Lee, J.-H.; Lee, J. Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 7225. https://doi.org/10.3390/ijms23137225
Kim Y, Kim S, Cho K-H, Lee J-H, Lee J. Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia coli and Staphylococcus aureus. International Journal of Molecular Sciences. 2022; 23(13):7225. https://doi.org/10.3390/ijms23137225
Chicago/Turabian StyleKim, Yeseul, Sanghun Kim, Kiu-Hyung Cho, Jin-Hyung Lee, and Jintae Lee. 2022. "Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia coli and Staphylococcus aureus" International Journal of Molecular Sciences 23, no. 13: 7225. https://doi.org/10.3390/ijms23137225
APA StyleKim, Y., Kim, S., Cho, K.-H., Lee, J.-H., & Lee, J. (2022). Antibiofilm Activities of Cinnamaldehyde Analogs against Uropathogenic Escherichia coli and Staphylococcus aureus. International Journal of Molecular Sciences, 23(13), 7225. https://doi.org/10.3390/ijms23137225







