Stem Cell Models for Cancer Therapy
Abstract
1. Introduction
2. Experimental Models
2.1. Mechanistic Assays
2.2. Pharmacological Agents
3. Characterization of Cancer Stem Cells
3.1. Breast
3.2. Colon
4. Experimental Modulation
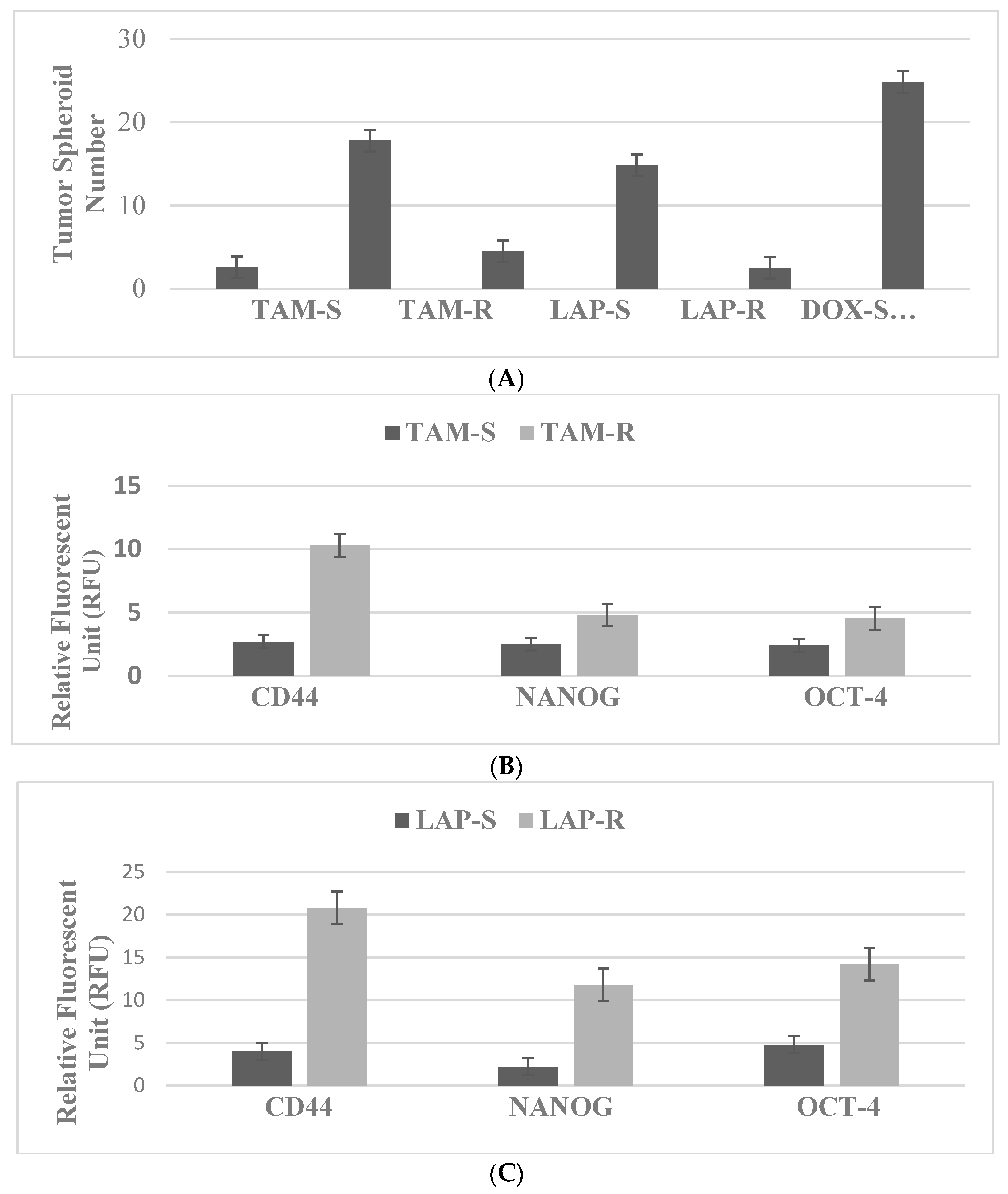
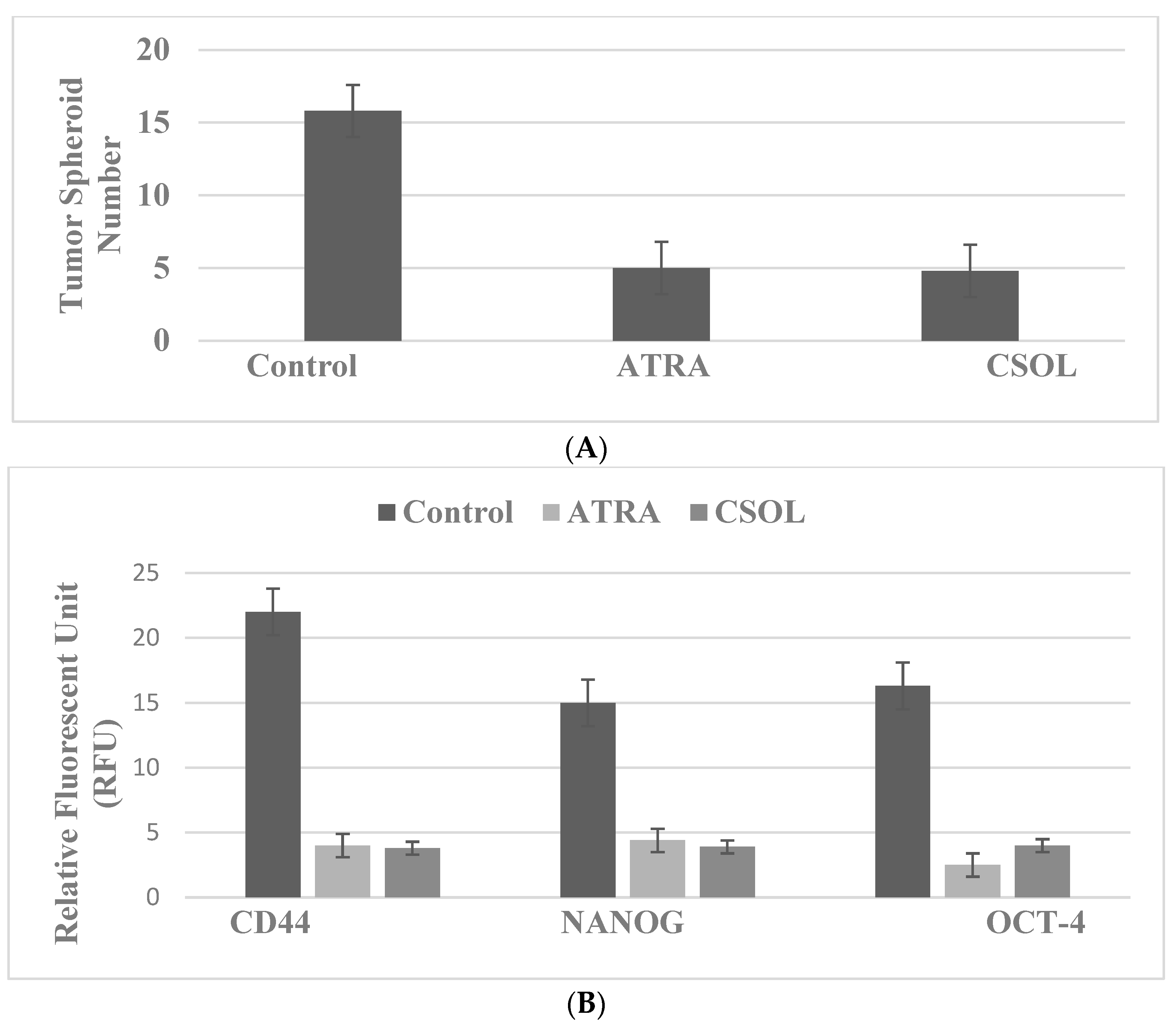
4.1. Breast: For These Experiments, the Natural Products Vitamin A Derivative ATRA and Terpene CSOL Are Used at Their Predetermined Maximally Cytostatic Concentrations
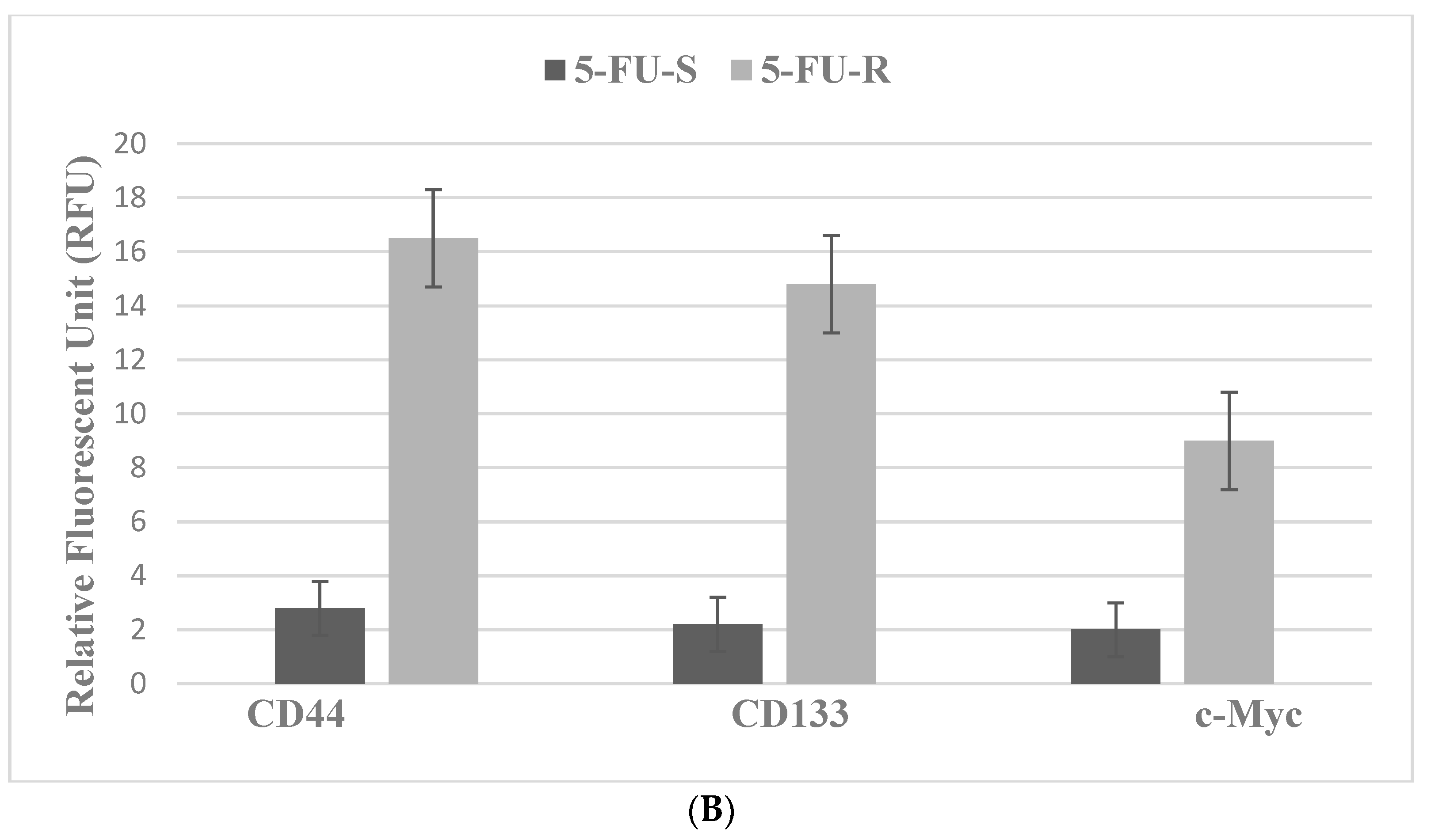
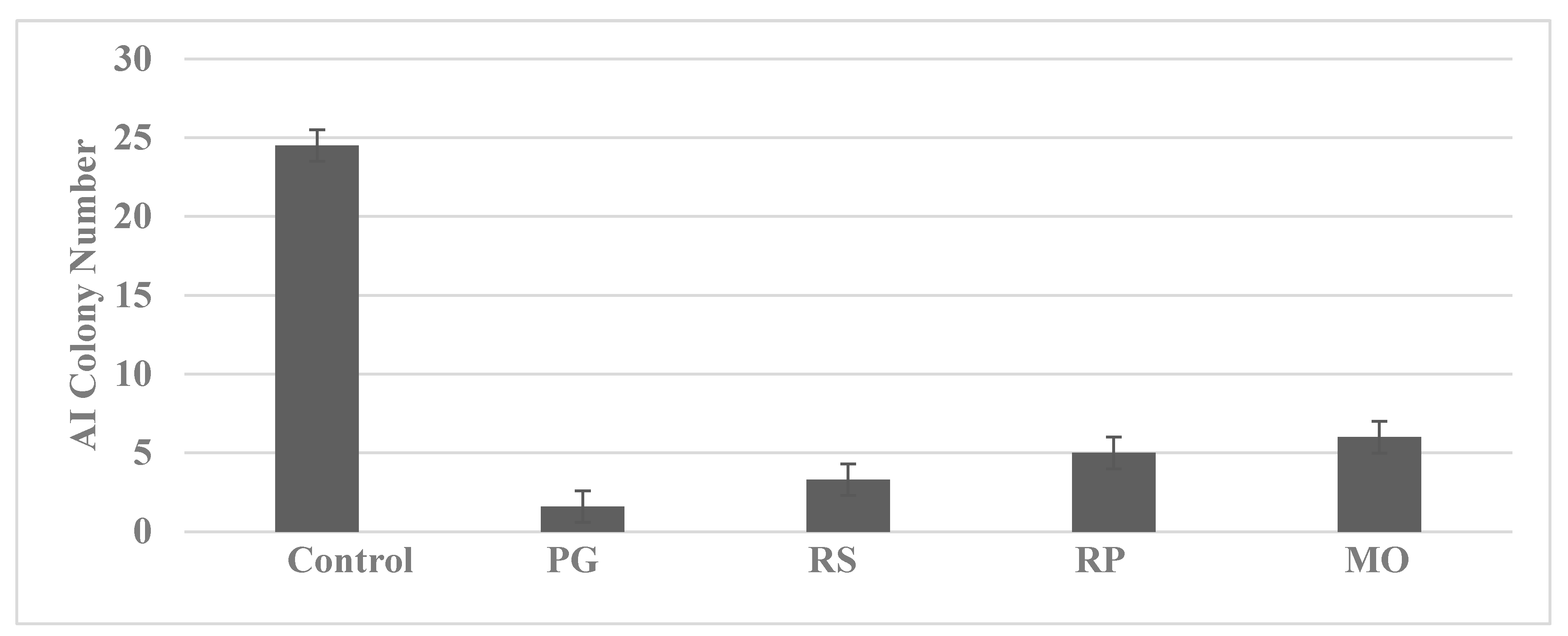
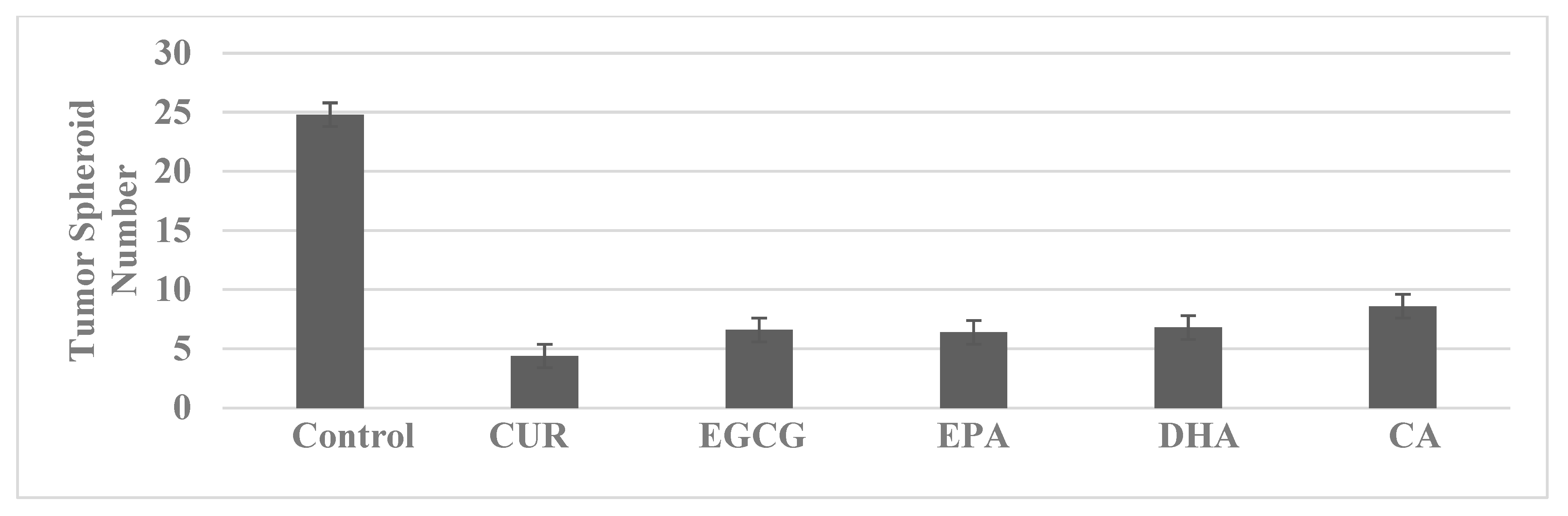
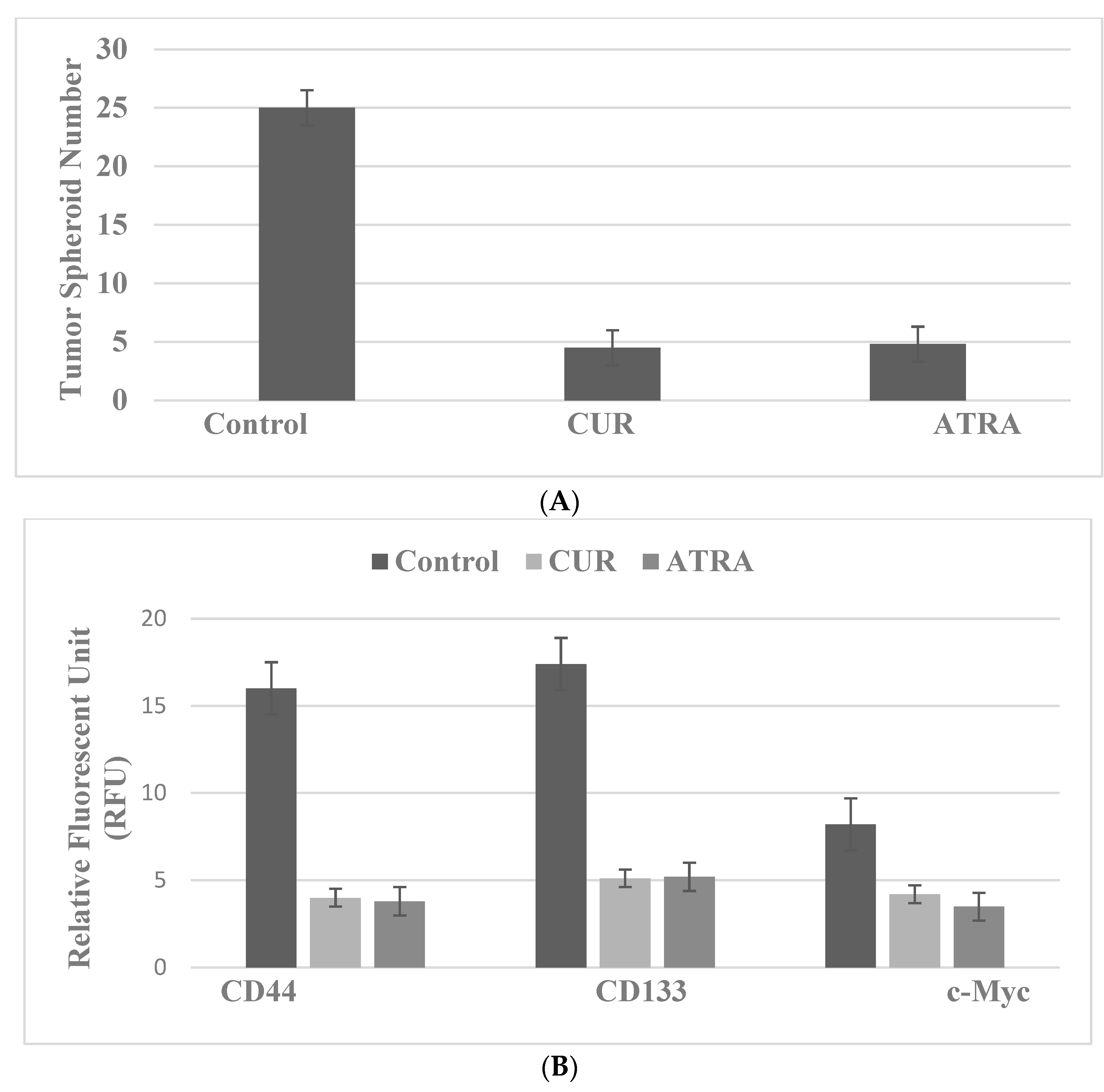
4.2. Colon
5. Stem Cell Models—Overview
6. Conclusions
7. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- National Comprehensive Cancer Network. 2010. Available online: http://www.nccn.org.NCCN (accessed on 10 April 2022).
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.-F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wicha, M.S.; Schwartz, S.J.; Sun, D. Implication of cancer stem theory for cancer chemoprevention by natural dietary compounds. J. Nutr. Biochem. 2011, 22, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Moy, B.; Goss, P.E. Estrogen receptor pathway: Resistance to endocrine therapy and new therapeutic approaches. Clin. Cancer Res. 2006, 12, 4790–4793. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. The role of estrogen in the initiation of breast cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Yue, W.; Wang, J.-P. Estrogen metabolites and breast cancer. Steroids 2015, 99, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Gustafson, J.-A. Estrogen signaling: A subtle balance between ER-α and ER-β. Mol. Interven. 2003, 3, 281–292. [Google Scholar] [CrossRef]
- Saji, S.; Hirose, M.; Toi, M. Clinical significance of estrogen receptor-β in breast cancer. Cancer Chemother. Pharmacol. 2005, 56 (Suppl. S1), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef]
- Heldering, N.; Pike, A.; Anderson, S.; Mathews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Stro, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 37, 905–935. [Google Scholar] [CrossRef]
- Cho, N.L.; Javid, S.H.; Carothers, A.M.; Redston, M.; Bertagnolli, M.M. Estrogen receptor α and β are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007, 67, 2366–2372. [Google Scholar] [CrossRef]
- Williams, C.; Di Leo, A.; Niv, Y.; Gustafson, J.-A. Estrogen receptor-β as a target for colorectal cancer prevention. Cancer Lett. 2016, 372, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Filho, R.P.S.; Junior, S.A.; Begnami, M.D.; Farrreira, F.O.; Nakagava, W.T. Estrogen receptor-β as a prognostic marker of tumor progression in colorectal cancer with familial adenomatous polyps and sporadic polyps. Pathol. Oncol. Res. 2018, 24, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell. Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Soteriou, D.; Fuchs, Y. A matter of life and death: Stem cell survival in tissue regeneration and tumor formation. Nat. Rev. Cancer 2018, 18, 187–201. [Google Scholar] [CrossRef]
- Lytle, N.K.; Barber, A.G.; Reya, T. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer 2018, 18, 669–680. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumor stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Patel, S.A.; Ndabahaliye, A.; Lim, P.K.; Milton, R.; Rameshwar, P. Challenges in the development of future treatments for breast cancer stem cells. Breast Cancer (Dove Med. Press) 2010, 2, 1–11. [Google Scholar] [CrossRef]
- Telang, N.; Li, G.; Katdare, M.; Sepkovic, D.; Bradlow, L.; Wong, G.Y.C. Inhibitory effects of Chinese nutritional herbs in isogenic breast carcinoma cells with modulated estrogen receptor function. Oncol. Lett. 2016, 12, 3949–3957. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Katdare, M.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y. The nutritional herb Epimedium grandiflorum inhibits the growth in a model for the Luminal A molecular subtype of breast cancer. Oncol. Lett. 2017, 13, 2477–2482. [Google Scholar] [CrossRef]
- Telang, N.T. The divergent effects of ovarian steroid hormones in the MCF-7 model for Luminal A breast cancer: Mechanistic leads for therapy. Int. J. Mol. Sci. 2022, 23, 4800. [Google Scholar] [CrossRef]
- Telang, N.T.; Nair, H.B.; Wong, G.Y.C. Growth inhibitory efficacy of Cornus officinalis in a cell culture model for triple-negative breast cancer. Oncol. Lett. 2019, 17, 5261–5266. [Google Scholar] [CrossRef] [PubMed]
- Telang, N.T.; Nair, H.B.; Wong, G.Y.C. Growth inhibitory efficacy of the nutritional herb Psoralea corylifolia in a model for triple-negative breast cancer. Int. J. Funct. Nutr. 2021, 2, 8. [Google Scholar] [CrossRef]
- Telang, N.T.; Nair, H.B.; Wong, G.Y.C. Growth inhibitory efficacy of Chinese herbs in a cellular model for triple-negative breast cancer. Pharmaceuticals 2021, 14, 1318. [Google Scholar] [CrossRef] [PubMed]
- Telang, N.; Nair, H.B.; Wong, G.Y.C. Anti-proliferative and pro-apoptotic effects of Dipsacus asperoides in a cellular model for triple-negative breast cancer. Arch. Breast Cancer 2022, 9, 66–75. [Google Scholar] [CrossRef]
- Telang, N. Stem cell models for genetically predisposed colon cancer (Review). Oncol. Lett. 2020, 20, 138. [Google Scholar] [CrossRef]
- Telang, N. Isolation and characterization of chemo-resistant stem cells from a mouse model of hereditary non-polyposis colon cancer. Stem Cells Cloning Adv. Appl. 2021, 14, 19–25. [Google Scholar] [CrossRef]
- Telang, N. Drug-resistant stem cells: Novel approach for colon cancer therapy. Int. J. Mol. Sci. 2022, 23, 2519. [Google Scholar] [CrossRef]
- Kundu, A.B.; Telang, N.T.; Banerjee, M.R. Binding of 7,12-dimethylbenz[α] anthracene to BALB/c mouse mammary gland DNA in organ culture. J. Natl. Cancer. Inst. 1978, 61, 465–469. [Google Scholar]
- Telang, N.T.; Banerjee, M.R.; Iyer, A.P.; Kundu, A.B. Neoplastic transformation of epithelial cells in whole mammary gland in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 5886–5890. [Google Scholar] [CrossRef]
- Garg, A.; Suto, A.; Osborne, M.P.; Gupta, R.C.; Telang, N.T. Expression of biomarkers for transformation in 7,12-dimethylbenz[α] anthracene-treated mammary epithelial cells. Int. J. Oncol. 1993, 3, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Telang, N.T.; Williams, G.M. Carcinogen-induced DNA damage and cellular alterations in F344 rat colon organ cultures. J. Natl. Cancer Inst. 1982, 68, 1015–1022. [Google Scholar] [PubMed]
- Telang, N.T.; Narayanan, R.; Bradlow, H.L.; Osborne, M.P. Coordinated expression of biomarkers for tumorigenic transformation in RAS-transfected mouse mammary epithelial cells. Breast Cancer Res. Treat. 1991, 18, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Telang, N.T.; Arcuri, P.; Granata, O.M.; Bradlow, H.L.; Osborne, M.P.; Castagnetta, L. Alterations of oestradiol metabolism myc oncogene-transfected mouse mammary epithelial cells. Br. J. Cancer 1998, 77, 1549–1554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pierce, J.H.; Arnstein, P.; DiMarco, E.; Artrip, J.; Fraus, M.H.; Lonardo, F.; DiFiorre, P.P.; Aaronson, A.A. Oncogenic potential of erbB2 in human mammary epithelial cells. Oncogene 1991, 6, 1189–1194. [Google Scholar] [PubMed]
- Zhai, Y.F.; Beittenmiller, H.; Wang, B.; Gould, M.N.; Oakley, C.; Esselman, E.; Welch, C.W. Increased expression of specific protein tyrosine phosphatases in human breast epithelial cells neoplastically transformed by the neu oncogene. Cancer Res. 1993, 53, 2273–2278. [Google Scholar]
- Park, I.H.; Zhou, R.; West, J.A.; Yabuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprograming of human somatic cells to pluripotency with defined factors. Nature 2008, 151, 141–146. [Google Scholar] [CrossRef]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stuart, R.; Slukvin, I.I.; Thompson, J.A. Human induced pluripotent cells free of vector and transgene sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef]
- Telang, N. Natural phytochemicals as testable therapeutic alternatives for HER-2-enriched breast cancer (Review). World Acad. Sci. J. 2020, 2, 19. [Google Scholar] [CrossRef]
- Ye, J.; Jia, Y.; Ji, K.-E.; Saunders, A.J.; Xue, K.; Ji, J.; Mason, M.D.; Jiang, W.G. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol. Lett. 2015, 10, 1240–1250. [Google Scholar] [CrossRef]
- Hong, M.; Tan, H.Y.; Li, S.; Cheung, F.; Wang, N.; Nagamatsu, T.; Feng, Y. Cancer stem cells: The potential targets of Chinese medicines and their active compounds. Int. J. Mol. Sci. 2016, 17, 893. [Google Scholar] [CrossRef]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoeffer, R.A.; Zeleva, V.; Siewertsz van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on triple-negative breast cancer: Current treatment strategies, unmet needs, and potential targets for future therapies. Cancer 2020, 12, 2392. [Google Scholar] [CrossRef]
- Manogaran, P.; Umapathy, D.; Karthikeyan, M.; Venkatachalam, K.; Singarvelu, A. Dietary phytochemicals as a potential source for targeting cancer stem cells. Cancer Investig. 2021, 39, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Meerson, A.; Khatib, S.; Mahjna, J. Natural products targeting cancer stem cells for augmenting cancer therapeutics. Int. J. Mol. Sci. 2021, 22, 13044. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; Mc Kee, D.L. The “big five” phytochemicals targeting the cancer stem cells: Curcumin, EGCG, sulforaphane, resveratrol, and genistein. Cur. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef] [PubMed]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Piero, K.; Mohamodian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal plants in the treatment and prevention of colon cancer. Oxidat. Med. Cell. Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-Y.; Hong, S.; Shin, S.; Kim, Y.A.; Kim, N.S.; Bang, O.-S. A new herbal formula BP10A exerted an anti-tumor effect and enhanced anti-cancer effects of irinotecan and oxaliplatin in the colon cancer PDTX model. Biomed. Pharmacother. 2019, 116, 108987. [Google Scholar] [CrossRef]
- Li, Y.; Pu, R.; Zhou, L.; Wang, D.; Li, X. Effects of a chlorogenic acid-containing herbal medicine (LAS NB) on colon cancer. Evid. Based Complement Alt. Med. 2021, 2021, 9923467. [Google Scholar]
- Kouri, J.; Zhong, L.; Hao, J. Targeting signaling pathways in cancer stem cells for cancer treatment. Stem Cell Int. 2017, 2017, 2925869. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Nunes, T.; Hamdan, D.; Leboeuf, C.; El Boutachtaoui, M.; Gapihan, G.; Ngyuen, T.T.; Meles, S.; Angeli, E.; Ratajczak, P.; Lu, H.; et al. Targeting cancer stem cells to overcome chemo-resistance. Int. J. Mol. Sci. 2018, 19, 4036. [Google Scholar] [CrossRef]
- Gooding, A.J.; Schiemann, W.P. Epithelial-mesenchymal transition programs and cancer stem cell phenotypes: Mediators of breast cancer therapy resistance. Mol. Cancer Res. 2020, 18, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Kudaravalli, S.; den Hollander, P.; Mani, S.A. Role of p38 MAP kinase in cancer stem cells and metastasis. Oncogene 2022, 41, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Keeton, A.B.; Salter, A.E.; Piaza, G.A. The RAS-effector interaction as a drug target. Cancer Res. 2017, 77, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Solit, D.B. Overcoming adaptive resistance to KRAS inhibitors through vertical pathway targeting. Clin Cancer Res. 2020, 26, 1538–1540. [Google Scholar] [CrossRef]
- Shetu, S.A.; Bandyopadhyay, D. Small-molecule RAS inhibitors as anticancer agents: Discovery, development and mechanistic studies. Int. J. Mol. Sci. 2022, 23, 3706. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Yu, R. Lycopene inhibits epithelial-mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m-TOR pathway. Drug Design Develop. Ther. 2020, 14, 2461–2471. [Google Scholar] [CrossRef]
- Liu, L.; Yan, B.; Cao, Y.; Yan, Y.; Shen, X.; Yu, B.; Tao, L.; Wang, S. Proliferation, migration and invasion of triple-negative breast cancer cells are suppressed by berbamine via PI3K/AKT/MDM2/p53 and PI3K/AKT/m-TOR signaling pathways. Oncol. Lett. 2021, 21, 70. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Q.; Yu, L.; Zhu, J.; Cao, Y.; Gao, X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2021, 264, 113249. [Google Scholar] [CrossRef]
- Kanazaki, H.; Chatterjee, H.; Nejad, H.H.; Ari Kanazaki, H.; Chatterjee, H.; Nejad, H.H.; Ariani Zhang, X.; Chung, S.; Deng, N.; Ramanujan, V.K.; et al. Disabling nuclear translocation of RelA/NF-kB by small molecule inhibits triple-negative breast cancer growth. Breast Cancer Targets Ther. 2021, 13, 419–430. [Google Scholar] [CrossRef]
- Armaghani, A.J.; Han, H.S. Alpesilib in the treatment of breast cancer: A short review on emerging clinical data. Breast Cancer Targets Ther. 2020, 12, 251–258. [Google Scholar] [CrossRef]
- Fragiadaki, P.; Ranieri, E.; Kalliantasi, K.; Kouvidi, E.; Apalaki, E.; Vakonaki, E.; Mamoulakis, C.; Spandidos, D.A.; Tsatsakis, A. Telomerase inhibitors and activators: A systematic review. Mol. Med. Rep. 2022, 25, 158. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; Rueda, O.M.; Greenwood, W.; Batra, A.S.; Callari, M.; Batra, R.N.; Pogrebniak, K.; Sandoval, J.; Cassidy, J.W.; Tufegdzic-Vidakovic, A.; et al. A biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anti-cancer compounds. Cell 2016, 167, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; De Ligt, J.; Kopper, O.; Gogola, E.; Buovnova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 2018, 172, 373–386. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chan, W.C. Use of conditional reprogramming cell, patient derived xenograft and organoid for drug screening for individualized prostate cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2022, 60, 52. [Google Scholar] [CrossRef]
- Hsu, K.-S.; Adileh, M.; Martin, M.L.; Makarov, V.; Chen, J.; Wu, C.; Bodo, S.; Klinger, S.; Gabriel, C.-E.; Szeglin, B.C.; et al. Colorectal cancer develops inherent radio-sensitivity that can be predicted using patient-derived organoids. Cancer Res. 2022, 82, 2298–2312. [Google Scholar] [CrossRef]
- Devall, M.A.M.; Drew, D.A.; Dampier, C.H.; Plummer, S.J.; Eaton, S.; Bryant, J.; Diez-Obrero, V.; Mo, J.; Kedrin, D.; Zerjav, D.C.; et al. Transcriptome-wide in vitro effects of aspirin in patient-derived normal colon organoids. Cancer Prev. Res. (Phila) 2021, 14, 1089–1100. [Google Scholar] [CrossRef]
- Crespo, M.; Villar, E.; Tsai, S.Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef]
- Gavert, N.; Zwang, Y.; Weiser, R.; Greenberg, O.; Halperin, S.; Jacoby, O.; Mallel, G.; Sandler, O.; Berger, A.J.; Stossel, E.; et al. Ex vivo organotypic cultures for synergistic therapy prioritization identify patient-specific responses to combined MEK and Src inhibition in colorectal cancer. Nat. Cancer 2022, 3, 219–231. [Google Scholar] [CrossRef]

| Model | Molecular Characteristics | Origin | Clinical Subtype | ||
|---|---|---|---|---|---|
| Breast | ER | PR | HER-2 | ||
| 184-B5 | - | - | - | Reduction mammoplasty | Female normal breast |
| MCF-7 | + | + | - | Female breast carcinoma | Luminal A |
| 184-B5/HER | - | - | + | HER-2 positive | HER-2-enriched |
| MDA-MB-231 | - | - | - | Female breast carcinoma | TNBC |
| Colon | Apc | Mlh1 | |||
| C57 COL | +/+ | +/+ | Female mouse colonic epithelium | Normal colon | |
| 850MIN COL | +/− | +/+ | Female mouse Apc codon 850 mutation | FAP | |
| Mlh1/1638N COL | +/− | −/− | Female mouse Apc codon 1638N mutation, Mlh1 allelic deletion | HNPCC | |
| HCT-116 | Apc | β-Cat. | Somatic mutation | Male sporadic colon cancer | |
| WT | MT | ||||
| SW480 | MT | WT | Somatic mutation | Male sporadic colon cancer | |
| Endpoint | Cellular Model | |||
|---|---|---|---|---|
| 184-B5 | MCF-7 | 184-B5/HER | MDA-MB-231 | |
| AI Colony Formation | 0/18 | 18/18 | 18/18 | 18/18 |
| Incidence | 0% | 100% | 100% | 100% |
| Colony Number | ND | 30.9 ± 2.4 | 23.0 ± 2.6 | 38.9 ± 1.6 |
| Tumor Formation | 0/10 | 10/10 | 10/10 | 10/10 |
| Incidence | 0% | 100% | 100% | 100% |
| Latent Period (weeks) | 24 | 3–5 | 3–5 | 3–5 |
| Endpoint | Cellular Model | ||
|---|---|---|---|
| C57COL | 850MINCOL | Mlh1/1638COL | |
| AI Colony Formation | 0/18 | 18/18 | 16/18 |
| Incidence | 0% | 100% | 88.9% |
| Colony Number | ND | 18.9 ± 2.5 | 15.2 ± 1.4 |
| Tumor Formation | 0/10 | 8/10 | 6/10 |
| Incidence | 0% | 80% | 60% |
| Latent Period (weeks) | 24 | 3–5 | 3–5 |
| Agent | Type | Molecular Target | Stem Cell Model |
|---|---|---|---|
| Breast | |||
| TAM | SERM | ER | MCF-7 TAM-R, Luminal A |
| LAP | Small-molecule inhibitor | EGFR, HER/2 | 184-B5/HER LAP-R, HER-2-enriched |
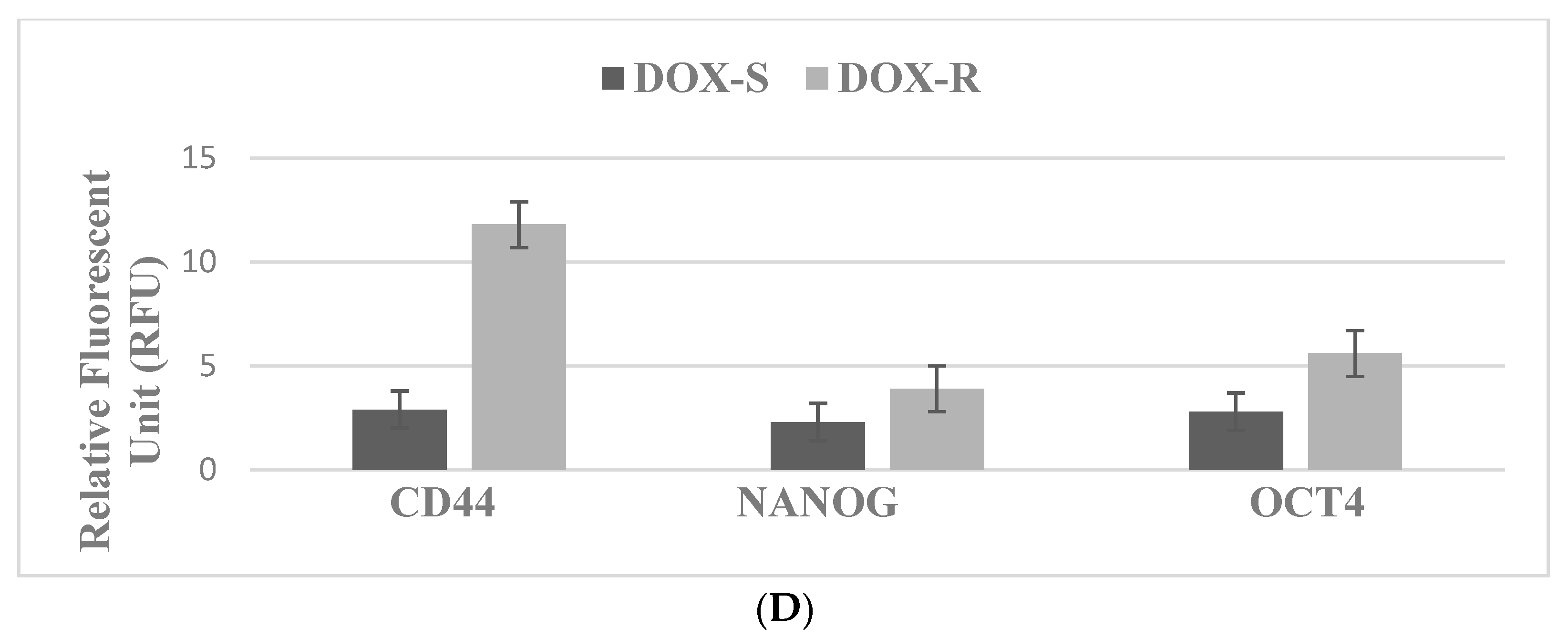
| DOX | DNA inhibitor | S-phase inhibition | MDA-MD-231, DOX-R, TNBC |
| Colon | |||
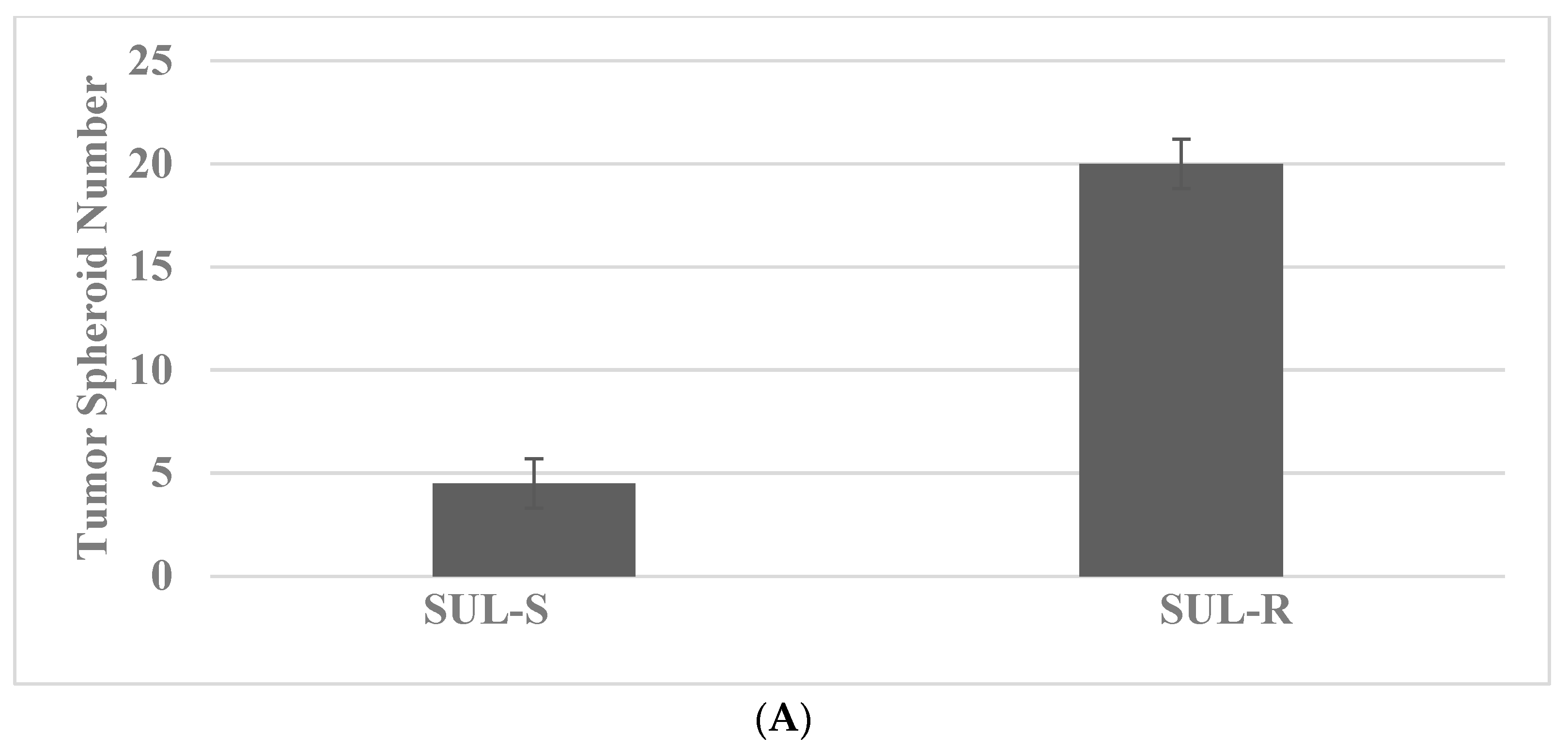
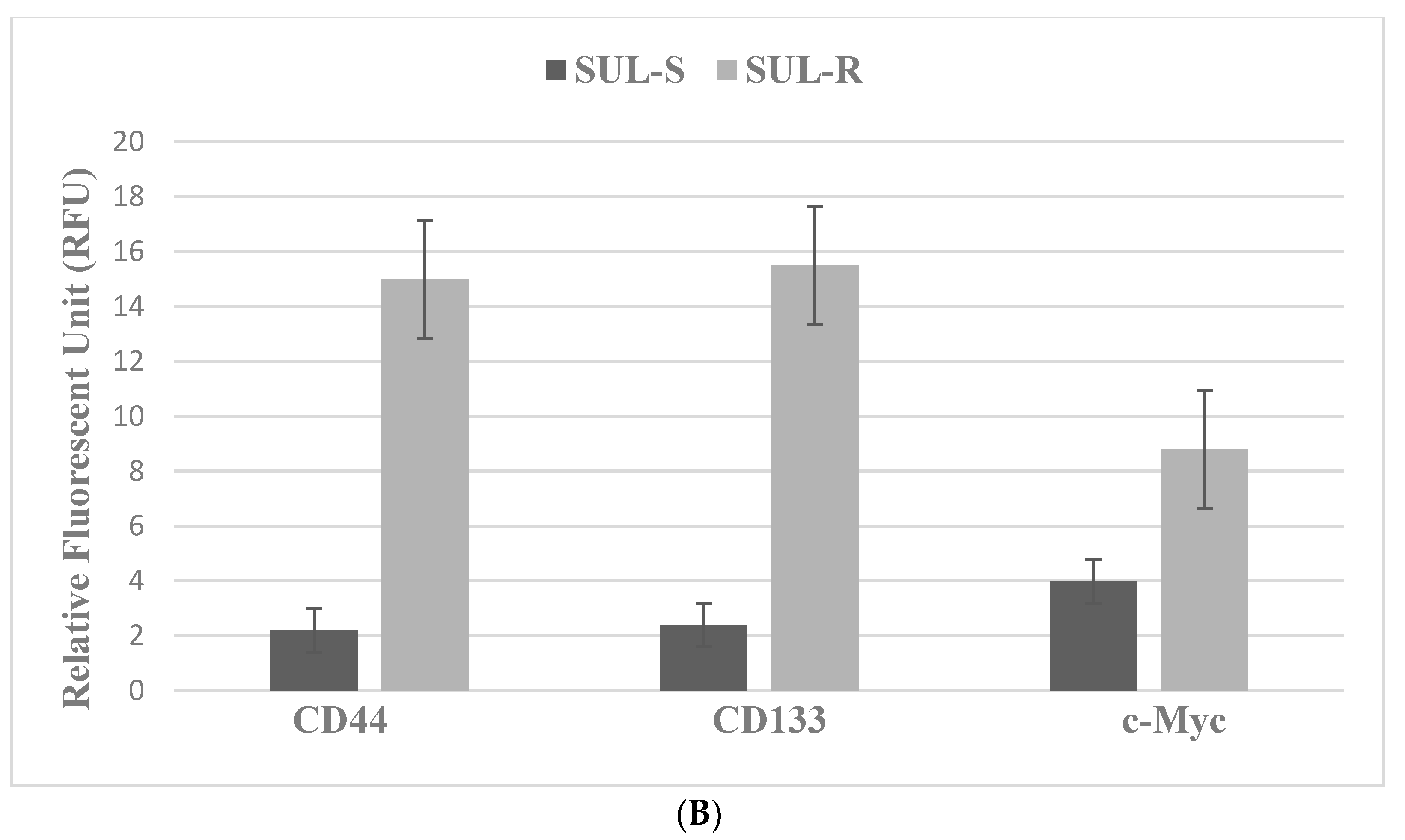
| SUL | NSAID | COX-1. COX-2 | 850MINCOL SUL-R, FAP |
| 5-FU | DNA inhibitor | S-phase inhibition | Mlh1/1638N COL, 5-FU-R, HNPCC |
| Therapeutic Options | Stem Cell Models | Testable Alternatives |
|---|---|---|
| Breast | ||
| Multi-drug chemotherapy | Drug resistance | Dietary phytochemicals, nutritional herbs, micro-nutrients |
| Endocrine therapy | TAM-R, LAP-R, DOX-R | LAP-R: ATRA, CSOL |
| Molecular-targeted therapy | Markers: TS, CD44, NANOG, OCT-4 | Marker downregulation |
| Selective inhibitors: SERM, SERD, AI, PI3K, AKT, m TOR | ||
| Colon | ||
| Multi-drug chemotherapy | Drug resistance | |
| Molecular-targeted therapy | SUL-R, 5-FU-R | SUL-R: CUR, EGCG, EPA, DHA, CA, ATRA |
| Selective inhibitors: EGFR, ODC, COX-2, NSAID | Markers: TS, CD44, CD133, c-Myc | Marker downregulation |
| Limitations: Systemic toxicity, therapy resistance, drug-resistant stem cell population | Advantages: Documented human consumption, low systemic toxicity | |
| Future research: PDTX, PDTO, stem cell models for clinical cancer | Future research: Bioactive constituents from nutritional herbs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telang, N. Stem Cell Models for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 7055. https://doi.org/10.3390/ijms23137055
Telang N. Stem Cell Models for Cancer Therapy. International Journal of Molecular Sciences. 2022; 23(13):7055. https://doi.org/10.3390/ijms23137055
Chicago/Turabian StyleTelang, Nitin. 2022. "Stem Cell Models for Cancer Therapy" International Journal of Molecular Sciences 23, no. 13: 7055. https://doi.org/10.3390/ijms23137055
APA StyleTelang, N. (2022). Stem Cell Models for Cancer Therapy. International Journal of Molecular Sciences, 23(13), 7055. https://doi.org/10.3390/ijms23137055






