Novel Insights into Factor D Inhibition
Abstract
:1. Introduction
1.1. PNH as the Disease Model of Complementopathies
1.2. Other Complementopathies
1.3. The Complement System
2. Factor D Inhibition
2.1. Factor D
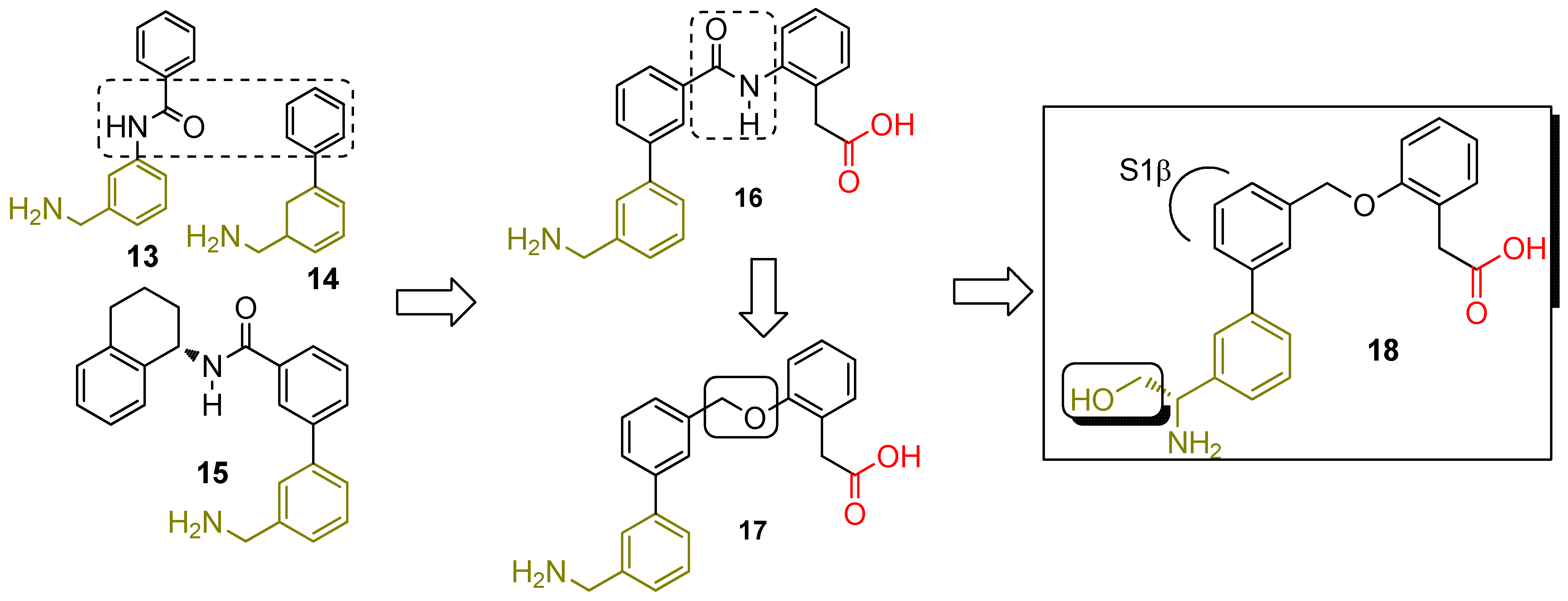
2.2. The Discovery of Small Molecule FD Inhibitors
- -
- Irreversible FD inhibitors
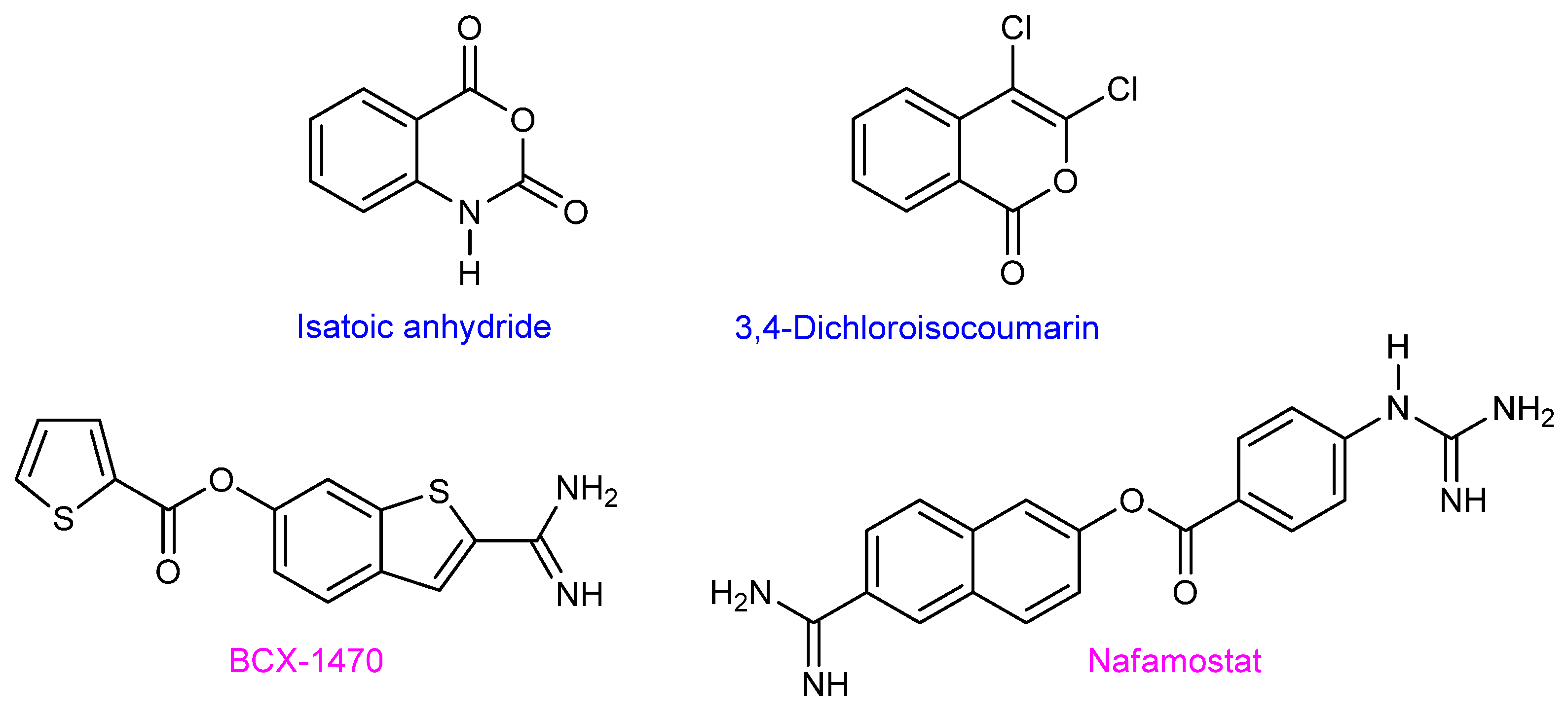
2.2.1. First-Generation of Latent FD Inhibitors

- -
- “Me too” latent FD inhibitors
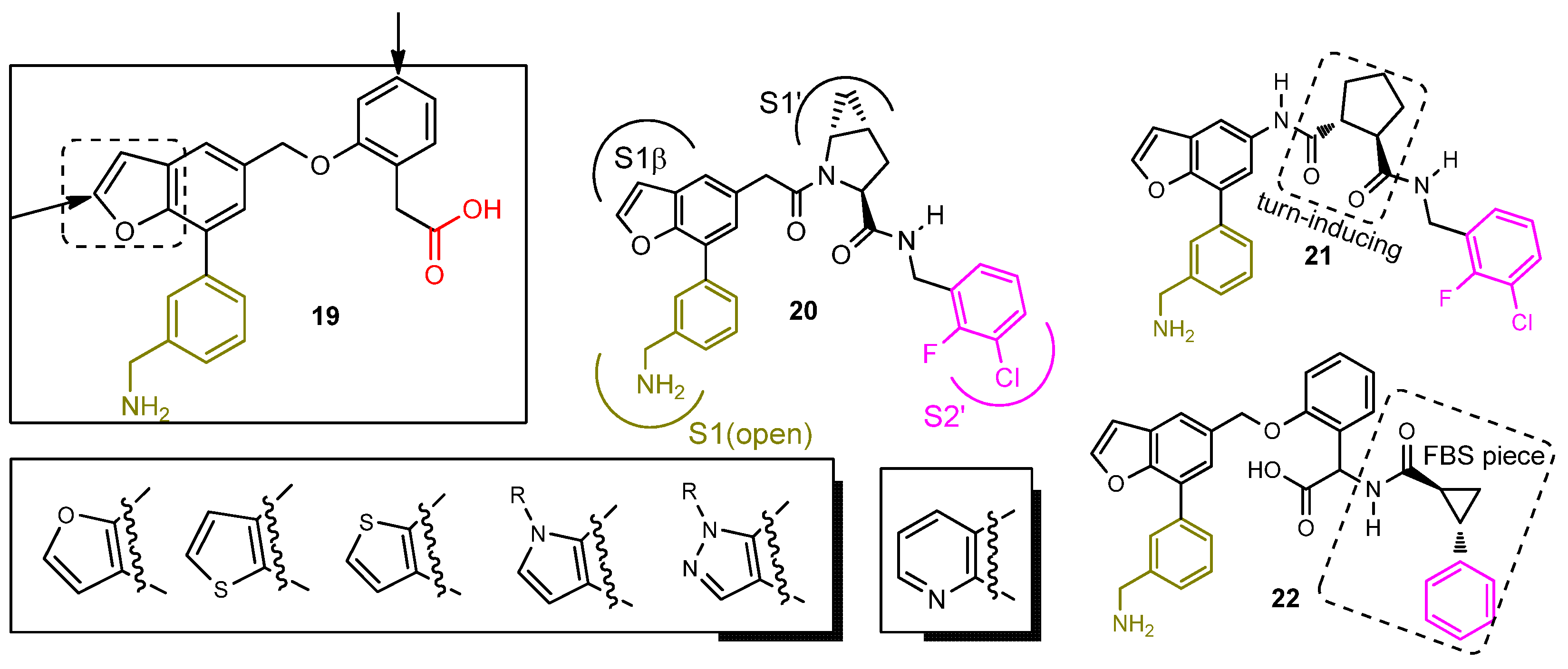
2.2.2. First-Generation of Inhibitors Targeting the “Unlocked” FD Conformation
- -
- “Me too” inhibitors targeting the “unlocked” FD conformation
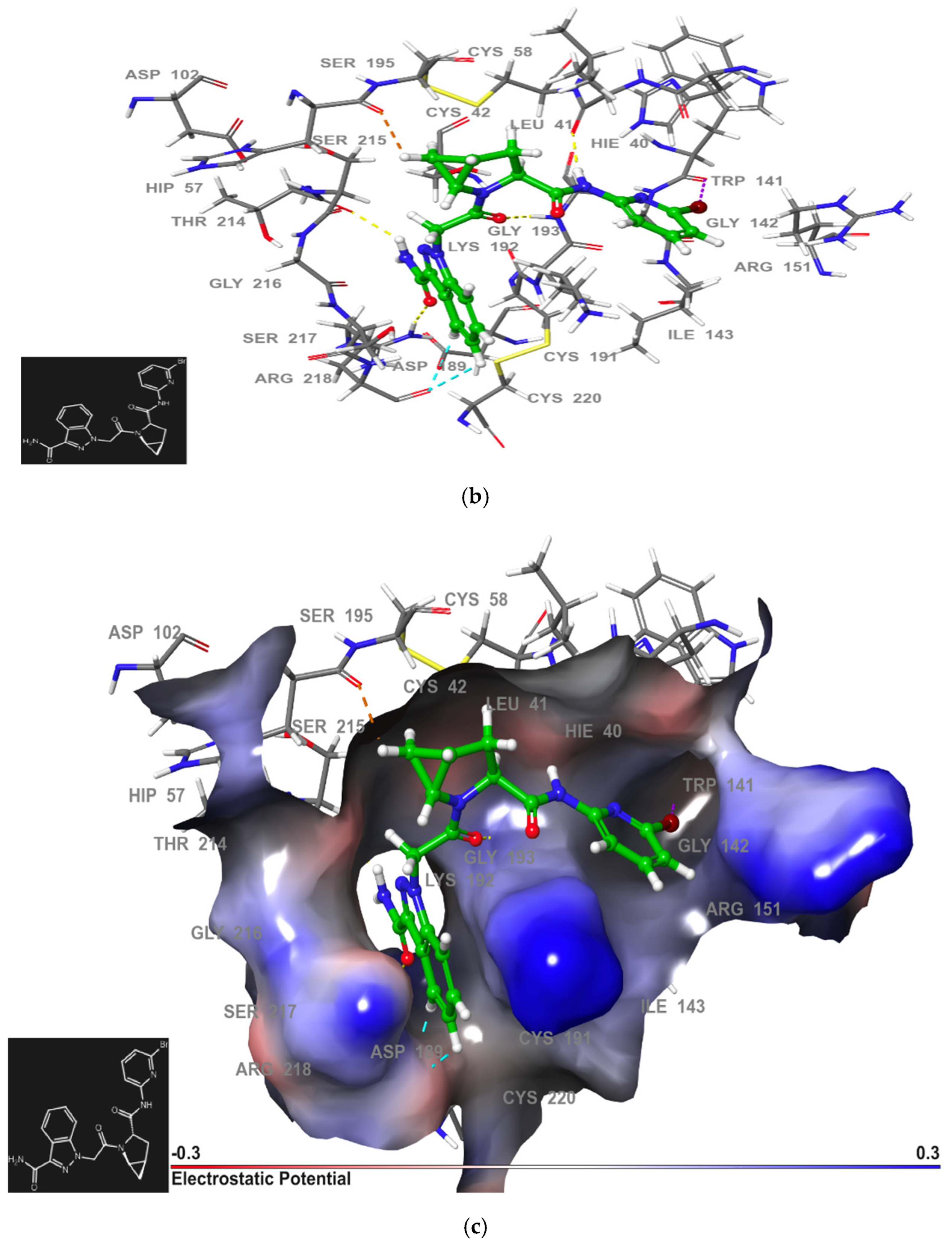
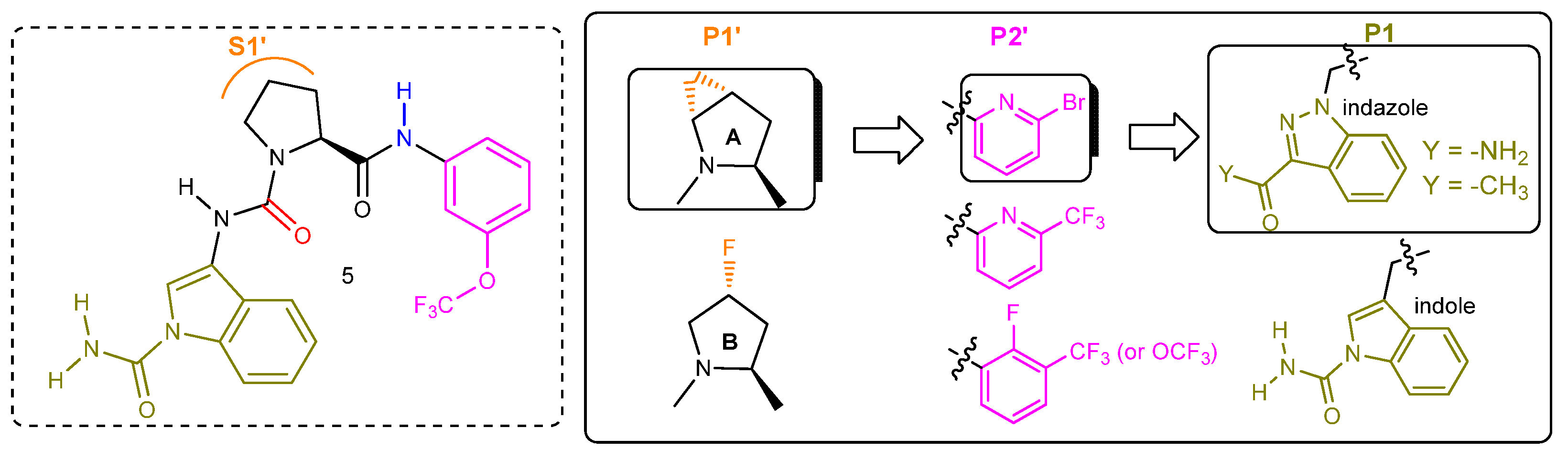
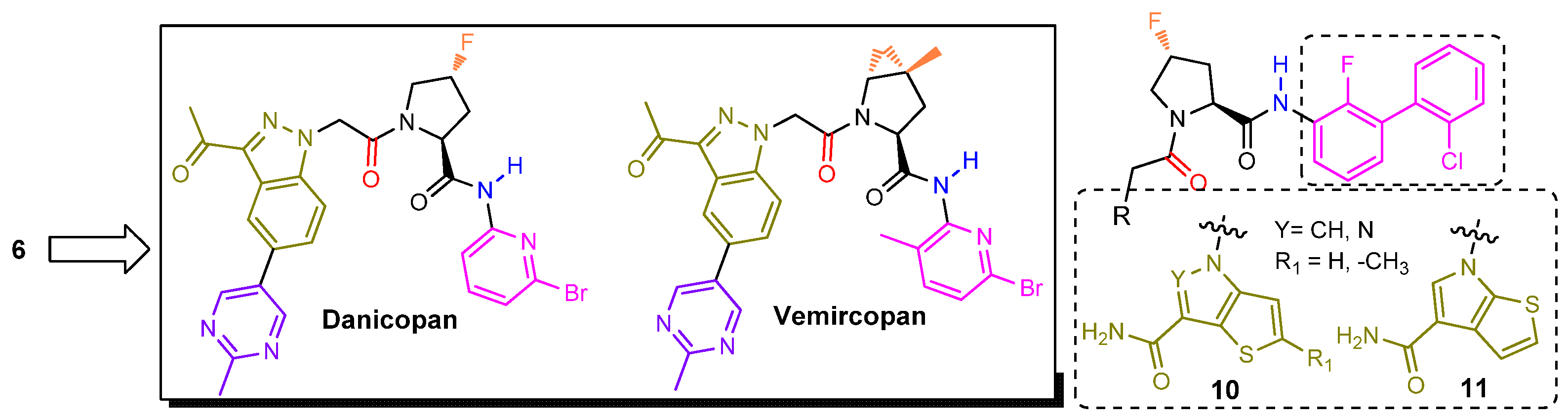
2.2.3. Second-Generation of Latent FD Inhibitors
3. Clinical Development of Complement Inhibitors
3.1. C5 Inhibitors in PNH
3.2. Development and Application of C3 Inhibitors and Similar Molecules
- -
- Factor D inhibitors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gavriilaki, E.; Anagnostopoulos, A.; Mastellos, D.C. Complement in Thrombotic Microangiopathies: Unraveling Ariadne’s Thread into the Labyrinth of Complement Therapeutics. Front. Immunol. 2019, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Takeda, J.; Miyata, T.; Kawagoe, K.; Iida, Y.; Endo, Y.; Fujita, T.; Takahashi, M.; Kitani, T.; Kinoshita, T. Deficiency of the GPI Anchor Caused by a Somatic Mutation of the PIG-A Gene in Paroxysmal Nocturnal Hemoglobinuria. Cell 1993, 73, 703–711. [Google Scholar] [CrossRef]
- Brodsky, R.A. How I Treat Paroxysmal Nocturnal Hemoglobinuria. Blood 2021, 137, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Effect of Eculizumab on Hemolysis and Transfusion Requirements in Patients with Paroxysmal Nocturnal Hemoglobinuria|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa031688 (accessed on 14 April 2022).
- Lee, J.W.; De Fontbrune, F.S.; Lee, L.W.L.; Pessoa, V.; Gualandro, S.; Füreder, W.; Ptushkin, V.; Rottinghaus, S.T.; Volles, L.; Shafner, L.; et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: The 301 study. Blood 2019, 133, 530–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavriilaki, E.; de Latour, R.P.; Risitano, A.M. Advancing Therapeutic Complement Inhibition in Hematologic Diseases: PNH and Beyond. Blood 2022, 139, 3571–3582. [Google Scholar] [CrossRef]
- Risitano, A.M.; Notaro, R.; Marando, L.; Serio, B.; Ranaldi, D.; Seneca, E.; Ricci, P.; Alfinito, F.; Camera, A.; Gianfaldoni, G.; et al. Complement Fraction 3 Binding on Erythrocytes as Additional Mechanism of Disease in Paroxysmal Nocturnal Hemoglobinuria Patients Treated by Eculizumab. Blood 2009, 113, 4094–4100. [Google Scholar] [CrossRef]
- Lin, Z.; Schmidt, C.Q.; Koutsogiannaki, S.; Ricci, P.; Risitano, A.M.; Lambris, J.D.; Ricklin, D. Complement C3dg-Mediated Erythrophagocytosis: Implications for Paroxysmal Nocturnal Hemoglobinuria. Blood 2015, 126, 891–894. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.; Rother, R.P.; Arnold, L.; Kelly, R.; Cullen, M.J.; Richards, S.J.; Hillmen, P. Eculizumab Prevents Intravascular Hemolysis in Patients with Paroxysmal Nocturnal Hemoglobinuria and Unmasks Low-Level Extravascular Hemolysis Occurring through C3 Opsonization. Haematologica 2010, 95, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Mastellos, D.C.; Reis, E.S.; Yancopoulou, D.; Risitano, A.M.; Lambris, J.D. Expanding Complement Therapeutics for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Semin. Hematol. 2018, 55, 167–175. [Google Scholar] [CrossRef]
- Pegcetacoplan versus Eculizumab in Paroxysmal Nocturnal Hemoglobinuria|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2029073 (accessed on 14 April 2022).
- Smith, R.J.H.; Appel, G.B.; Blom, A.M.; Cook, H.T.; D’Agati, V.D.; Fakhouri, F.; Fremeaux-Bacchi, V.; Józsi, M.; Kavanagh, D.; Lambris, J.D.; et al. C3 Glomerulopathy—Understanding a Rare Complement-Driven Renal Disease. Nat. Rev. Nephrol. 2019, 15, 129–143. [Google Scholar] [CrossRef]
- Riedl, M.; Thorner, P.; Licht, C. C3 Glomerulopathy. Pediatr. Nephrol. 2017, 32, 43–57. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Bomback, A.S. C3 Glomerulopathy: Pathogenesis and Treatment. Adv. Chronic Kidney Dis. 2020, 27, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Connor, K.M.; Lambris, J.D. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front. Immunol. 2019, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Berendschot, T.T.J.M. Age-Related Macular Degeneration: A Complementopathy? Ophthalmic Res. 2015, 54, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Halawa, O.A.; Lin, J.B.; Miller, J.W.; Vavvas, D.G. A Review of Completed and Ongoing Complement Inhibitor Trials for Geographic Atrophy Secondary to Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 2580. [Google Scholar] [CrossRef]
- Thurman, J.M.; Holers, V.M. The Central Role of the Alternative Complement Pathway in Human Disease. J. Immunol. 2006, 176, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Harboe, M.; Ulvund, G.; Vien, L.; Fung, M.; Mollnes, T.E. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 2004, 138, 439–446. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, M.; Ippolito, G.C.; Schroeder, H.W.; Carroll, M.C.; Volanakis, J.E. Complement Activation in Factor D-Deficient Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 14577–14582. [Google Scholar] [CrossRef] [Green Version]
- Abrogation of the Alternative Complement Pathway by Targeted Deletion of Murine Factor B-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/9238044/ (accessed on 14 April 2022).
- Schubart, A.; Anderson, K.; Mainolfi, N.; Sellner, H.; Ehara, T.; Adams, C.M.; Mac Sweeney, A.; Liao, S.-M.; Crowley, M.; Littlewood-Evans, A.; et al. Small-Molecule Factor B Inhibitor for the Treatment of Complement-Mediated Diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 7926–7931. [Google Scholar] [CrossRef] [Green Version]
- Maibaum, J.; Liao, S.-M.; Vulpetti, A.; Ostermann, N.; Randl, S.; Rüdisser, S.; Lorthiois, E.; Erbel, P.; Kinzel, B.; Kolb, F.A.; et al. Small-Molecule Factor D Inhibitors Targeting the Alternative Complement Pathway. Nat. Chem. Biol. 2016, 12, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Avacopan for the Treatment of ANCA-Associated Vasculitis|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa2023386 (accessed on 14 April 2022).
- Volanakis, J.E.; Barnum, S.R.; Giddens, M.; Galla, J.H. Renal Filtration and Catabolism of Complement Protein D. N. Engl. J. Med. 1985, 312, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Stevens, J.W.; Macon, K.J.; Volanakis, J.E. Recombinant and Native Zymogen Forms of Human Complement Factor D. J. Immunol. 1994, 152, 3645–3653. [Google Scholar] [PubMed]
- Volanakis, J.E.; Narayana, S.V. Complement Factor D, a Novel Serine Protease. Protein Sci. 1996, 5, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Jing, H.; Babu, Y.S.; Moore, D.; Kilpatrick, J.M.; Liu, X.Y.; Volanakis, J.E.; Narayana, S.V. Structures of Native and Complexed Complement Factor D: Implications of the Atypical His57 Conformation and Self-Inhibitory Loop in the Regulation of Specific Serine Protease Activity. J. Mol. Biol. 1998, 282, 1061–1081. [Google Scholar] [CrossRef]
- Forneris, F.; Ricklin, D.; Wu, J.; Tzekou, A.; Wallace, R.S.; Lambris, J.D.; Gros, P. Structures of C3b in Complex with Factors B and D Give Insight into Complement Convertase Formation. Science 2010, 330, 1816–1820. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.B.; Kilpatrick, J.M.; Chu, N.; Babu, Y.S. Structure of 3,4-Dichloroisocoumarin-Inhibited Factor D. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998, 54, 711–717. [Google Scholar] [CrossRef]
- Ponnuraj, K.; Xu, Y.; Macon, K.; Moore, D.; Volanakis, J.E.; Narayana, S.V.L. Structural Analysis of Engineered Bb Fragment of Complement Factor B: Insights into the Activation Mechanism of the Alternative Pathway C3-Convertase. Mol. Cell 2004, 14, 17–28. [Google Scholar] [CrossRef]
- Moorman, A.R.; Abeles, R.H. New Class of Serine Protease Inactivators Based on Isatoic Anhydride. J. Am. Chem. Soc. 1982, 104, 6785–6786. [Google Scholar] [CrossRef]
- Harper, J.W.; Hemmi, K.; Powers, J.C. Reaction of Serine Proteases with Substituted Isocoumarins: Discovery of 3,4-Dichloroisocoumarin, a New General Mechanism Based Serine Protease Inhibitor. Biochemistry 1985, 24, 1831–1841. [Google Scholar] [CrossRef]
- Szalai, A.J.; Digerness, S.B.; Agrawal, A.; Kearney, J.F.; Bucy, R.P.; Niwas, S.; Kilpatrick, J.M.; Babu, Y.S.; Volanakis, J.E. The Arthus Reaction in Rodents: Species-Specific Requirement of Complement. J. Immunol. 2000, 164, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Ikari, N.; Sakai, Y.; Hitomi, Y.; Fujii, S. New Synthetic Inhibitor to the Alternative Complement Pathway. Immunology 1983, 49, 685–691. [Google Scholar] [PubMed]
- Sanderson, P.E. Small, Noncovalent Serine Protease Inhibitors. Med. Res. Rev. 1999, 19, 179–197. [Google Scholar] [CrossRef]
- Vulpetti, A.; Randl, S.; Rüdisser, S.; Ostermann, N.; Erbel, P.; Mac Sweeney, A.; Zoller, T.; Salem, B.; Gerhartz, B.; Cumin, F.; et al. Structure-Based Library Design and Fragment Screening for the Identification of Reversible Complement Factor D Protease Inhibitors. J. Med. Chem. 2017, 60, 1946–1958. [Google Scholar] [CrossRef] [PubMed]
- Kam, C.M.; McRae, B.J.; Harper, J.W.; Niemann, M.A.; Volanakis, J.E.; Powers, J.C. Human Complement Proteins D, C2, and B. Active Site Mapping with Peptide Thioester Substrates. J. Biol. Chem. 1987, 262, 3444–3451. [Google Scholar] [CrossRef]
- Lorthiois, E.; Anderson, K.; Vulpetti, A.; Rogel, O.; Cumin, F.; Ostermann, N.; Steinbacher, S.; Mac Sweeney, A.; Delgado, O.; Liao, S.-M.; et al. Discovery of Highly Potent and Selective Small-Molecule Reversible Factor D Inhibitors Demonstrating Alternative Complement Pathway Inhibition In Vivo. J. Med. Chem. 2017, 60, 5717–5735. [Google Scholar] [CrossRef]
- Ganguly, H.K.; Basu, G. Conformational Landscape of Substituted Prolines. Biophys. Rev. 2020, 12, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Phillips, J.G.; Stuckey, J.A.; Bai, L.; Sun, H.; Delproposto, J.; Brown, W.C.; Chinnaswamy, K. Buried Hydrogen Bond Interactions Contribute to the High Potency of Complement Factor D Inhibitors. ACS Med. Chem. Lett. 2016, 7, 1092–1096. Available online: https://pubs.acs.org/doi/10.1021/acsmedchemlett.6b00299 (accessed on 14 April 2022). [CrossRef] [PubMed] [Green Version]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef] [PubMed]
- Glide|Schrödinger. Available online: https://www.schrodinger.com/products/glide (accessed on 14 April 2022).
- 4-Fluoroprolines: Conformational Analysis and Effects on the Stability and Folding of Peptides and Proteins|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/7081_2015_196 (accessed on 14 April 2022).
- Yuan, X.; Gavriilaki, E.; Thanassi, J.A.; Yang, G.; Baines, A.C.; Podos, S.D.; Huang, Y.; Huang, M.; Brodsky, R.A. Small-Molecule Factor D Inhibitors Selectively Block the Alternative Pathway of Complement in Paroxysmal Nocturnal Hemoglobinuria and Atypical Hemolytic Uremic Syndrome. Haematologica 2017, 102, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Vulpetti, A.; Ostermann, N.; Randl, S.; Yoon, T.; Mac Sweeney, A.; Cumin, F.; Lorthiois, E.; Rüdisser, S.; Erbel, P.; Maibaum, J. Discovery and Design of First Benzylamine-Based Ligands Binding to an Unlocked Conformation of the Complement Factor D. ACS Med. Chem. Lett. 2018, 9, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.G.; Powers, J.; Mainolfi, N.; Anderson, K.; Belanger, D.B.; Liu, D.; Ji, N.; Jendza, K.; Gelin, C.F.; Mac Sweeney, A.; et al. Design, Synthesis, and Preclinical Characterization of Selective Factor D Inhibitors Targeting the Alternative Complement Pathway. J. Med. Chem. 2019, 62, 4656–4668. [Google Scholar] [CrossRef] [PubMed]
- Ravulizumab (ALXN1210) vs Eculizumab in C5-Inhibitor–Experienced Adult Patients with PNH: The 302 Study|Blood|American Society of Hematology. Available online: https://ashpublications.org/blood/article/133/6/540/260562/Ravulizumab-ALXN1210-vs-eculizumab-in-C5-inhibitor (accessed on 14 April 2022).
- Röth, A.; Nishimura, J.; Nagy, Z.; Gaàl-Weisinger, J.; Panse, J.; Yoon, S.-S.; Egyed, M.; Ichikawa, S.; Ito, Y.; Kim, J.S.; et al. The Complement C5 Inhibitor Crovalimab in Paroxysmal Nocturnal Hemoglobinuria. Blood 2020, 135, 912–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risitano, A.M.; Marotta, S.; Ricci, P.; Marano, L.; Frieri, C.; Cacace, F.; Sica, M.; Kulasekararaj, A.; Calado, R.T.; Scheinberg, P.; et al. Anti-Complement Treatment for Paroxysmal Nocturnal Hemoglobinuria: Time for Proximal Complement Inhibition? A Position Paper from the SAAWP of the EBMT. Front. Immunol. 2019, 10, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debureaux, P.-E.; Kulasekararaj, A.G.; Cacace, F.; Silva, B.G.P.; Calado, R.T.; Barone, F.; de Fontbrune, F.S.; Prata, P.H.; Soret, J.; Sica, M.; et al. Categorizing Hematological Response to Eculizumab in Paroxysmal Nocturnal Hemoglobinuria: A Multicenter Real-Life Study. Bone Marrow Transplant. 2021, 56, 2600–2602. [Google Scholar] [CrossRef]
- Risitano, A.M.; Ricklin, D.; Huang, Y.; Reis, E.S.; Chen, H.; Ricci, P.; Lin, Z.; Pascariello, C.; Raia, M.; Sica, M.; et al. Peptide Inhibitors of C3 Activation as a Novel Strategy of Complement Inhibition for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Blood 2014, 123, 2094–2101. [Google Scholar] [CrossRef]
- Risitano, A.M.; Marotta, S. Therapeutic Complement Inhibition in Complement-Mediated Hemolytic Anemias: Past, Present and Future. Semin. Immunol. 2016, 28, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Kulasekararaj, A.G.; Lee, J.W.; Maciejewski, J.P.; Notaro, R.; Brodsky, R.; Huang, M.; Geffner, M.; Browett, P. Danicopan: An Oral Complement Factor D Inhibitor for Paroxysmal Nocturnal Hemoglobinuria. Haematologica 2021, 106, 3188. Available online: https://www.haematologica.org/article/view/haematol.2020.261826 (accessed on 14 April 2022). [CrossRef]
- Kulasekararaj, A.G.; Risitano, A.M.; Maciejewski, J.P.; Notaro, R.; Browett, P.; Lee, J.W.; Huang, M.; Geffner, M.; Brodsky, R.A. Phase 2 Study of Danicopan in Patients with Paroxysmal Nocturnal Hemoglobinuria with an Inadequate Response to Eculizumab. Blood 2021, 138, 1928–1938. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavriilaki, E.; Papakonstantinou, A.; Agrios, K.A. Novel Insights into Factor D Inhibition. Int. J. Mol. Sci. 2022, 23, 7216. https://doi.org/10.3390/ijms23137216
Gavriilaki E, Papakonstantinou A, Agrios KA. Novel Insights into Factor D Inhibition. International Journal of Molecular Sciences. 2022; 23(13):7216. https://doi.org/10.3390/ijms23137216
Chicago/Turabian StyleGavriilaki, Eleni, Anna Papakonstantinou, and Konstantinos A. Agrios. 2022. "Novel Insights into Factor D Inhibition" International Journal of Molecular Sciences 23, no. 13: 7216. https://doi.org/10.3390/ijms23137216
APA StyleGavriilaki, E., Papakonstantinou, A., & Agrios, K. A. (2022). Novel Insights into Factor D Inhibition. International Journal of Molecular Sciences, 23(13), 7216. https://doi.org/10.3390/ijms23137216







