Fibroblast Growth Factor 7 Suppresses Fibrosis and Promotes Epithelialization during Wound Healing in Mouse Fetuses
Abstract
:1. Introduction
2. Results
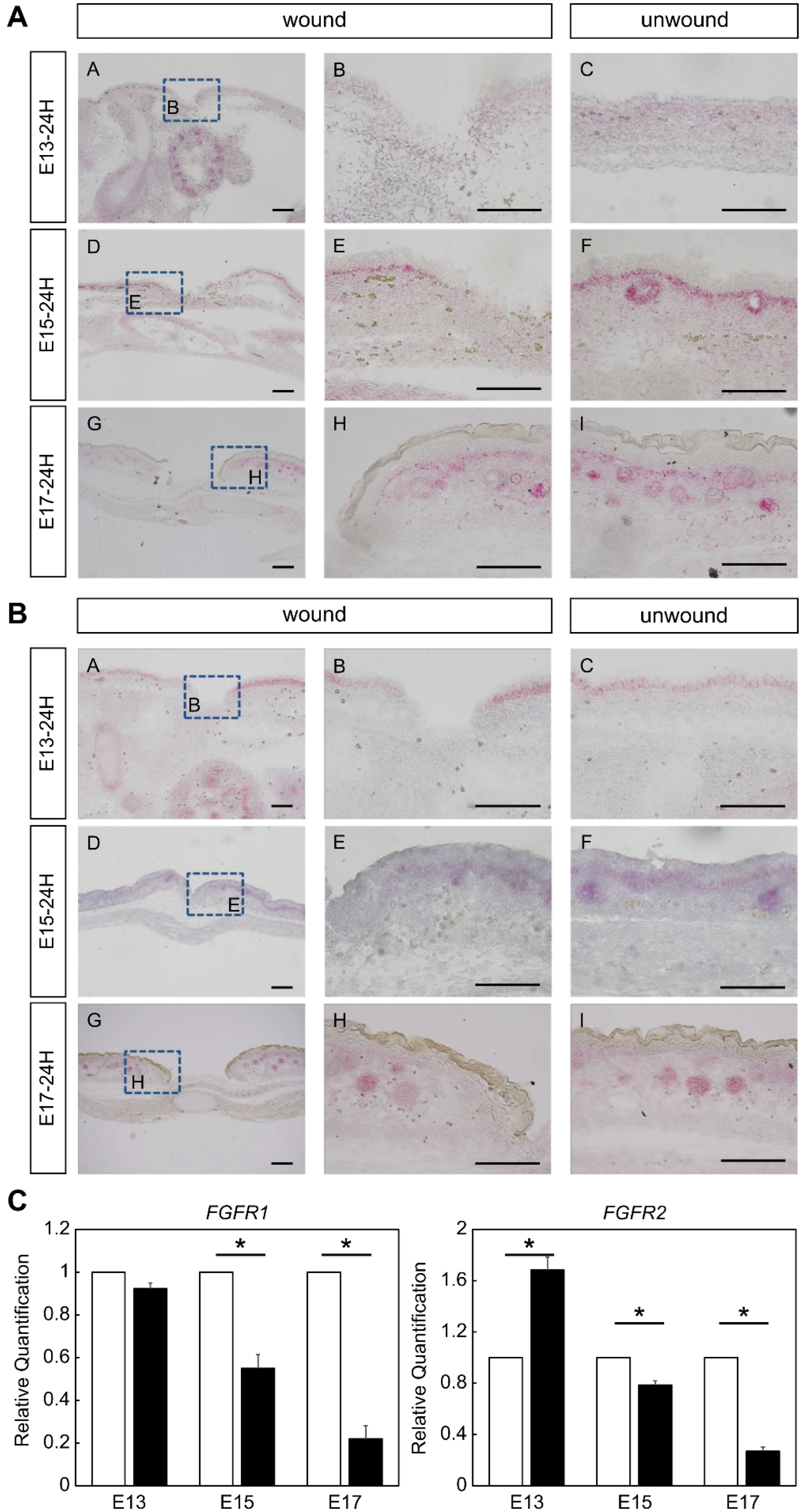
2.1. Expression of FGFR Members during Fetal Wound Healing in Mice
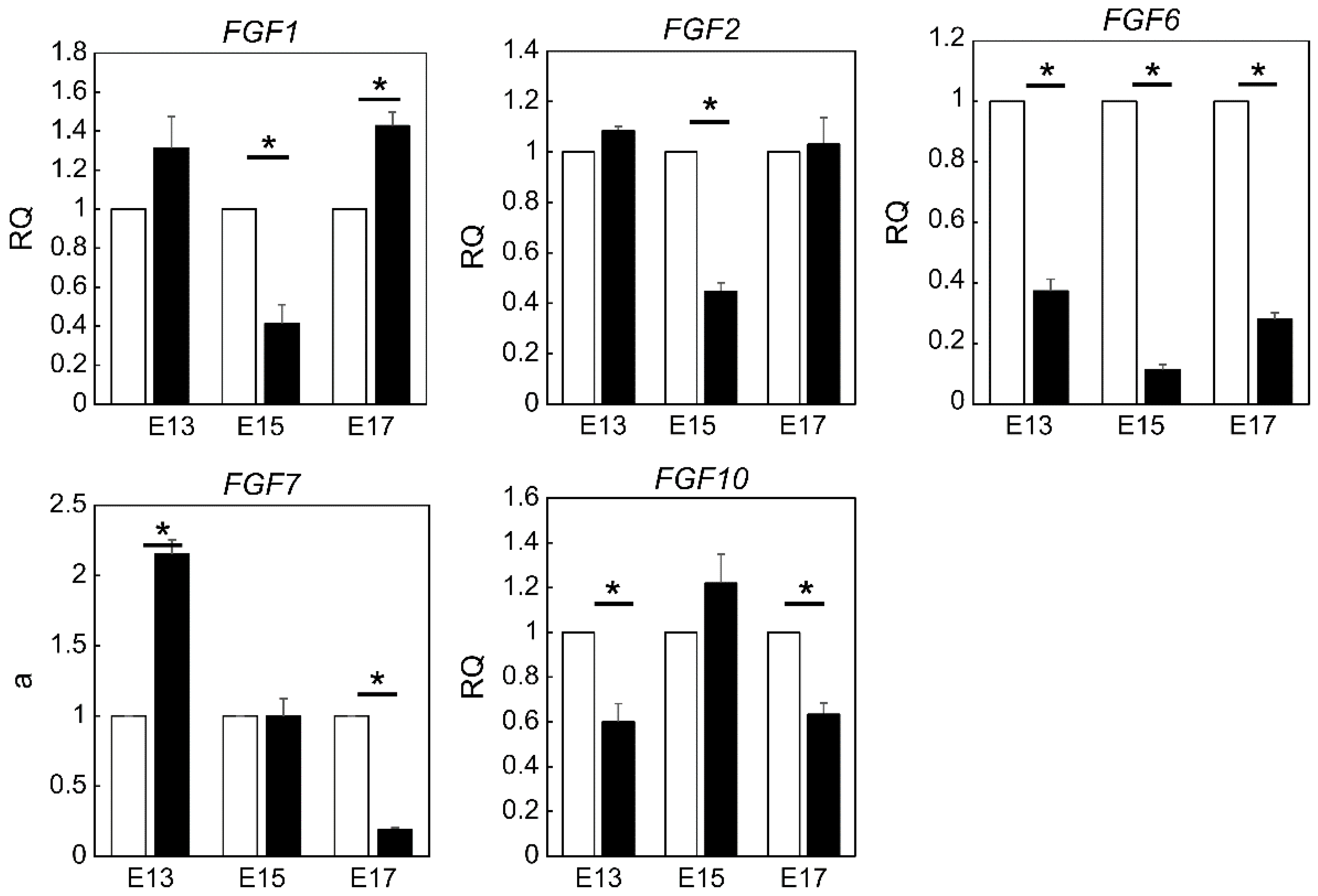
2.2. Expression of FGF Family Members during Fetal Wound Healing in Mice
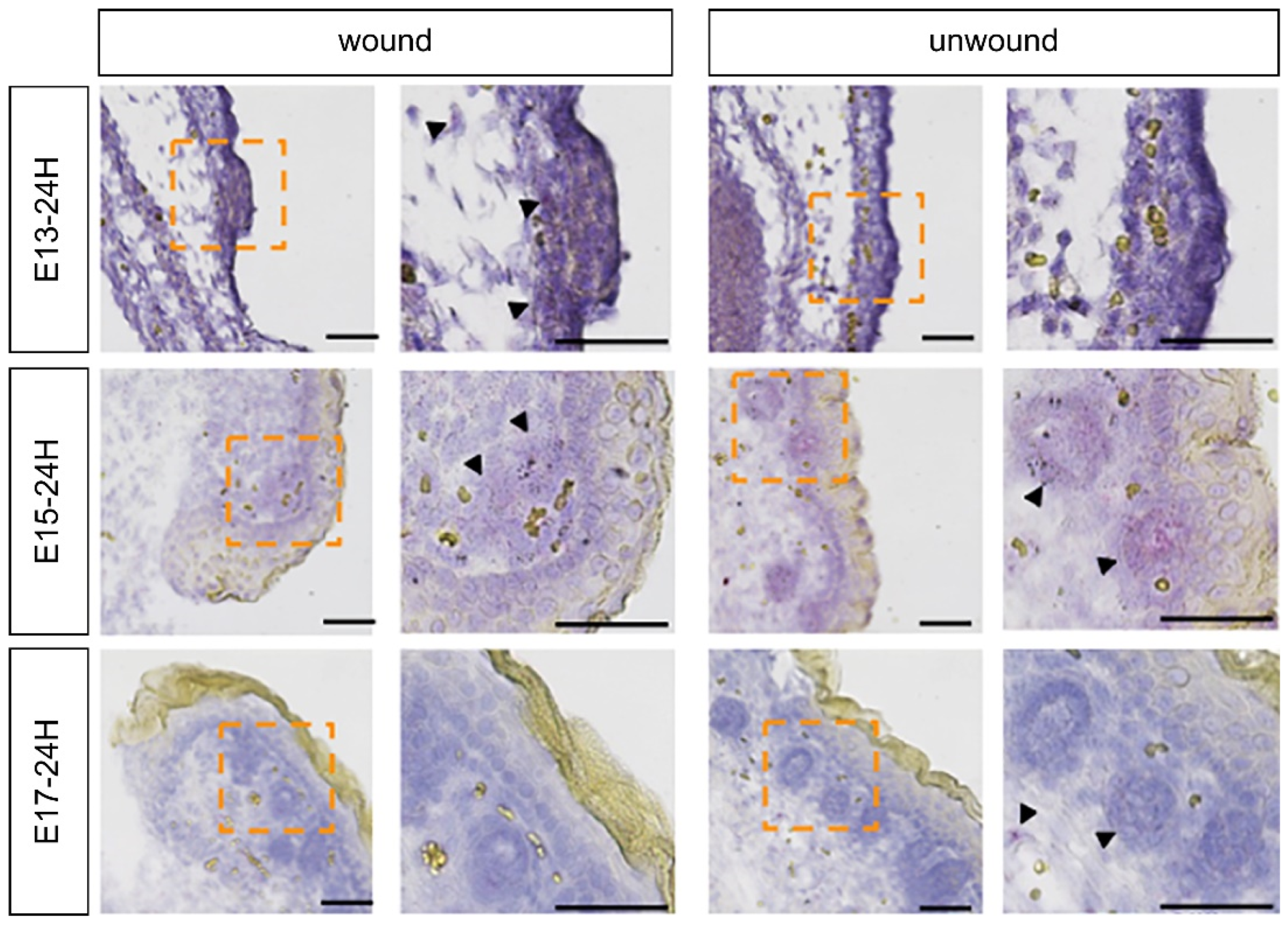
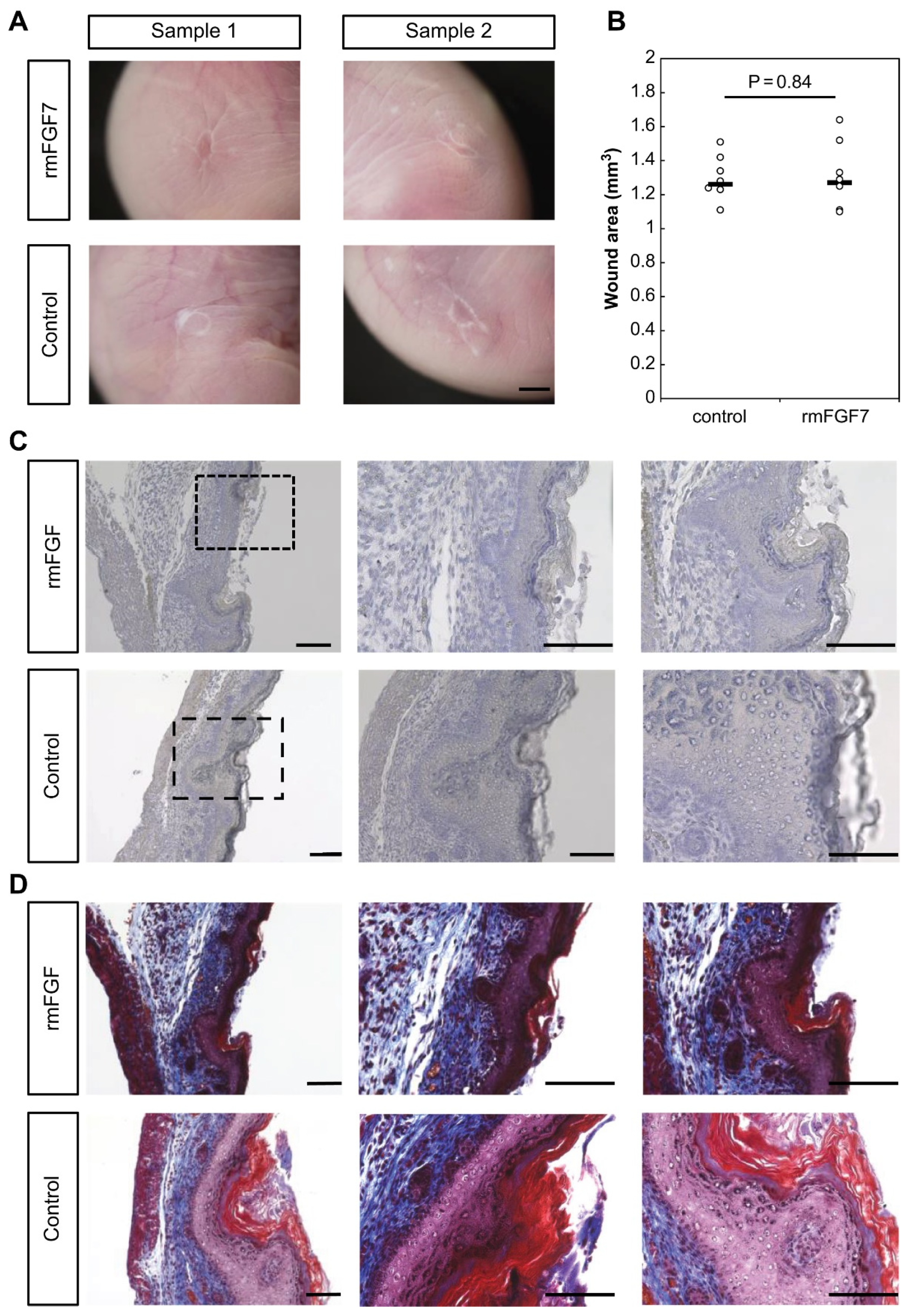
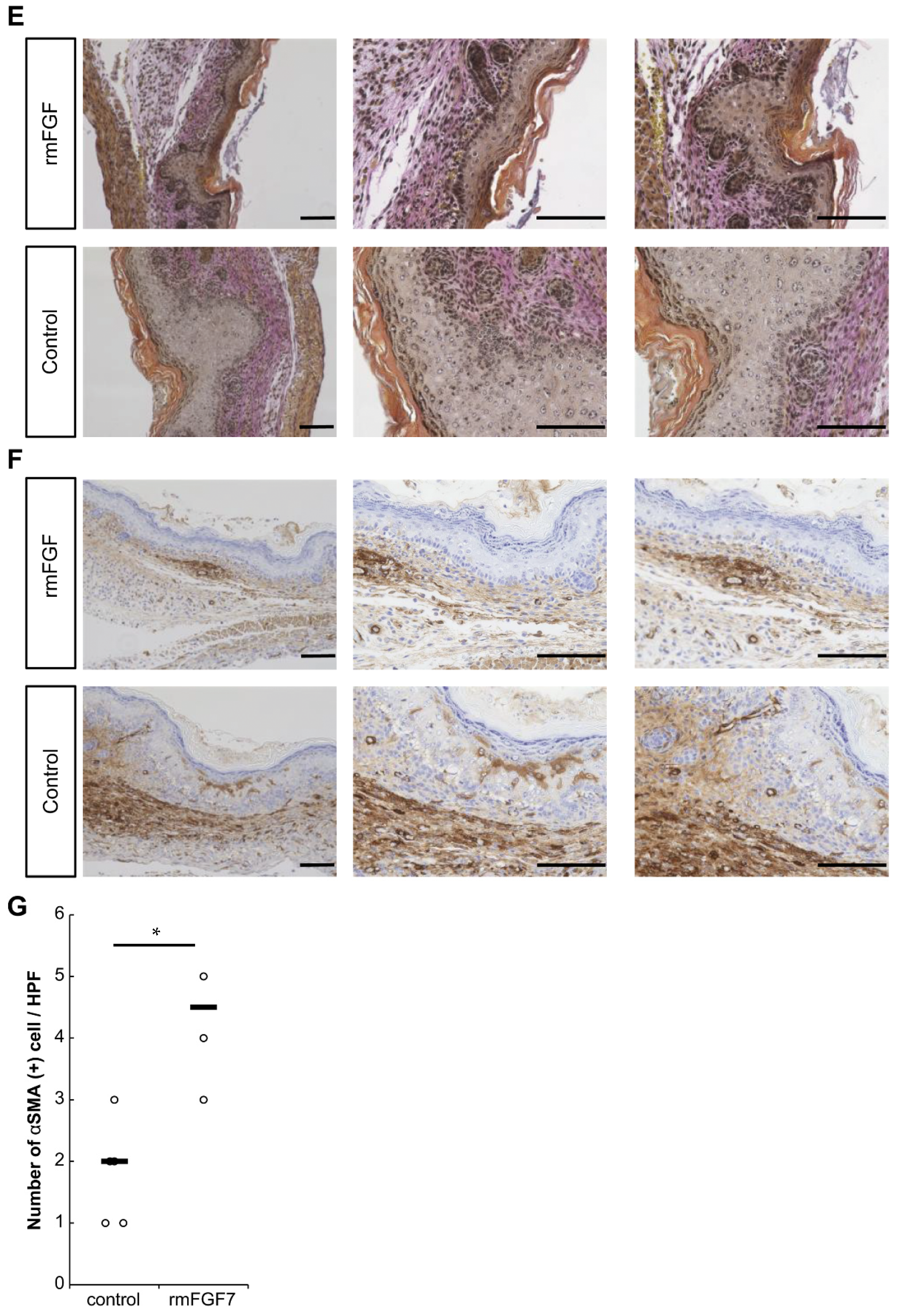
2.3. Role of FGF7 in the Wound Healing Process in Mouse Fetuses
3. Discussion
4. Materials and Methods
4.1. Ethical Consideration
4.2. Fetal-Wounding Procedure
4.3. Immunohistochemistry
4.4. In Situ Hybridization
4.5. LMD, RNA Isolation, and Reverse Transcription
4.6. Quantitative Real-Time PCR
4.7. rmFGF Administration into Mouse Fetuses
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hess, A. Reactions of mammalian fetal tissues to injury. II. Skin. Anat. Rec. 1954, 119, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Cass, D.L.; Bullard, K.M.; Sylvester, K.G.; Yang, E.Y.; Longaker, M.T.; Adzick, N.S. Wound size and gestational age modulate scar formation in fetal wound repair. J. Pediatr. Surg. 1997, 32, 411–415. [Google Scholar] [CrossRef]
- Longaker, M.T.; Adzick, N.S. The biology of fetal wound healing: A review. Plast. Reconstr. Surg. 1991, 87, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Zhang, W.; Li, L.; He, Y.; Wei, Y.; Dang, Y.; Nie, S.; Guo, Z. Signaling pathway and small-molecule drug discovery of FGFR: A comprehensive review. Front. Chem. 2022, 10, 860985. [Google Scholar] [CrossRef]
- Ortega, S.; Ittmann, M.; Tsang, S.H.; Ehrlich, M.; Basilico, C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA 1998, 95, 5672–5677. [Google Scholar] [CrossRef] [Green Version]
- Steiling, H.; Werner, S. Fibroblast growth factors: Key players in epithelial morphogenesis, repair and cytoprotection. Curr. Opin. Biotechnol. 2003, 14, 533–537. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.; Müller, A.K.; Yang, J.; Moik, D.; Ponzio, G.; Ornitz, D.M.; Grose, R.; Werner, S. FGF receptors 1 and 2 are key regulators of keratinocyte migration in vitro and in wounded skin. J. Cell Sci. 2012, 125, 5690–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Meyer, M.; Müller, A.K.; Böhm, F.; Grose, R.; Dauwalder, T.; Verrey, F.; Kopf, M.; Partanen, J.; Bloch, W.; et al. Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J. Cell Biol. 2010, 188, 935–952. [Google Scholar] [CrossRef] [Green Version]
- Kishi, K.; Okabe, K.; Shimizu, R.; Kubota, Y. Fetal skin possesses the ability to regenerate completely: Complete regeneration of skin. Keio J. Med. 2012, 61, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinholz, M.; Poetschke, J.; Schwaiger, H.; Epple, A.; Ruzicka, T.; Gauglitz, G.G. The dermatology life quality index as a means to assess life quality in patients with different scar types. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2112–2119. [Google Scholar] [CrossRef]
- Putra, A.; Alif, I.; Hamra, N.; Santosa, O.; Kustiyah, A.R.; Muhar, A.M.; Lukman, K. MSC-released TGF-β regulate α-SMA expression of myofibroblast during wound healing. J. Stem Cells Regen. Med. 2020, 16, 73–79. [Google Scholar] [PubMed]
- Usui, M.L.; Mansbridge, J.N.; Carter, W.G.; Fujita, M.; Olerud, J.E. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J. Histochem. Cytochem. 2008, 56, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Meyer, M.; Müller, A.K.; Yang, J.; Ŝulcová, J.; Werner, S. The role of chronic inflammation in cutaneous fibrosis: Fibroblast growth factor receptor deficiency in keratinocytes as an example. J. Investig. Dermatol. Symp. Proc. 2011, 15, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Akatsu, Y.; Takahashi, N.; Yoshimatsu, Y.; Kimuro, S.; Muramatsu, T.; Katsura, A.; Maishi, N.; Suzuki, H.I.; Inazawa, J.; Hida, K.; et al. Fibroblast growth factor signals regulate transforming growth factor-β-induced endothelial-to-myofibroblast transition of tumor endothelial cells via Elk1. Mol. Oncol. 2019, 13, 1706–1724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zheng, L.; Cheng, S.; Peng, Y.; Fu, L.; Zhang, X.; Linhardt, R.J. Comparison of the interactions of different growth factors and glycosaminoglycans. Molecules 2019, 24, 3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognoni, E.; Pisco, A.O.; Hiratsuka, T.; Sipilä, K.H.; Belmonte, J.M.; Mobasseri, S.A.; Philippeos, C.; Dilão, R.; Watt, F.M. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 2018, 14, e8174. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, S.; desJardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S.; et al. Preventing engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heumüller, M.; Glock, C.; Rangaraju, V.; Biever, A.; Schuman, E.M. A genetically encodable cell-type-specific protein synthesis inhibitor. Nat. Methods 2019, 16, 699–702. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am. J. Pathol. 2005, 167, 1575–1586. [Google Scholar] [CrossRef] [Green Version]
- Martinello, T.; Pascoli, F.; Caporale, G.; Perazzi, A.; Iacopetti, I.; Patruno, M. Might the Masson trichrome stain be considered a useful method for categorizing experimental tendon lesions? Histol. Histopathol. 2015, 30, 963–969. [Google Scholar]
- Kazlouskaya, V.; Malhotra, S.; Lambe, J.; Idriss, M.H.; Elston, D.; Andres, C. The utility of elastic Verhoeff-Van Gieson staining in dermatopathology. J. Cutan. Pathol. 2013, 40, 211–225. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaya, K.; Aramaki-Hattori, N.; Sakai, S.; Okabe, K.; Asou, T.; Kishi, K. Fibroblast Growth Factor 7 Suppresses Fibrosis and Promotes Epithelialization during Wound Healing in Mouse Fetuses. Int. J. Mol. Sci. 2022, 23, 7087. https://doi.org/10.3390/ijms23137087
Takaya K, Aramaki-Hattori N, Sakai S, Okabe K, Asou T, Kishi K. Fibroblast Growth Factor 7 Suppresses Fibrosis and Promotes Epithelialization during Wound Healing in Mouse Fetuses. International Journal of Molecular Sciences. 2022; 23(13):7087. https://doi.org/10.3390/ijms23137087
Chicago/Turabian StyleTakaya, Kento, Noriko Aramaki-Hattori, Shigeki Sakai, Keisuke Okabe, Toru Asou, and Kazuo Kishi. 2022. "Fibroblast Growth Factor 7 Suppresses Fibrosis and Promotes Epithelialization during Wound Healing in Mouse Fetuses" International Journal of Molecular Sciences 23, no. 13: 7087. https://doi.org/10.3390/ijms23137087
APA StyleTakaya, K., Aramaki-Hattori, N., Sakai, S., Okabe, K., Asou, T., & Kishi, K. (2022). Fibroblast Growth Factor 7 Suppresses Fibrosis and Promotes Epithelialization during Wound Healing in Mouse Fetuses. International Journal of Molecular Sciences, 23(13), 7087. https://doi.org/10.3390/ijms23137087





