Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging
Abstract
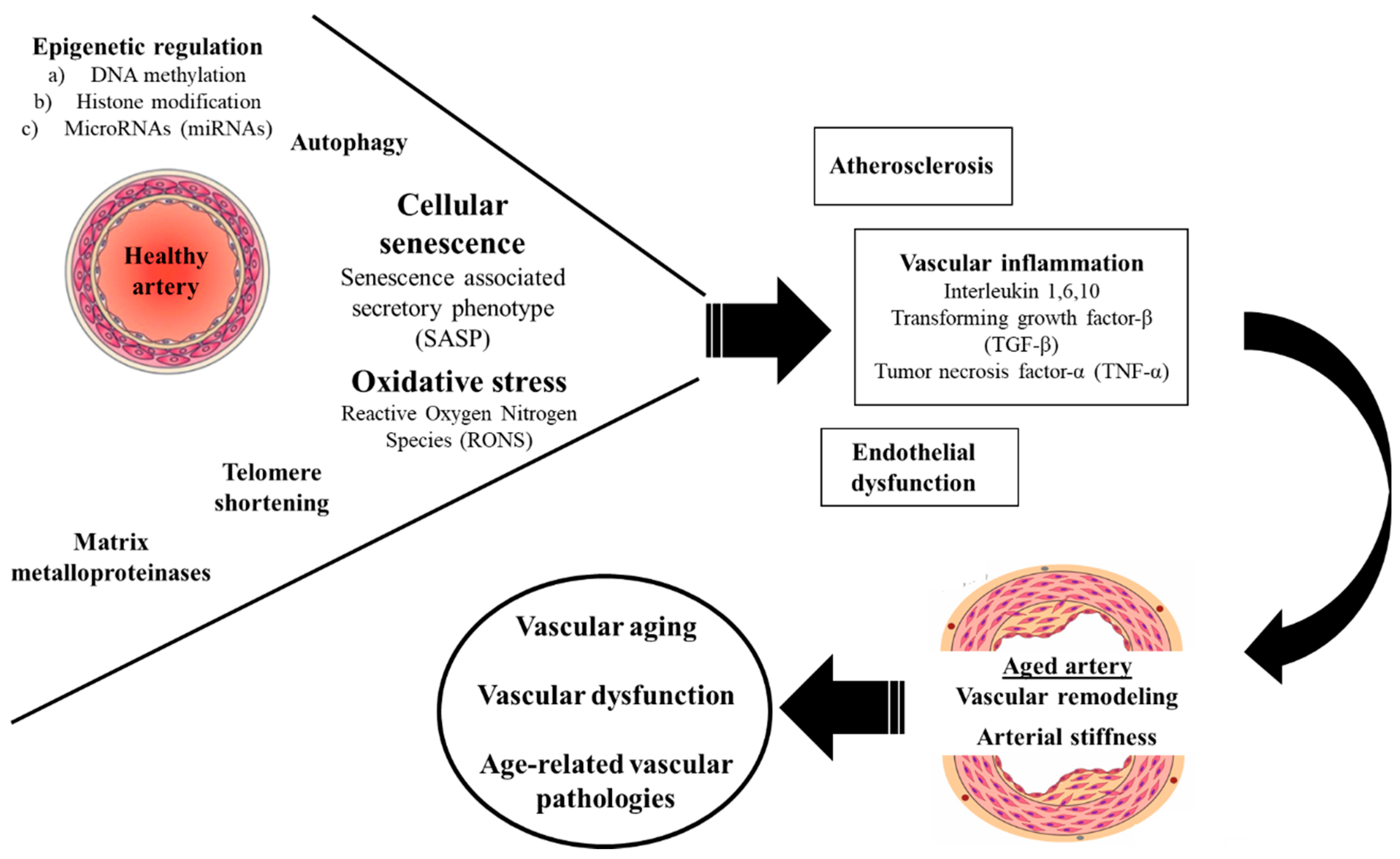
1. Introduction
2. Methodology
2.1. Oxidative Stress
2.1.1. Role of Oxidative and Nitrosative Stress
2.1.2. Endogenous Sources of RONS
2.1.3. The Renin/Angiotensin Signaling Pathway
2.1.4. Exogenous Sources of RONS
2.2. Inflammation
2.2.1. Interleukin-1
2.2.2. Ιnterleukin-6
2.2.3. Ιnterleukin-10
2.2.4. Transforming Growth Factor Beta
2.2.5. Tumor Necrosis Factor-α
2.3. Extracellular Matrix Metalloproteinases
2.3.1. MMP-2
2.3.2. MMP-3
2.3.3. MMP-7
2.3.4. MMP-9
2.4. Epigenetic Regulation
2.4.1. DNA Methylation
2.4.2. Histone Modification
2.4.3. Non-Coding RNAs
2.5. Telomere Shortening
2.6. Cellular Senescence
2.7. Autophagy
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin converting enzyme |
| AMPK | AMP-activated protein kinase |
| AngII | angiotensin II |
| AP-1 | activator protein-1 |
| ApoE | apolipoprotein E |
| BCL6 | B-cell lymphoma 6 protein |
| BP | blood pressure |
| CAT | catalase |
| COX-2 | cyclooxygenase-2 |
| CRP | C-reactive protein |
| CTGF | connective tissue growth factor |
| CVD | cardiovascular disease |
| DDR | DNA damage response |
| DNMT | DNA methyltransferases |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| EGFR | epidermal growth factor receptor |
| EPCs | endothelial progenitor cells |
| eNOS | endothelial nitric oxide synthase |
| FOXO | Forkhead box |
| GPX | glutathione-peroxidase |
| HATs | histone acetyltransferases |
| HB-EGF | heparin-binding epidermal growth factor |
| HDACs | histone deacetylases |
| HDMs | histone demethylases |
| HDL | high density lipoprotein |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| HMTs | histone methyltransferases |
| Hpx | hemopexin |
| HUVECs | human umbilical endothelial cells |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFN | interferon |
| IL | interleukin |
| IMT | intima-media thickness |
| KLF2 | Kruppel-like Factor 2 |
| LDL | low density lipoprotein |
| LKB1 | liver kinase B1 |
| LOX-1 | lectin-like oxidized low-density lipoprotein receptor-1 |
| LPOs | lipoxygenases |
| Lys | lysine |
| MAPKs | mitogen-activated protein kinases |
| MCP-1 | monocyte chemotactic protein-1 |
| MIP-1a | macrophage inflammatory protein-1 alpha |
| miRNAs | microRNAs |
| MMPs | matrix metalloproteinases |
| MPOs | myeloperoxidases |
| mTOR | mammalian target of rapamycin |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| ncRNAs | non-coding RNAs |
| NF-κB | nuclear factor-kb |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| oxLDL | oxidized low-density lipoprotein |
| PAI-1 | plasminogen activator inhibitor-type 1 |
| PAR-1 | protease activated receptor-1 |
| PARP | poly (ADP-ribose) polymerase |
| PDGF | platelet-derived growth factor |
| PCNA | proliferating cell nuclear antigen |
| PGC-1a | peroxisome proliferator-activated receptor-γ coactivator-1α |
| RAS | renin/angiotensin system |
| RONS | reactive oxygen and nitrogen species |
| ROS | reactive oxygen species |
| RSPO3 | pro-permeability factor R-spondin 3 |
| SASP | senescence-associated secretory phenotype |
| sGCβ1 | soluble guanylyl cyclase β1 |
| Sirt | sirtuin |
| SOD | superoxide dismutase |
| TGF-β | transforming growth factor-β |
| TIMPs | tissue inhibitors of matrix metalloproteinases |
| TLR | toll-like receptor |
| TNF | tumor necrosis factor |
| TNFS4 | tumor necrosis factor superfamily member 4 |
| TRF-2 | telomeric repeat-binding factor 2 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| VSMC | vascular smooth muscle cell |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Freedman, V.A.; Martin, L.G.; Schoeni, R.F. Recent Trends in Disability and Functioning among Older Adults in the United States: A Systematic Review. JAMA 2002, 288, 3137–3146. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A “Set up” for Vascular Disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Paneni, F.; Diaz Cañestro, C.; Libby, P.; Lüscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, L. Alterations in Viscoelastic Properties Following Premature Birth May Lead to Hypertension and Cardiovascular Disease Development in Later Life. Acta Paediatr. Int. J. Paediatr. 2015, 104, 19–26. [Google Scholar] [CrossRef]
- Martyn, C.N.; Greenwald, S.E. Impaired Synthesis of Elastin in Walls of Aorta and Large Conduit Arteries during Early Development as an Initiating Event in Pathogenesis of Systemic Hypertension. Lancet 1997, 350, 953–955. [Google Scholar] [CrossRef]
- Martin, H.; Hu, J.; Gennser, G.; Norman, M. Impaired Endothelial Function and Increased Carotid Stiffness in 9-Year-Old Children with Low Birthweight. Circulation 2000, 102, 2739–2744. [Google Scholar] [CrossRef]
- Brodszki, J.; Länne, T.; Maršál, K.; Ley, D. Impaired Vascular Growth in Late Adolescence after Intrauterine Growth Restriction. Circulation 2005, 111, 2623–2628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-Related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef] [PubMed]
- Laina, A.; Stellos, K.; Stamatelopoulos, K. Vascular Ageing: Underlying Mechanisms and Clinical Implications. Exp. Gerontol. 2018, 109, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-morte, D.; Testa, G.; Cacciatore, F.; Bonaduce, D.; Abete, P. Oxidative Stress and Diseases. Oxidative Stress Dis. 2018, 47, 770–773. [Google Scholar] [CrossRef]
- Martínez-Revelles, S.; García-Redondo, A.B.; Avendaño, M.S.; Varona, S.; Palao, T.; Orriols, M.; Roque, F.R.; Fortuño, A.; Touyz, R.M.; Martínez-González, J.; et al. Lysyl Oxidase Induces Vascular Oxidative Stress and Contributes to Arterial Stiffness and Abnormal Elastin Structure in Hypertension: Role of P38MAPK. Antioxid. Redox Signal. 2017, 27, 379–397. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Durackova, Z. Systems Biology of Free Radicals and Antioxidants; Springer: Berlin/Heidelberg, Germany, 2014; Volume 9783642300. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-morte, D.; Testa, G.; Cacciatore, F.; Bonaduce, D.; Abete, P. Oxidative Stress and Diseases; IntechOpen: London, UK, 2012; pp. 757–772. [Google Scholar] [CrossRef]
- Genestra, M. Oxyl Radicals, Redox-Sensitive Signalling Cascades and Antioxidants. Cell. Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef]
- Kubala, L.; Schmelzer, K.R.; Klinke, A.; Kolarova, H.; Baldus, S.; Hammock, B.D.; Eiserich, J.P. Modulation of Arachidonic and Linoleic Acid Metabolites in Myeloperoxidase-Deficient Mice during Acute Inflammation. Free Radic. Biol. Med. 2010, 48, 1311–1320. [Google Scholar] [CrossRef]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a Catalyst for Lipoprotein Oxidation, Is Expressed in Human Atherosclerotic Lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase, Modified Lipoproteins, and Atherogenesis. J. Lipid Res. 2009, 50, S346–S351. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Albrett, A.M.; Chapman, A.L.; Dickerhof, N.; Forbes, L.V.; Khalilova, I.; Turner, R. Measuring Chlorine Bleach in Biology and Medicine. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a Key Enzyme for Leukotriene Biosynthesis in Health and Disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Kim, E.N.; Kim, M.Y.; Lim, J.H.; Jang, I.A.; Ban, T.H.; Shin, S.J.; Park, C.W.; Chang, Y.S.; Choi, B.S. Age-Associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxid. Med. Cell. Longev. 2016, 2016, 6731093. [Google Scholar] [CrossRef]
- Touyz, R.M. Reactive Oxygen Species and Angiotensin II Signaling in Vascular Cells—Implications in Cardiovascular Disease. Brazilian J. Med. Biol. Res. 2004, 37, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. Free Radical Production and Angiotensin. Curr. Hypertens. Rep. 2000, 2, 167–173. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Harrison, D.G. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, T.M.; Monteiro, M.M.O.; Braga, V.A. Angiotensin-II-Derived Reactive Oxygen Species on Baroreflex Sensitivity during Hypertension: New Perspectives. Front. Physiol. 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Alberts-Grill, N.; Denning, T.L.; Rezvan, A.; Jo, H. The Role of the Vascular Dendritic Cell Network in Atherosclerosis. Am. J. Physiol. Cell Physiol. 2013, 305, C1–C21. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, G.; Morieri, M.L.; Volpato, S.; Maggio, M.; Cherubini, A.; Francesconi, D.; Bandinelli, S.; Paolisso, G.; Guralnik, J.M.; Ferrucci, L. Insulin Resistance and Systemic Inflammation, but Not Metabolic Syndrome Phenotype, Predict 9 Years Mortality in Older Adults. Atherosclerosis 2014, 235, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.-C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 Level and the Development of Disability in Older Persons. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; An, Y.; Zoli, M.; Simonsick, E.M.; Guralnik, J.M.; Bandinelli, S.; Boyd, C.M.; Ferrucci, L. Aging and the Burden of Multimorbidity: Associations with Inflammatory and Anabolic Hormonal Biomarkers. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 63–70. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and Frailty in the Elderly: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Montero, I.; Orbe, J.; Varo, N.; Beloqui, O.; Monreal, J.I.; Rodréguez, J.A.; Díez, J.; Libby, P.; Páramo, J.A. C-Reactive Protein Induces Matrix Metalloproteinase-1 and -10 in Human Endothelial Cells: Implications for Clinical and Subclinical Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, 1369–1378. [Google Scholar] [CrossRef]
- Scicali, R.; Di Pino, A.; Urbano, F.; Ferrara, V.; Marchisello, S.; Di Mauro, S.; Scamporrino, A.; Filippello, A.; Piro, S.; Rabuazzo, A.M.; et al. Analysis of S100A12 Plasma Levels in Hyperlipidemic Subjects with or without Familial Hypercholesterolemia. Acta Diabetol. 2019, 56, 899–906. [Google Scholar] [CrossRef]
- Wang, W.; Deng, Z.; Li, L.; Li, J.; Jin, X. Association of Hyper-Sensitive C-Reactive Protein with Arterial Stiffness and Endothelial Function in Patients with Hyperlipidemia. Int. J. Clin. Exp. Med. 2016, 9, 23416–23424. [Google Scholar]
- Vicenová, B.; Vopálenský, V.; Burýšek, L.; Pospíšek, M. Emerging Role of Interleukin-1 in Cardiovascular Diseases. Physiol. Res. 2009, 58, 481–498. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The Origins of Age-Related Proinflammatory State. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of Interleukin-1ß Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef]
- Merhi-Soussi, F.; Kwak, B.R.; Magne, D.; Chadjichristos, C.; Berti, M.; Pelli, G.; James, R.W.; Mach, F.; Gabay, C. Interleukin-1 Plays a Major Role in Vascular Inflammation and Atherosclerosis in Male Apolipoprotein E-Knockout Mice. Cardiovasc. Res. 2005, 66, 583–593. [Google Scholar] [CrossRef]
- Nicklin, M.J.H.; Hughes, D.E.; Barton, J.L.; Ure, J.M.; Duff, G.W. Arterial Inflammation in Mice Lacking the Interleukin 1 Receptor Antagonist Gene. J. Exp. Med. 2000, 191, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Libby, P.; Everett, B.M. Novel Antiatherosclerotic Therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 538–545. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and Atherosclerosis: Vascular Intrinsic and Extrinsic Factors and Potential Role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Song, Y.; Shen, H.; Schenten, D.; Shan, P.; Lee, P.J.; Goldstein, D.R. Aging Enhances the Basal Production of IL-6 and CCL2 in Vascular Smooth Muscle Cells. Arter. Thromb Vasc Biol. 2013, 32, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Hung, M.J.; Cherng, W.J.; Hung, M.Y.; Wu, H.T.; Pang, J.H.S. Interleukin-6 Inhibits Endothelial Nitric Oxide Synthase Activation and Increases Endothelial Nitric Oxide Synthase Binding to Stabilized Caveolin-1 in Human Vascular Endothelial Cells. J. Hypertens. 2010, 28, 940–951. [Google Scholar] [CrossRef]
- Schrader, L.I.; Kinzenbaw, D.A.; Johnson, A.W.; Faraci, F.M.; Didion, S.P. IL-6 Deficiency Protects against Angiotensin II-Induced Endothelial Dysfunction and Hypertrophy. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2576–2581. [Google Scholar] [CrossRef]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 Induces Oxidative Stress and Endothehal Dysfunction by Overexpression of the Angiotensin II Type 1 Receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W.; Kinzenbaw, D.A.; Modrick, M.L.; Faraci, F.M. Small-Molecule Inhibitors of Signal Transducer and Activator of Transcription 3 Protect against Angiotensin Ii-Induced Vascular Dysfunction and Hypertension. Hypertension 2013, 61, 437–442. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Ciechomska, M.; Cant, R.; Van Laar, J.M. Interleukin-6 (IL-6) Trans Signaling Drives a STAT3-Dependent Pathway That Leads to Hyperactive Transforming Growth Factor-β (TGF-β) Signaling Promoting SMAD3 Activation and Fibrosis via Gremlin Protein. J. Biol. Chem. 2014, 289, 9952–9960. [Google Scholar] [CrossRef] [PubMed]
- Mingomataj, E.; Bakiri, A.H. Regulator versus Effector Paradigm: Interleukin-10 as Indicator of the Switching Response. Clin. Rev. Allergy Immunol. 2016, 50, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Kinzenbaw, D.A.; Chu, Y.; Peña Silva, R.A.; Didion, S.P.; Faraci, F.M. Interleukin-10 Protects against Aging-Induced Endothelial Dysfunction. Physiol. Rep. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Lima, V.V.; Zemse, S.M.; Chiao, C.W.; Bomfim, G.F.; Tostes, R.C.; Clinton Webb, R.; Giachini, F.R. Interleukin-10 Limits Increased Blood Pressure and Vascular RhoA/Rho-Kinase Signaling in Angiotensin II-Infused Mice. Life Sci. 2016, 145, 137–143. [Google Scholar] [CrossRef]
- Sikka, G.; Miller, K.L.; Steppan, J.; Pandey, D.; Jung, S.M.; Fraser, C.D.; Ellis, C.; Ross, D.; Vandegaer, K.; Bedja, D.; et al. Interleukin 10 Knockout Frail Mice Develop Cardiac and Vascular Dysfunction with Increased Age. Exp. Gerontol. 2013, 48, 128–135. [Google Scholar] [CrossRef]
- Strom, A.C.; Cross, A.J.; Cole, J.E.; Blair, P.A.; Leib, C.; Goddard, M.E.; Rosser, E.C.; Park, I.; Nilsson, A.H.; Nilsson, J.; et al. B Regulatory Cells Are Increased in Hypercholesterolemic Mice and Pro- Tect from Lesion Development via IL-10. Thromb Haemost. 2015, 114, 835–847. [Google Scholar]
- Arjuman, A.; Chandra, N.C. Effect of IL-10 on LOX-1 Expression, Signalling and Functional Activity: An Atheroprotective Response. Diabetes Vasc. Dis. Res. 2013, 10, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Rubic, T.; Lorenz, R.L. Downregulated CD36 and OxLDL Uptake and Stimulated ABCA1/G1 and Cholesterol Efflux as Anti-Atherosclerotic Mechanisms of Interleukin-10. Cardiovasc. Res. 2006, 69, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Boisvert, W.A. Interleukin-10 Protects against Atherosclerosis by Modulating Multiple Atherogenic Macrophage Function. Thromb. Haemost. 2015, 113, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Lustig, A.; Liu, H.B.; Metter, E.J.; An, Y.; Swaby, M.A.; Elango, P.; Ferrucci, L.; Hodes, R.J.; Weng, N.P. Telomere Shortening, Inflammatory Cytokines, and Anti-Cytomegalovirus Antibody Follow Distinct Ageassociated Trajectories in Humans. Front. Immunol. 2017, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Firth, C.M.; Phillips, A.C.; Moss, P.; Baylis, D.; Syddall, H.; Sayer, A.A.; Cooper, C.; Lord, J.M. The Age-Related Increase in Low-Grade Systemic Inflammation (Inflammaging) Is Not Driven by Cytomegalovirus Infection. Aging Cell 2012, 11, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rodríguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-β Signaling in Vascular Fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. TGF-Β1-Induced Plasminogen Activator Inhibitor-1 Expression in Vascular Smooth Muscle Cells Requires Pp60c-Src /EGFRY845 and Rho/ ROCK Signaling. J. Mol. Cell. Cardiol. 2008, 44, 527–538. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, D.; Spinetti, G.; Zhang, J.; Jiang, L.Q.; Pintus, G.; Monticone, R.; Lakatta, E.G. Matrix Metalloproteinase 2 Activation of Transforming Growth Factor-Β1 (TGF-Β1) and TGF-Β1-Type II Receptor Signaling within the Aged Arterial Wall. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1503–1509. [Google Scholar] [CrossRef]
- Wang, W.; Huang, X.R.; Canlas, E.; Oka, K.; Truong, L.D.; Bhowmick, N.A.; Ju, W.; Bottinger, E.P.; Lan, H.Y. Essential Role of Smad3 in Angiotensin II–Induced Vascular Fibrosis. Circ Res. 2006, 98, 1032–1039. [Google Scholar] [CrossRef]
- Toma, I.; McCaffrey, T.A. Transforming Growth Factor-β and Atherosclerosis: Interwoven Atherogenic and Atheroprotective Aspects. Cell Tissue Res. 2012, 347, 155–175. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.P.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in Vascular Dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.L.; Pendleton, L.C.; Levy, M.M.; Solomonson, L.P.; Eichler, D.C. Tumor Necrosis Factor-α Reduces Argininosuccinate Synthase Expression and Nitric Oxide Production in Aortic Endothelial Cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Smith, K.; Rivera, A.; Orosz, Z.; Ungvari, Z. Vasculoprotective Effects of Anti-Tumor Necrosis Factor-α Treatment in Aging. Am. J. Pathol. 2007, 170, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, L.; López-Hoyos, M.; Muñoz-Cacho, P.; Martínez-Taboada, V.M. Aging Is Associated with Circulating Cytokine Dysregulation. Cell. Immunol. 2012, 273, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Ungvari, Z.; Koller, A.; Edwards, J.G.; Kaley, G. Proinflammatory Phenotype of Coronary Arteries Promotes Endothelial Apoptosis in Aging. Physiol. Genomics 2004, 17, 21–30. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Avolio, A.P. Smooth Muscle Cell and Arterial Aging: Basic and Clinical Aspects. Cardiovasc. Res. 2018, 114, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Foote, K.; Bennett, M.R. Molecular Insights into Vascular Aging. Aging 2018, 10, 3647–3649. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Helena Laronha, J.C. Structure and Function Function of of Human. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Rajput, P.S.; Vasudevan, H.; McClure, B.; Kumar, U.; MacLeod, K.M.; McNeill, J. Inhibition of Matrix Metalloproteinase-2 Improves Endothelial Function and Prevents Hypertension in Insulin-Resistant Rats. Br. J. Pharmacol. 2012, 165, 705–715. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Telljohann, R.; Jiang, L.; Wu, J.; Monticone, R.E.; Kapoor, K.; Talan, M.; Lakatta, E.G. Chronic Matrix Metalloproteinase Inhibition Retards Age-Associated Arterial Proinflammation and Increase in Blood Pressure. Hypertension 2012, 60, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, Y.M.; Diaz-Canestro, C.; Liberale, L.; Guzik, T.J.; Flammer, A.J.; Bonetti, N.R.; Wüst, P.; Constantino, S.; Paneni, F.; Akhmedov, A.; et al. Therapeutic MMP-2 Knockdown Blunts Age-Dependent Carotid Stiffness by Decreasing Elastin Degradation and Augmenting Enos Activation. Atherosclerosis 2021, 331, e29–e30. [Google Scholar] [CrossRef]
- Lee, H.Y.; You, H.J.; Won, J.Y.; Youn, S.W.; Cho, H.J.; Park, K.W.; Park, W.Y.; Seo, J.S.; Park, Y.B.; Walsh, K.; et al. Forkhead Factor, FOXO3a, Induces Apoptosis of Endothelial Cells through Activation of Matrix Metalloproteinases. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 302–308. [Google Scholar] [CrossRef]
- Silence, J.; Lupu, F.; Collen, D.; Lijnen, H.R. Persistence of Atherosclerotic Plaque but Reduced Aneurysm Formation in Mice with Stromelysin-1 (MMP-3) Gene Inactivation. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; George, S.J.; Newby, A.C.; Jackson, C.L. Divergent Effects of Matrix Metalloproteinases 3, 7, 9, and 12 on Atherosclerotic Plaque Stability in Mouse Brachiocephalic Arteries. Proc. Natl. Acad. Sci. USA 2005, 102, 15575–15580. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Aukrust, P.; Russell, D.; Krohg-Sørensen, K.; Almås, T.; Bundgaard, D.; Bjerkeli, V.; Sagen, E.L.; Michelsen, A.E.; Dahl, T.B.; et al. Matrix Metalloproteinase 7 Is Associated with Symptomatic Lesions and Adverse Events in Patients with Carotid Atherosclerosis. PLoS ONE 2014, 9, e84935. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, J.H.; Jang, W.; Hwang, I.K.; Kim, I.J.; Kim, H.J.; Cho, K.H.; Lee, S.T. Apolipoprotein A-IV Is a Novel Substrate for Matrix Metalloproteinases. J. Biochem. 2012, 151, 291–298. [Google Scholar] [CrossRef]
- Williams, H.; Johnson, J.L.; Jackson, C.L.; White, S.J.; George, S.J. MMP-7 Mediates Cleavage of N-Cadherin and Promotes Smooth Muscle Cell Apoptosis. Cardiovasc. Res. 2010, 87, 137–146. [Google Scholar] [CrossRef]
- Hao, L.; Du, M.; Lopez-Campistrous, A.; Fernandez-Patron, C. Agonist-Induced Activation of Matrix Metalloproteinase-7 Promotes Vasoconstriction through the Epidermal Growth Factor-Receptor Pathway. Circ. Res. 2004, 94, 68–76. [Google Scholar] [CrossRef]
- Florence, J.M.; Krupa, A.; Booshehri, L.M.; Allen, T.C.; Kurdowska, A.K. Metalloproteinase-9 Contributes to Endothelial Dysfunction in Atherosclerosis via Protease Activated Receptor-1. PLoS ONE 2017, 12, e0171427. [Google Scholar] [CrossRef] [PubMed]
- Tziakas, D.N.; Lazarides, M.K.; Tentes, I.K.; Georgiadis, G.S.; Eleftheriadou, E.; Chalikias, G.K.; Kortsaris, A.; Hatseras, D.I. Gelatinases [Matrix Metalloproteinase-2 (MMP-2) and MMP-9] Induce Carotid Plaque Instability but Their Systemic Levels Are Not Predictive of Local Events. Ann. Vasc. Surg. 2005, 19, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Lutgens, E.; Manderveld, A.; Maris, K.; Collen, D.; Carmeliet, P.; Moons, L. Loss of Matrix Metalloproteinase-9 or Matrix Metalloproteinase-12 Protects Apolipoprotein E-Deficient Mice against Atherosclerotic Media Destruction but Differentially Affects Plaque Growth. Circulation 2004, 109, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.T.; Collins, E.T.; Marine, L.A.; Uberti, M.G.; Uchida, H.; Leidenfrost, J.E.; Khan, F.F.; Boc, K.P.; Abendschein, D.R.; Parks, W.C. Matrix Metalloproteinase-9 Modulation by Resident Arterial Cells Is Responsible for Injury-Induced Accelerated Atherosclerotic Plaque Development in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1020–1025. [Google Scholar] [CrossRef]
- Galis, Z.S.; Johnson, C.; Godin, D.; Magid, R.; Shipley, J.M.; Senior, R.M.; Ivan, E. Targeted Disruption of the Matrix Metalloproteinase-9 Gene Impairs Smooth Muscle Cell Migration and Geometrical Arterial Remodeling. Circ. Res. 2002, 91, 852–859. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted Gene Disruption of Matrix Metalloproteinase-9 (Gelatinase B) Suppresses Development of Experimental Abdominal Aortic Aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Correa, G.A.; Zacchigna, S.; Arsic, N.; Zentilin, L.; Salvi, A.; Sinagra, G.; Giacca, M. Potent Inhibition of Arterial Intimal Hyperplasia by TIMP1 Gene Transfer Using AAV Vectors. Mol. Ther. 2004, 9, 876–884. [Google Scholar] [CrossRef]
- Allaire, E.; Forough, R.; Clowes, M.; Starcher, B.; Clowes, A.W. Local Overexpression of TIMP-1 Prevents Aortic Aneurysm Degeneration and Rupture in a Rat Model. J. Clin. Investig. 1998, 102, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Baker, A.H.; Oka, K.; Chan, L.; Newby, A.C.; Jackson, C.L.; George, S.J. Suppression of Atherosclerotic Plaque Progression and Instability by Tissue Inhibitor of Metalloproteinase-2: Involvement of Macrophage Migration and Apoptosis. Circulation 2006, 113, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Kassiri, Z. Biology of Tissue Inhibitor of Metalloproteinase 3 (TIMP3), and Its Therapeutic Implications in Cardiovascular Pathology. Front. Physiol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Son, D.J.; Jung, Y.Y.; Seo, Y.S.; Park, H.; Lee, D.H.; Kim, S.; Roh, Y.S.; Han, S.B.; Yoon, D.Y.; Hong, J.T. Interleukin-32α Inhibits Endothelial Inflammation, Vascular Smooth Muscle Cell Activation, and Atherosclerosis by Upregulating Timp3 and Reck through Suppressing MicroRNA-205 Biogenesis. Theranostics 2017, 7, 2186–2203. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, R.; Cavalera, M.; Menini, S.; Mavilio, M.; Casagrande, V.; Rossi, C.; Urbani, A.; Cardellini, M.; Pugliese, G.; Menghini, R.; et al. Loss of TIMP3 Exacerbates Atherosclerosis in ApoE Null Mice. Atherosclerosis 2014, 235, 438–443. [Google Scholar] [CrossRef]
- Basu, R.; Lee, J.; Morton, J.S.; Takawale, A.; Fan, D.; Kandalam, V.; Wang, X.; Davidge, S.T.; Kassiri, Z. TIMP3 Is the Primary TIMP to Regulate Agonist-Induced Vascular Remodelling and Hypertension. Cardiovasc. Res. 2013, 98, 360–371. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.; Spiers, P.; McGovern, E.; Feely, J. Aging Is Associated with Increased Matrix Metalloproteinase-2 Activity in the Human Aorta. Am. J. Hypertens. 2005, 18, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Beaudeux, J.L.; Giral, P.; Bruckert, E.; Bernard, M.; Foglietti, M.J.; Chapman, M.J. Serum Matrix Metalloproteinase-3 and Tissue Inhibitor of Metalloproteinases-1 as Potential Markers of Carotid Atherosclerosis in Infraclinical Hyperlipidemia. Atherosclerosis 2003, 169, 139–146. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix Metalloproteinases: They’re Not Just for Matrix Anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Loftus, I.M.; Naylor, A.R.; Goodall, S.; Crowther, M.; Jones, L.; Bell, P.R.F.; Thompson, M.M. Increased Matrix Metalloproteinase-9 Activity in Unstable Carotid Plaques. Stroke 2000, 31, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Feng, Y.; Dong, G.; Wang, Y.; Yang, J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediators Inflamm. 2020, 2020, 3872367. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Pushpakumar, S.; Muradashvili, N.; Kundu, S.; Tyagi, S.C.; Sen, U. Regulation and Involvement of Matrix Metalloproteinases in Vascular Diseases. Front. Biosci. 2016, 21, 89–118. [Google Scholar]
- Raffetto, J.D.; Khalil, R.A. Matrix Metalloproteinases and Their Inhibitors in Vascular Remodeling and Vascular Disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Tabaei, S.; Tabaee, S.S. DNA Methylation Abnormalities in Atherosclerosis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, S.; Liu, Y.S. Roles and Mechanisms of DNA Methylation in Vascular Aging and Related Diseases. Front. Cell Dev. Biol. 2021, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Vikram, A.; Hoffman, T.A.; Naqvi, A.; Lewarchik, C.M.; Kim, Y.R.; Irani, K. Histone and DNA Methylation-Mediated Epigenetic Downregulation of Endothelial Kruppel-like Factor 2 by Low-Density Lipoprotein Cholesterol. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1936–1942. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Huang, Y.T.; Huang, T.S.; Pang, W.; Zhu, J.J.; Liu, Y.F.; Tang, R.Z.; Zhao, C.R.; Yao, W.J.; Li, Y.S.; et al. The Mammalian Target of Rapamycin and DNA Methyltransferase 1 Axis Mediates Vascular Endothelial Dysfunction in Response to Disturbed Flow. Sci. Rep. 2017, 7, 14996. [Google Scholar] [CrossRef]
- Xu, L.; Hao, H.; Hao, Y.; Wei, G.; Li, G.; Ma, P.; Xu, L.; Ding, N.; Ma, S.; Chen, A.F.; et al. Aberrant MFN2 Transcription Facilitates Homocysteine-Induced VSMCs Proliferation via the Increased Binding of c-Myc to DNMT1 in Atherosclerosis. J. Cell. Mol. Med. 2019, 23, 4611–4626. [Google Scholar] [CrossRef]
- Zhu, L.; Jia, L.; Liu, Z.; Zhang, Y.; Wang, J.; Yuan, Z.; Hui, R. Elevated Methylation of FOXP3 (Forkhead Box P3)-TSDR (Regulatory T-Cell-Specific Demethylated Region) Is Associated with Increased Risk for Adverse Outcomes in Patients with Acute Coronary Syndrome. Hypertension 2019, 74, 581–589. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, B.G.; Jelinek, J.; Kim, D.H.; Lee, S.H.; Cho, K.; Rha, S.H.; Lee, Y.H.; Jin, H.S.; Choi, D.K.; et al. Promoter Methylation Changes in ALOX12 and AIRE1: Novel Epigenetic Markers for Atherosclerosis. Clin. Epigenetics 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Aavik, E.; Lumivuori, H.; Leppänen, O.; Wirth, T.; Häkkinen, S.K.; Bräsen, J.H.; Beschorner, U.; Zeller, T.; Braspenning, M.; Van Criekinge, W.; et al. Global DNA Methylation Analysis of Human Atherosclerotic Plaques Reveals Extensive Genomic Hypomethylation and Reactivation at Imprinted Locus 14q32 Involving Induction of a MiRNA Cluster. Eur. Heart J. 2015, 36, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Yang, M.; Yang, H.; Xu, N.; Fan, X.; Liu, G.; Jiang, X.; Fan, J.; Zhang, L.; et al. DNA Methylome Profiling Reveals Epigenetic Regulation of Lipoprotein-Associated Phospholipase A2 in Human Vulnerable Atherosclerotic Plaque. Clin. Epigenetics 2021, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Istas, G.; Declerck, K.; Pudenz, M.; Szic, K.S.V.; Lendinez-Tortajada, V.; Leon-Latre, M.; Heyninck, K.; Haegeman, G.; Casasnovas, J.A.; Tellez-Plaza, M.; et al. Identification of Differentially Methylated BRCA1 and CRISP2 DNA Regions as Blood Surrogate Markers for Cardiovascular Disease. Sci. Rep. 2017, 7, 5120. [Google Scholar] [CrossRef]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.H.; Di Guo, D.; Guan, Y.; Chi, C.Y.; Liu, B.D. Changes of DNA Methylation of Isoetes Sinensis under Pb and Cd Stress. Environ. Sci. Pollut. Res. 2019, 26, 3428–3435. [Google Scholar] [CrossRef] [PubMed]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The Many Roles of Histone Deacetylases in Development and Physiology: Implications for Disease and Therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Wagner, R.; Rzucidlo, E.M. Age-Related Loss of SirT1 Expression Results in Dysregulated Human Vascular Smooth Muscle Cell Function. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, 533–541. [Google Scholar] [CrossRef]
- Halasa, M.; Adamczuk, K.; Adamczuk, G.; Afshan, S.; Stepulak, A.; Cybulski, M.; Wawruszak, A. Deacetylation of Transcription Factors in Carcinogenesis. Int. J. Mol. Sci. 2021, 22, 1810. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. The Protective Role of Sirt1 in Vascular Tissue: Its Relationship to Vascular Aging and Atherosclerosis. Aging 2016, 8, 2290–2307. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 Promotes Endothelium-Dependent Vascular Relaxation by Activating Endothelial Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Donato, A.J.; Magerko, K.A.; Lawson, B.R.; Durrant, J.R.; Lesniewski, L.A.; Seals, D.R. SIRT-1 and Vascular Endothelial Dysfunction with Ageing in Mice and Humans. J. Physiol. 2011, 589, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Akishita, M.; Eto, M.; Iijima, K.; Kaneki, M.; Ouchi, Y. Sirt1 Modulates Premature Senescence-like Phenotype in Human Endothelial Cells. J. Mol. Cell. Cardiol. 2007, 43, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Man, A.W.C.; Yang, K.; Guo, Y.; Xu, C.; Tse, H.F.; Han, W.; Bloksgaard, M.; De Mey, J.G.R.; Vanhoutte, P.M.; et al. Endothelial SIRT1 Prevents Adverse Arterial Remodeling by Facilitating HERC2-Mediated Degradation of Acetylated LKB1. Oncotarget 2016, 7, 39065–39081. [Google Scholar] [CrossRef]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 Regulation of Antioxidant Genes Is Dependent on the Formation of a FoxO3a/PGC-1α Complex. Antioxid. Redox Signal. 2013, 19, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.X.; Van Tits, L.J.; Lohmann, C.; Arsiwala, T.; Winnik, S.; Tailleux, A.; Stein, S.; Gomes, A.P.; Suri, V.; Ellis, J.L.; et al. The Sirt1 Activator SRT3025 Provides Atheroprotection in Apoe−/− Mice by Reducing Hepatic Pcsk9 Secretion and Enhancing Ldlr Expression. Eur. Heart J. 2015, 36, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Gillum, M.P.; Erion, D.M.; Shulman, G.I. Sirtuin-1 Regulation of Mammalian Metabolism. Trends Mol. Med. 2011, 17, 8–13. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Gao, P.; Zhou, S.; Zhang, Z.Q.; Hao, D.L.; Lian, L.S.; Li, Y.J.; Chen, H.Z.; Liu, D.P. SIRT1-Mediated Epigenetic Downregulation of Plasminogen Activator Inhibitor-1 Prevents Vascular Endothelial Replicative Senescence. Aging Cell 2014, 13, 890–899. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.Z.; Wan, Y.Z.; Zhang, Q.J.; Wei, Y.S.; Huang, S.; Liu, J.J.; Lu, Y.B.; Zhang, Z.Q.; Yang, R.F.; et al. Repression of P66Shc Expression by SIRT1 Contributes to the Prevention of Hyperglycemia-Induced Endothelial Dysfunction. Circ. Res. 2011, 109, 639–648. [Google Scholar] [CrossRef]
- Cutolo, M.; Soldano, S.; Contini, P.; Sulli, A.; Seriolo, B.; Montagna, P.; Brizzolara, R. Intracellular NF-KκB Decrease and IKBaα Increase in Human Macrophages Following CTLA4-Ig Treatment. Clin. Exp. Rheumatol. 2013, 31, 943–946. [Google Scholar] [PubMed]
- Zhang, R.; Chen, H.Z.; Liu, J.J.; Jia, Y.Y.; Zhang, Z.Q.; Yang, R.F.; Zhang, Y.; Xu, J.; Wei, Y.S.; Liu, D.P.; et al. SIRT1 Suppresses Activator Protein-1 Transcriptional Activity and Cyclooxygenase-2 Expression in Macrophages. J. Biol. Chem. 2010, 285, 7097–7110. [Google Scholar] [CrossRef] [PubMed]
- Fry, J.L.; Al Sayah, L.; Weisbrod, R.M.; Van Roy, I.; Weng, X.; Cohen, R.A.; Bachschmid, M.M.; Seta, F. Vascular Smooth Muscle Sirtuin-1 Protects against Diet-Induced Aortic Stiffness. Hypertension 2016, 68, 775–784. [Google Scholar] [CrossRef]
- Pan, P.W.; Feldman, J.L.; Devries, M.K.; Dong, A.; Edwards, A.M.; Denu, J.M. Structure and Biochemical Functions of SIRT6. J. Biol. Chem. 2011, 286, 14575–14587. [Google Scholar] [CrossRef] [PubMed]
- Cardus, A.; Uryga, A.K.; Walters, G.; Erusalimsky, J.D. SIRT6 Protects Human Endothelial Cells from DNA Damage, Telomere Dysfunction, and Senescence. Cardiovasc. Res. 2013, 97, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Woo, Y.M.; Moon, S.; Lee, J.; Park, H.; Jang, H.; Bae, S.; Park, K.; Heo, J.H.; Choi, Y. Sirtuin 6 Deficiency Induces Endothelial Cell Senescence via Downregulation of Forkhead Box M1 Expression. Aging 2020, 12, 20946–20967. [Google Scholar] [CrossRef]
- Xu, S.; Yin, M.; Koroleva, M.; Mastrangelo, M.A.; Zhang, W.; Bai, P.; Little, P.J.; Jin, Z.G. SIRT6 Protects against Endothelial Dysfunction and Atherosclerosis in Mice. Aging 2016, 8, 1064–1082. [Google Scholar] [CrossRef]
- Wang, T.; Sun, C.; Hu, L.; Gao, E.; Li, C.; Wang, H.; Sun, D. Sirt6 Stabilizes Atherosclerosis Plaques by Promoting Macrophage Autophagy and Reducing Contact with Endothelial Cells. Biochem. Cell Biol. 2020, 98, 120–129. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Li, N.; Wang, S.; Bu, P. Role of SIRT3 in Angiotensin II-Induced Human Umbilical Vein Endothelial Cells Dysfunction. BMC Cardiovasc. Disord. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T.; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase SIRT3 Reduces Vascular Dysfunction and Hypertension While SIRT3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Wang, X.; Zhang, Y.; Bu, P.; Zhang, Q.; Jiang, F. Global Gene Expression Profiling Reveals Functional Importance of Sirt2 in Endothelial Cells under Oxidative Stress. Int. J. Mol. Sci. 2013, 14, 5633–5649. [Google Scholar] [CrossRef]
- Araki, S.; Izumiya, Y.; Rokutanda, T.; Ianni, A.; Hanatani, S.; Kimura, Y.; Onoue, Y.; Senokuchi, T.; Yoshizawa, T.; Yasuda, O.; et al. Sirt7 Contributes to Myocardial Tissue Repair by Maintaining Transforming Growth Factor-β Signaling Pathway. Circulation 2015, 132, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-Coding Rnas in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef]
- Ding, Q.; Shao, C.; Rose, P.; Zhu, Y.Z. Epigenetics and Vascular Senescence–Potential New Therapeutic Targets? Front. Pharmacol. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; Terrinoni, A.; Amati, F.; Vasa-Nicotera, M.; Ippoliti, A.; Novelli, G.; Melino, G.; et al. MicroRNA 217 Modulates Endothelial Cell Senescence via Silent Information Regulator 1. Circulation 2009, 120, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- De Yébenes, V.G.; Briones, A.M.; Martos-Folgado, I.; Mur, S.M.; Oller, J.; Bilal, F.; González-Amor, M.; Méndez-Barbero, N.; Silla-Castro, J.C.; Were, F.; et al. Aging-Associated MiR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2408–2424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, J.; Chen, A.F. MicroRNA-34a Induces Endothelial Progenitor Cell Senescence and Impedes Its Angiogenesis via Suppressing Silent Information Regulator 1. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 110–116. [Google Scholar] [CrossRef]
- Zhu, S.; Deng, S.; Ma, Q.; Zhang, T.; Jia, C.; Zhuo, D.; Yang, F.; Wei, J.; Wang, L.; Dykxhoorn, D.M.; et al. MicroRNA-10A* and MicroRNA-21 Modulate Endothelial Progenitor Cell Senescence via Suppressing High-Mobility Group A2. Circ. Res. 2013, 112, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.G.; Zheng, B.; Han, M.; Fang, X.M.; Li, H.X.; Miao, S.B.; Su, M.; Han, Y.; Shi, H.J.; Wen, J.K. MiR-146a and Krüppel-like Factor 4 Form a Feedback Loop to Participate in Vascular Smooth Muscle Cell Proliferation. EMBO Rep. 2011, 12, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Cordes, K.R.; Sheehy, N.T.; White, M.; Berry, E.; Sarah, U.; Muth, A.N.; Lee, T.; Miano, J.M.; Ivey, K.N. MiR-145 and MiR-143 Regulate Smooth Muscle Cell Fate Decisions. Nature 2010, 460, 705–710. [Google Scholar] [CrossRef]
- Farina, F.M.; Hall, I.F.; Serio, S.; Zani, S.; Climent, M.; Salvarani, N.; Carullo, P.; Civilini, E.; Condorelli, G.; Elia, L.; et al. MiR-128-3p Is a Novel Regulator of Vascular Smooth Muscle Cell Phenotypic Switch and Vascular Diseases. Circ. Res. 2020, 126, e120–e135. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Q.; He, S.; Yang, M.; Maguire, E.M.; An, W.; Afzal, T.A.; Luong, L.A.; Zhang, L.; Xiao, Q. MiR-22 Is a Novel Mediator of Vascular Smooth Muscle Cell Phenotypic Modulation and Neointima Formation. Circulation 2018, 137, 1824–1841. [Google Scholar] [CrossRef]
- Olivieri, F.; Rippo, M.R.; Monsurrò, V.; Salvioli, S.; Capri, M.; Procopio, A.D.; Franceschi, C. MicroRNAs Linking Inflamm-Aging, Cellular Senescence and Cancer. Ageing Res. Rev. 2013, 12, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, S.; Kim, S.; Kim, J.; Lee, D.K.; Park, W.; Kim, T.; Jung, J.; Hwang, J.Y.; Won, M.H.; et al. NF-ΚB-Responsive MiR-155 Induces Functional Impairment of Vascular Smooth Muscle Cells by Downregulating Soluble Guanylyl Cyclase. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Jahantigh, M.; Wei, Y.; Noels, H.; Akhtar, S.; Zhou, Z.; Koenen, R.R.; Heyll, K.; Gremse, F.; Kiessling, F.; Grommes, J.; et al. MicroRNA-155 Promotes Atherosclerosis by Repressing Bcl6 in Macrophages. J. Clin. Investig. 2012, 122, 4190–4202. [Google Scholar] [CrossRef] [PubMed]
- Uryga, A.K.; Bennett, M.R. Ageing Induced Vascular Smooth Muscle Cell Senescence in Atherosclerosis. J. Physiol. 2016, 594, 2115–2124. [Google Scholar] [CrossRef]
- Fyhrquist, F.; Saijonmaa, O.; Strandberg, T. The Roles of Senescence and Telomere Shortening in Cardiovascular Disease. Nat. Rev. Cardiol. 2013, 10, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Samuel, I.; Bloom, A.J.D. The Role of Senescence, Telomere Dysfunction and Shelterin in Vascular Aging. Microcirculation 2019, 26, e12487. [Google Scholar] [CrossRef]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular Smooth Muscle Cells Undergo Telomere-Based Senescence in Human Atherosclerosis: Effects of Telomerase and Oxidative Stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef]
- Bhayadia, R.; Schmidt, B.M.W.; Melk, A.; Hömme, M. Senescence-Induced Oxidative Stress Causes Endothelial Dysfunction. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 161–169. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial Cell Senescence in Human Atherosclerosis: Role of Telomere in Endothelial Dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef]
- Ogami, M.; Ikura, Y.; Ohsawa, M.; Matsuo, T.; Kayo, S.; Yoshimi, N.; Hai, E.; Shirai, N.; Ehara, S.; Komatsu, R.; et al. Telomere Shortening in Human Coronary Artery Diseases. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 546–550. [Google Scholar] [CrossRef]
- Nzietchueng, R.; Elfarra, M.; Nloga, J.; Labat, C.; Carteaux, J.P.; Maureira, P.; Lacolley, P.; Villemot, J.P.; Benetos, A. Telomere Length in Vascular Tissues from Patients with Atherosclerotic Disease. J. Nutr. Health Aging 2011, 15, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.R.; Norton, G.R.; Woodiwiss, A.J.; Brooksbank, R.L. Impact of Gender and Menopausal Status on Relationships between Biological Aging, as Indexed by Telomere Length, and Aortic Stiffness. Am. J. Hypertens. 2015, 28, 623–630. [Google Scholar] [CrossRef]
- McDonnell, B.J.; Yasmin; Butcher, L.; Cockcroft, J.R.; Wilkinson, I.B.; Erusalimsky, J.D.; McEniery, C.M. The Age-Dependent Association between Aortic Pulse Wave Velocity and Telomere Length. J. Physiol. 2017, 595, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Jeanclos, E.; Schork, N.J.; Kyvik, K.O.; Kimura, M.; Skurnick, J.H.; Aviv, A. Telomere Length Inversely Correlates with Pulse Pressure and Is Highly Familial. Hypertension 2000, 36, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chen, A.F.; Wang, H.Z.; Xie, L.Y.; Sui, K.X.; Zhang, Q.Y. Association of Shorter Mean Telomere Length with Large Artery Stiffness in Patients with Coronary Heart Disease. Aging Male 2011, 14, 27–32. [Google Scholar] [CrossRef] [PubMed]
- van der Harst, P.; van der Steege, G.; de Boer, R.A.; Voors, A.A.; Hall, A.S.; Mulder, M.J.; van Gilst, W.H.; van Veldhuisen, D.J. Telomere Length of Circulating Leukocytes Is Decreased in Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 49, 1459–1464. [Google Scholar] [CrossRef]
- Brouilette, S.; Singh, R.K.; Thompson, J.R.; Goodall, A.H.; Samani, N.J. White Cell Telomere Length and Risk of Premature Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Gardner, J.P.; Zureik, M.; Labat, C.; Xiaobin, L.; Adamopoulos, C.; Temmar, M.; Bean, K.E.; Thomas, F.; Aviv, A. Short Telomeres Are Associated with Increased Carotid Atherosclerosis in Hypertensive Subjects. Hypertension 2004, 43, 182–185. [Google Scholar] [CrossRef]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte Telomere Length and Risk of Cardiovascular Disease: Systematic Review and Meta- Analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef]
- D’Mello, M.J.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between Shortened Leukocyte Telomere Length and Cardiometabolic Outcomes: Systematic Review and Meta-Analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh-Far, R.; Cawthon, R.M.; Na, B.; Browner, W.S.; Schiller, N.B.; Whooley, M.A. Prognostic Value of Leukocyte Telomere Length in Patients with Stable Coronary Artery Disease: Data from the Heart and Soul Study (R1). Arter. Thromb. Vasc. Biol. 2008, 28, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and Aging: Causes, Consequences, and Therapeutic Avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Schmeer, C.; Kretz, A.; Wengerodt, D.; Stojiljkovic, M.; Witte, O.W. Dissecting Aging and Senescence-Current Concepts and Open Lessons. Cells 2019, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Morton, J.P.; Athineos, D.; Kang, T.; Lasitschka, F.; Andrulis, M.; Pascual, G.; et al. A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2014, 15, 978–990. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Machado-Oliveira, G.; Ramos, C.; Marques, A.R.; Vieira, O.V. Cell Senescence, Multiple Organelle Dysfunction and Atherosclerosis. Cells 2020, 9, 2146. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; Van Deursen, J.M. Senescent Intimal Foam Cells Are Deleterious at All Stages of Atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Gardner, S.E.; Humphry, M.; Bennett, M.R.; Clarke, M.C.H. Senescent Vascular Smooth Muscle Cells Drive Inflammation through an Interleukin-1α-Dependent Senescence-Associated Secretory Phenotype. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1963–1974. [Google Scholar] [CrossRef]
- Alique, M.; Ruíz-torres, M.P.; Bodega, G.; Noci, M.V.; Bohórquez, L.; Luna, C.; Luque, R.; Carmona, A.; Ramírez, R. Microvesicles from the Plasma of Elderly Subjects and from Senescent Endothelial Cells Promote Vascular Calcification. Aging 2017, 9, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The Senescence-Associated Secretome as an Indicator of Age and Medical Risk. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Lesniewski, L.A.; Seals, D.R.; Walker, A.E.; Henson, G.D.; Blimline, M.W.; Trott, D.W.; Bosshardt, G.C.; LaRocca, T.J.; Lawson, B.R.; Zigler, M.C.; et al. Dietary Rapamycin Supplementation Reverses Age-Related Vascular Dysfunction and Oxidative Stress, While Modulating Nutrient-Sensing, Cell Cycle, and Senescence Pathways. Aging Cell 2017, 16, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Wang, M.; Bernier, M.; Zhang, J.; Park, S.S.; Maudsley, S.; An, S.S.; Santhanam, L.; Martin, B.; Faulkner, S.; et al. Resveratrol Prevents High Fat/Sucrose Diet-Induced Central Arterial Wall Inflammation and Stiffening in Nonhuman Primates Julie. Cell Metab. 2014, 20, 183–190. [Google Scholar] [CrossRef]
- Yin, Z.; Pascual, C.; Klionsky, D.J. Autophagy: Machinery and Regulation. Microb. Cell 2016, 3, 588–596. [Google Scholar] [CrossRef]
- Madeo, F.; Zimmermann, A.; Maiuri, M.C.; Kroemer, G. Essential Role for Autophagy in Life Span Extension. J. Clin. Investig. 2015, 125, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.; Sedej, S.; Carmona-Gutierrez, D.; Madeo, F.; Kroemer, G. Autophagy in Cardiovascular Aging. Circ. Res. 2018, 123, 803–824. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a Promoter of Longevity: Insights from Model Organisms. Nat. Rev. Mol. Cell Biol. 2019, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Nussenzweig, S.C.; Verma, S.; Finkel, T. The Role of Autophagy in Vascular Biology. Circ. Res. 2015, 116, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed Interplay between Autophagy and Inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef]
- Tai, H.; Wang, Z.; Gong, H.; Han, X.; Zhou, J.; Wang, X.; Wei, X.; Ding, Y.; Huang, N.; Qin, J.; et al. Autophagy Impairment with Lysosomal and Mitochondrial Dysfunction Is an Important Characteristic of Oxidative Stress-Induced Senescence. Autophagy 2017, 13, 99–113. [Google Scholar] [CrossRef]
- Larocca, T.J.; Henson, G.D.; Thorburn, A.; Sindler, A.L.; Pierce, G.L.; Seals, D.R. Translational Evidence That Impaired Autophagy Contributes to Arterial Ageing. J. Physiol. 2012, 590, 3305–3316. [Google Scholar] [CrossRef]
- Bharath, L.P.; Cho, J.M.; Park, S.K.; Ruan, T.; Li, Y.; Mueller, R.; Bean, T.; Reese, V.; Richardson, R.S.; Cai, J.; et al. Endothelial Cell Autophagy Maintains Shear-Stress-Induced Nitric Oxide Generation via Glycolysis-Dependent Purinergic Signaling to ENOS. Arter. Thromb Vasc Biol. 2017, 37, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; da Costa Martins, P.A.; Bitsch, N.; Pintelon, I.; de Meyer, G.R.Y.; Martinet, W.; Schrijvers, D.M. Defective Autophagy in Vascular Smooth Muscle Cells Accelerates Senescence and Promotes Neointima Formation and Atherogenesis. Autophagy 2015, 11, 2014–2032. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Oba, M.; Suzuki, M.; Takahashi, A.; Yamamuro, T.; Fujiwara, M.; Ikenaka, K.; Minami, S.; Tabata, N.; Yamamoto, K.; et al. Suppression of Autophagic Activity by Rubicon Is a Signature of Aging. Nat. Commun. 2019, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; Lawson, B.R.; Lesniewski, L.A.; Seals, D.R. Life-Long Caloric Restriction Reduces Oxidative Stress and Preserves Nitric Oxide Bioavailability and Function in Arteries of Old Mice. Aging Cell 2013, 12, 772–783. [Google Scholar] [CrossRef]
- Martens, C.R.; Seals, D.R. Practical Alternatives to Chronic Caloric Restriction for Optimizing Vascular Function with Ageing. J. Physiol. 2016, 594, 7177–7195. [Google Scholar] [CrossRef] [PubMed]
| MMP and TIMP Class | Overexpression | Deficiency |
|---|---|---|
| MMP-2 | (a) Increased TGF-β1 and SMAD signaling leading to
(b) Endothelial dysfunction due to decreased NO production [83] (c) Increased intima-media thickening and vascular fibrosis [70] | (a) Reduced elastin fiber degeneration and collagen deposition [84] (b) Enhanced eNOS activation [85] |
| MMP-3 | Apoptosis of ECs [86] | Accelerated plaque growth rate with increased macrophage and decreased VSMC composition [87,88] |
| MMP-7 | (a) Atherosclerosis and plaque instability through collagen and matrix modulation and cleavage of apolipoprotein A-IV [89,90] (b) VSMC apoptosis through cleavage of n-cadherin [91] (c) Vasoconstriction through shedding of the HB-EGF and subsequent activation of EGFR [92] | Increased accumulation of VSMCs within the atherosclerotic plaques |
| MMP-9 | (a) Apoptosis in ECs through cleavage of PAR-1 [93] (b) Migration of VSMCs and contribution to atherosclerotic plaque instability and intraplaque hemorrhage [94] | (a) Reduction in size of atherosclerotic lesions and plaque burden [95,96] (b) Inhibition of VSMCs’ migration and restriction of vascular remodeling [97] (c) Prevention of formation of abdominal aortic aneurysms [98] |
| TIMP-1 | (a) Reduction in intimal formation through decreased collagen deposition and increased elastin accumulation [99] (b) Protection against aneurysm formation and rupture through prevention of elastin degradation [100] | |
| TIMP-2 | Suppression of atherosclerotic plaque progression through inhibition of migration and apoptosis of macrophages and foam cells [101] | |
| TIMP-3 | (a) Reduced intimal formation through apoptosis of VSMC [102] (b) Atherosclerosis through inhibition of EC inflammation and VSMC proliferation and migration [103] (c) Accumulation of inflammatory monocytes/macrophages within the vascular wall [104] | (a) Enhanced inflammation and atherosclerosis through increased (b) Adverse vascular remodeling and vascular aneurysm formation through loss of elastic lamellae and inflammation [105] |
| Recruitment of EC migration [139] |
| Delay of the aging and dysfunction of EPCs [140] |
| Inhibition of aging of ECs by binding the PAI-1 promoter and by deacetylation of histone H4K16 [141] |
| Promotion of endothelial KLF2 expression which enables transition of ECs to a “vaso-protective” state [141] |
| Mitigation of hyperglycaemia-induced endothelial dysfunction due to ROS production by inhibiting vascular p66Shc gene transcription [142] |
| Alleviation of oxidative stress and inflammation by the inhibition of NF-κB signaling pathway [143] |
| Activation of eNOS and promotion of NO production by the deacetylation of eNOS on Lys496 and Lys506 [132] |
| Reduction of COX-2 expression through downregulation of transcription factor AP-1 in macrophages [144] |
| Reduction of arterial remodeling and stiffness by alleviation of oxidative stress in VSMCs [145] |
| Deacetylation and activation of the FOXO 1, 3, and 4 transcription factors leading to the expression of several antioxidant genes [136] |
| miR-10A | Propagation of senescence of EPCs through suppression of the high-mobility group A2 molecule [160] |
| miR-21 | Propagation of senescence of EPCs through suppression of the high-mobility group A2 molecule [160] |
| miR-22 | Inhibition of VSMC proliferation and migration and neointima formation 164 |
| miR-34a | Suppression of EC proliferation and promotion of EC senescence in part through Sirt1 inhibition [159] Impairment of EPC-mediated angiogenesis through suppression of silent information regulator 1 [159] |
| miR-126 | Reduction of endothelial inflammation through inhibition of VCAM-1 expression [165] |
| miR-128 | Reduction of VSMC proliferation, migration, and contractility [163] |
| miR-143 | Inhibition of VSMC proliferation through targeting the transcription factor Elk-1 [162] |
| miR-145 | Inhibition of VSMC proliferation through targeting the transcription factor myocardin [162] |
| miR-146a | Promotion of VSMC proliferation and vascular neointimal hyperplasia through targeting KLF4 [161] |
| miR-155 | Promotion of atherosclerosis through repression of macrophage BCL6 expression [167] Endothelial dysfunction and vasoconstriction through downregulation of eNOS and sGCβ1 expression [166] |
| miR-217 | Acceleration of EC senescence, endothelial dysfunction and development of atherosclerosis through Sirt1 downregulation [157,158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkaliagkousi, E.; Lazaridis, A.; Dogan, S.; Fraenkel, E.; Tuna, B.G.; Mozos, I.; Vukicevic, M.; Yalcin, O.; Gopcevic, K. Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8672. https://doi.org/10.3390/ijms23158672
Gkaliagkousi E, Lazaridis A, Dogan S, Fraenkel E, Tuna BG, Mozos I, Vukicevic M, Yalcin O, Gopcevic K. Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging. International Journal of Molecular Sciences. 2022; 23(15):8672. https://doi.org/10.3390/ijms23158672
Chicago/Turabian StyleGkaliagkousi, Eugenia, Antonios Lazaridis, Soner Dogan, Emil Fraenkel, Bilge Guvenc Tuna, Ioana Mozos, Milica Vukicevic, Ozlem Yalcin, and Kristina Gopcevic. 2022. "Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging" International Journal of Molecular Sciences 23, no. 15: 8672. https://doi.org/10.3390/ijms23158672
APA StyleGkaliagkousi, E., Lazaridis, A., Dogan, S., Fraenkel, E., Tuna, B. G., Mozos, I., Vukicevic, M., Yalcin, O., & Gopcevic, K. (2022). Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging. International Journal of Molecular Sciences, 23(15), 8672. https://doi.org/10.3390/ijms23158672







