Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops
Abstract
1. Introduction
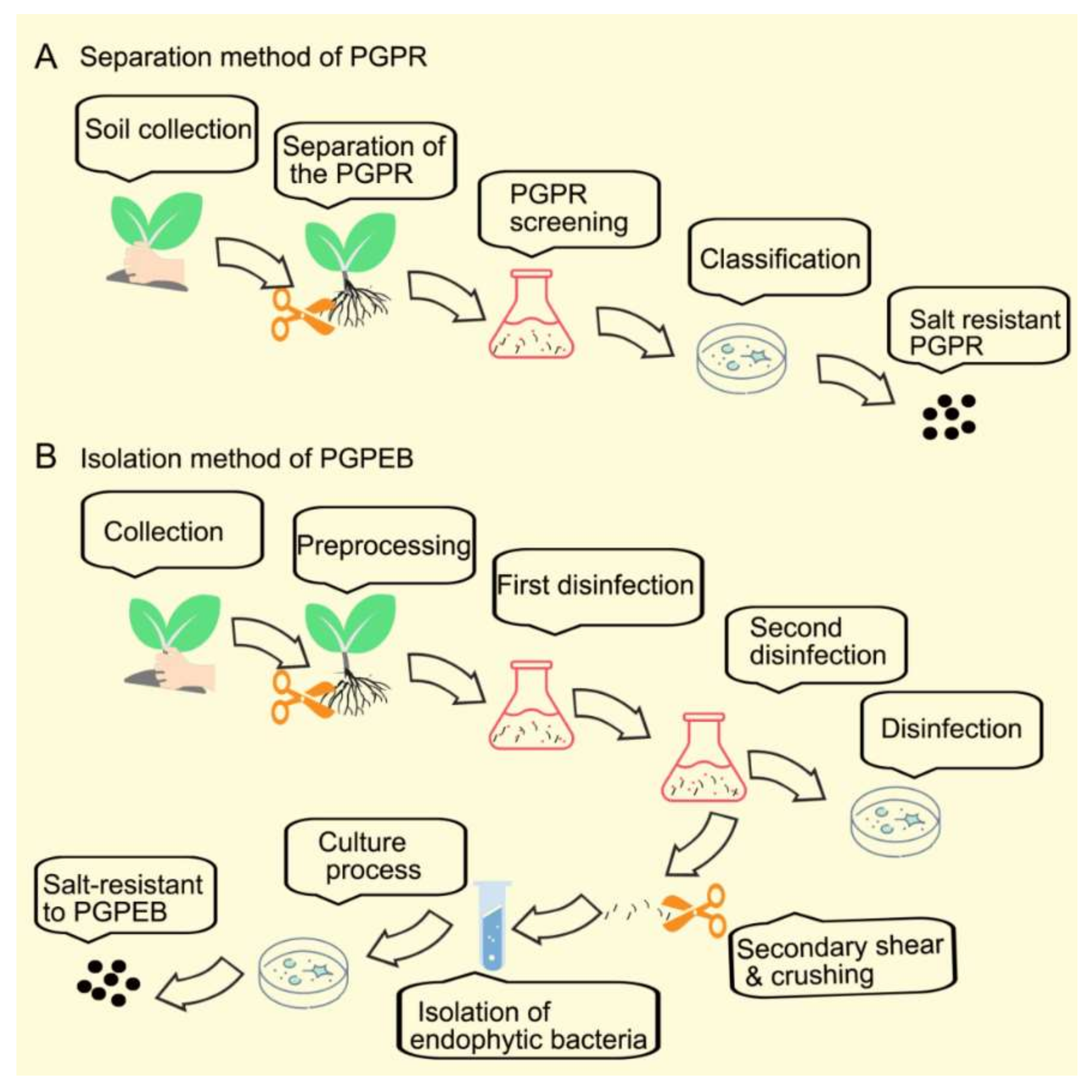
2. Summary of Plant Growth-Promoting Bacteria Isolation Procedures
3. Growth-Promoting Bacteria Related to Salt Stress Currently Found in Plants
| Plant Species | PGPR Species | Effect or Mechanism | Reference |
|---|---|---|---|
| Avena sativa | Klebsiella sp. | Regulating ion contents and proline levels | [27] |
| Barley (Hordeum vulgare) | Curtobacterium sp. | Regulating proline content | [28] |
| Hartmannibacter diazotrophicus | Enhancing ACC deaminase activity | [29] | |
| Maize (Zea mays) | Bacillus atrophaeus | Relieving salt stress | [30] |
| Azotobacter sp. | Promoting nutrient absorption in plants | [31] | |
| Bacillus amyloliquefaciens SQR9 | Enhancing antioxidant enzyme activity and increasing the expression of salt-stress-response genes | [32] | |
| Bacillus sp. | Increasing enzyme activities and proline and soluble sugar contents under salt stress; regulating ACC deaminase activity | [33,34] | |
| Enterobacter cloacae PM23 | Modulating plant physiology, antioxidant defense, compatible solute accumulation, and bio-surfactant-producing genes | [35] | |
| Geobacillus sp. | Regulating proline content | [36] | |
| Pseudomonas sp. | Enhancing proline and IAA content and EPS production | [37] | |
| Rhizobium sp. | Regulating pigment biosynthesis | [38] | |
| Rice (Oryza sativa) | Bacillus aryabhattai, Achromobacter denitrificans, and Ochrobactrum intermedium | Relieving salt stress | [39,40] |
| Bacillus pumilus and Pseudomonas pseudoalcaligenes | Increasing the absorption of nutrients | [41] | |
| Enterobacter sp. | Reducing ethylene production | [42] | |
| Glutamicibacter sp. YD01 | Adjusting ethylene contents | [43] | |
| Micrococcus sp. | Increasing IAA levels | [44] | |
| Rhizobacteria pseudomonas | Regulating ACC deaminase activity | [40] | |
| Wheat (Triticum aestivum) | Aeromonas sp. | Regulating ethylene content and alleviating salt stress | [45] |
| Arthrobacter sp. | Maintaining plant nutrient absorption | [46] | |
| Bacillus sp. | Regulating ACC deaminase activity | [47,48] | |
| Enterobacter sp. | Reducing ethylene production | [49] | |
| Microbacterium sp. | Regulating K+ content | [45] | |
| Planococcus rifietoensis | Regulating phosphate production and ACC deaminase activity | [50] | |
| Pseudomonas fluorescence, Bacillus pumilus, and Exiguobacterium aurantiacum | Adjusting osmotic substances | [51] | |
| Klebsiella sp. | Regulating ion contents, proline levels, and antioxidant enzyme activity | [52,53,54] | |
| Serratia sp. | Production of exopolysaccharides | [49,55] | |
| Serratia marcescens CDP-13 | Enhancing ACC deaminase activity and reducing salt-induced oxidative damage | [55] | |
| Arabidopsis thaliana | Acillus atropheus | Relieving salt stress | [30] |
| Bacillus sp. | Adjusting ACC deaminase activity | [56] | |
| Enterobacter sp. | Reducing ethylene production by promoting ACC deaminase activity | [37,57] | |
| Enterobacter sp. SA187 | Enhancing sulfur metabolism | [58] | |
| Micrococcus sp. | Increasing IAA levels | [44] | |
| Arachis hypogaea L. | Brachybacterium sp. | Regulating K+ content | [59] |
| Brevibacterium sp. | Regulating K+ content | [59] | |
| Haererohalobacter sp. | Regulating K+ content | [59] | |
| Ochrobactrum sp. | Regulating IAA levels and ACC deaminase activity | [60] | |
| Stenotrophomonas maltophilia BJ01 | Modulating physiology and biochemical activities | [61] | |
| Casuarina obesa (Miq.) | Pantoea agglomerans and Bacillus sp. | Increasing total chlorophyll production and proline accumulation | [62] |
| Codonopsis pilosula | Bacillus sp. | Adjusting ACC deaminase activity | [63] |
| Common bean (Phaseolus vulgaris) | Aneurinibacillus Aneurinilyticus and Paenibacillus sp. | Adjusting ACC deaminase activity | [64] |
| Cucumber (Cucumis sativus) | Bacillus sp. | Adjusting ACC deaminase activity | [65] |
| Burkholdera sp. | Maintaining the water balance and regulating photosynthetic pigment content | [8] | |
| Strawberry (Fragaria ananassa) | Kocuria sp. | Maintaining phosphate | [66] |
| Cotton (Gossypium hirsutum) | Pseudomonas sp. | Enhancing proline, IAA, and EPS content production | [67] |
| Helianthus Annuus L. | Azospirillum sp. | Regulating chlorophyll content and improving photosynthesis | [68] |
| Lens esculenta | Oceanobacillus sp. | Production of exopolysaccharides | [69] |
| Lettuce (Lactuca sativa) | Pseudomonas mendocina Palleroni, arbuscular mycorrhizal (AM) fungus | Improving antioxidase activity | [70] |
| Limonium sinense | Streptomyces sp. | Enhancing proline production | [71] |
| Medicago cilitaris | Sinorhizobium sp. | Promoting proline production | [72] |
| Mentha arvensis | Exiguobacterium sp. | Production of exopolysaccharides | [73] |
| Pistacia vera L. | Arthrobacter endophyticus,Zobellella denitrificans and Staphylococcus sciuri | Improving photosynthesis | [74] |
| Pea (Pisum sativum) | Arthrobacter sp. | Increasing nutrient uptake | [75] |
| Rhizobium sp. | Regulating pigment synthesis | [75] | |
| Variovorax sp. | Enhancing ACC deaminase activity | [76] | |
| Radish (Raphanus sativus) | Lactobacillus sp., P. putida, and Azotobacter chroococcum | Mitigating salinity stress at the time of germination | [77] |
| Sesuvium portulacastrum | Halobacillus sp. | Production of ammonia and cyanide (HCN) | [78] |
| Silybum marianum | Pseudomonas sp. | Enhancing proline and IAA content and EPS production | [79] |
| Soybean (Glycine max) | Arthrobacter woluwensis, Microbacterium oxydans, Arthrobacter aurescens, Bacillus megaterium, and Bacillus aryabhattai | Maintaining osmotic balance and regulating salt tolerance | [80] |
| Tomato (Solanum lycopersicum) | Achromobacter sp. | Adjusting ethylene content | [81] |
| Enterobacter sp. | Reducing ethylene production | [57] | |
| Growth-promoting rhizobacteria | Relieving water stress and increasing K+ absorption | [82] | |
| Leclercia adecarboxylata MO1 | Promoting the production of IAA and ACC | [83] | |
| Sphingomonas sp. | Exopolysaccharides and proline production | [84,85] | |
| Vigna radiata L. | Enterococcus sp. | Reducing sodium uptake | [86] |
| Pantoea sp. | Improving ACC deaminase activity | [86] | |
| Rhizobium sp. | Increasing chlorophyll and photosynthesis | [87] |
4. The Mechanism of PGPR in Improving Stress Tolerance
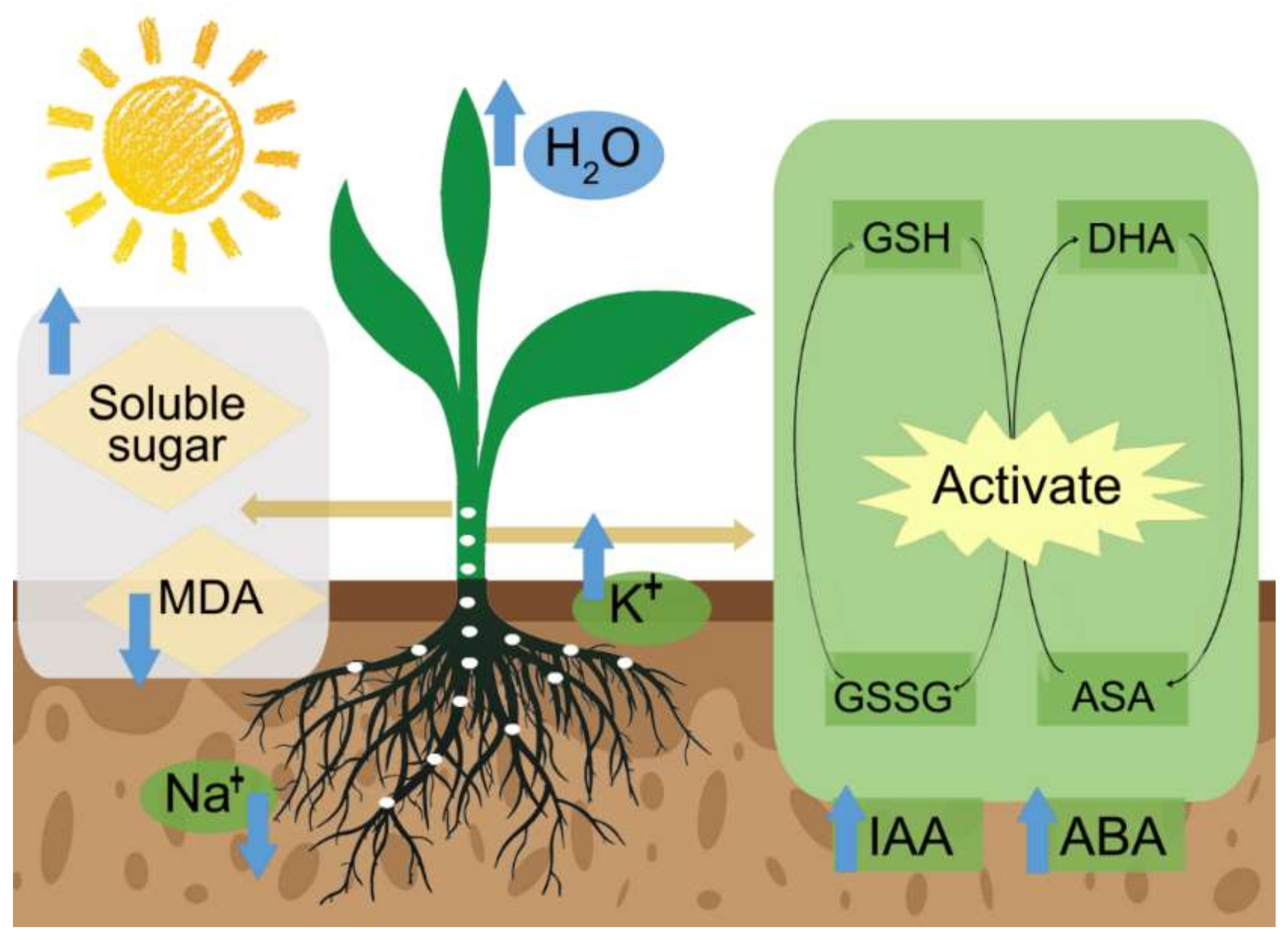
4.1. Inducing the Antioxidant System
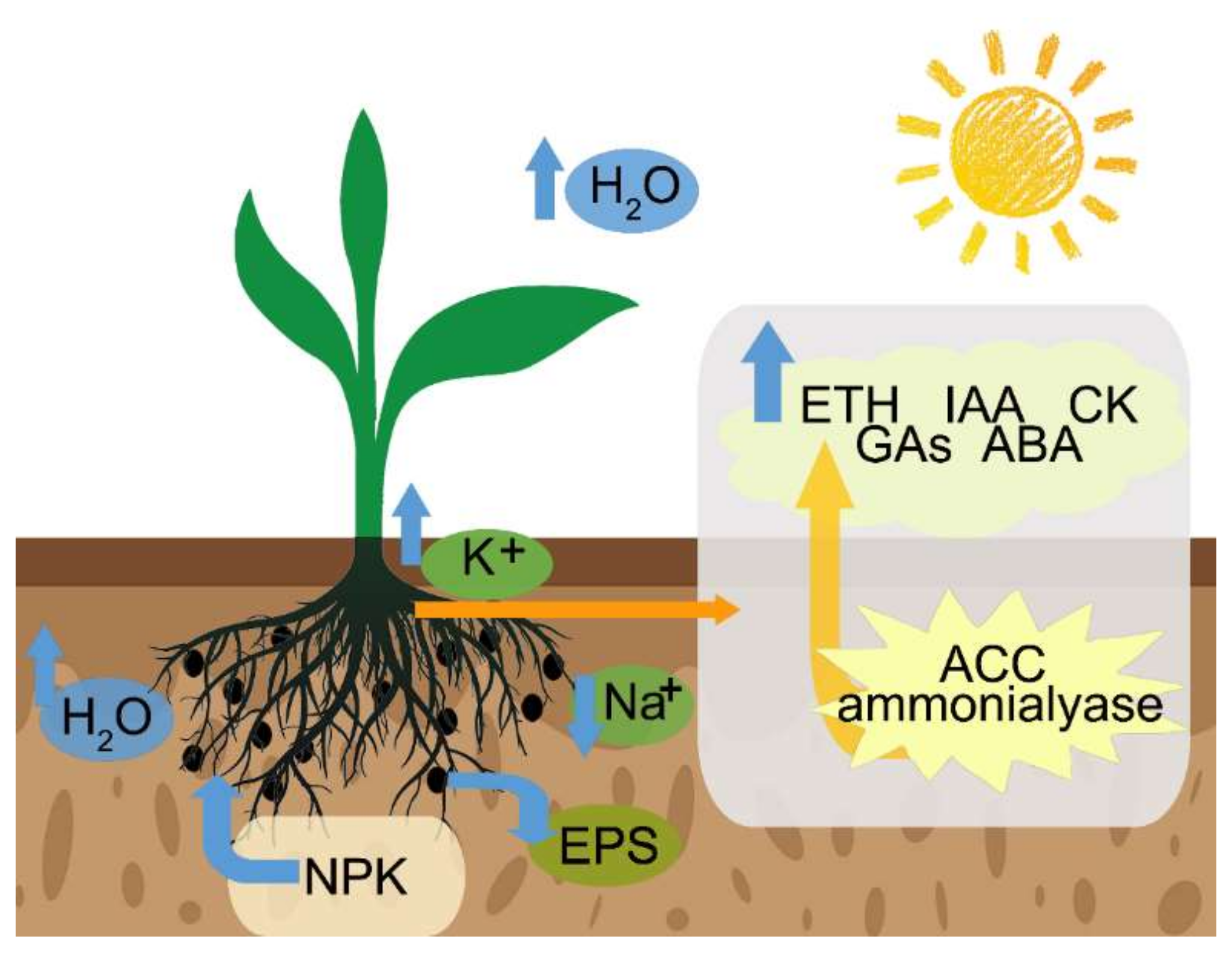
4.2. Maintaining the Water Balance and Access to Nutrients
4.3. Maintaining Ion Homeostasis
4.4. Production of Exopolysaccharides
4.5. Induction of Plant Hormones
4.6. Increasing Osmotic Substances
5. The Role of PGPEB in Alleviating Salt Stress
| Plant Species | PGPEB Species | Effect or Mechanism | Reference |
|---|---|---|---|
| Cape (Aloe ferox Mill) | Achromobacter xylosoxidans | Enhancing ACC deaminase activity | [156] |
| Millet (Pennisetum glaucum) | Bacillus subtilis, Bacillus cereus, and Bacillus amyloliquefaciens | Participating in ACC deaminase synthesis and enhancing IAA content | [157] |
| Onion (Allium cepa) | Bacillus subtilis, Bacillus megaterium, and Burkholderia phytofirmans | Participating in ACC deaminase synthesis and enhancing IAA content | [158,159] |
| Rice (Oryza sativa) | Pantoea ananatis | Enhancing IAA content and siderophore production | [160] |
| Sugarcane (Saccharum officinarum) | Gluconacetobacter diazotrophicus | Enhancing IAA content and nitrogen fixation | [161,162] |
| Wheat (Triticum aestivum) | Paraburkholderia, phytofirmans, and Bacillus cabrialessi | Recovery of nitrogen, phosphorus, and potassium | [163,164] |
| Arabidopsis thaliana | Serratia proteamaculans Para and burkholderia phytofirmans | Enhancing IAA content and enhancing ACC deaminase activity | [165,166] |
| Arachis hypogaea | Chryseobacterium indologenes, Enterobacter cloacae, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterobacter ludwigii | Nitrogen fixation, enhancing IAA content and ACC deaminase production, siderophore production, and phosphate solubilization | [167] |
| Cotton (Gossypium hirsutum) | Pantoea spp., Empedobacter spp., Enterobacter spp., Rhizobium spp., and Klebsiella spp. | Adjusting ACC deaminase activity | [168,169] |
| Dodonaea viscosa | Streptomyces alboniger, Bacillus idriensis, Pseudomonas taiwanensis, and Pseudomonas geniculate | Siderophore production, phosphate solubilization, enhancing IAA content and ACC deaminase production | [170] |
| Helianthus Annuus L. | Stentotrophomonas indicatrix | Enhancing IAA content, phosphate solubilization, siderophore and secondary metabolite synthesis | [171] |
| Poplar (Populus) | Stenotrophomonas maltophilia, and Pseudomonas putida | Enhancing IAA content and ACC deaminase synthesis | [172] |
| Potato (Solanum tuberosum) | Klebsiella oxytoca, Pseudomonas marginalis, Pseudomonas Viridilivida, Bacillus endophyticu, and Bacillus atrophaeus | Nitrogen fixation and phosphatase production | [173,174] |
| Soybean (Glycine max) | Bradyrhizobium japonicum | Enhancing IAA content and ACC deaminase production, nitrogen fixation | [172,175] |
| Tomato (Solanum lycopersicum) | Pseudomonas fluorescens and Pseudomonas migulae | Enhancing IAA content and ACC deaminase synthesis | [176,177] |
6. Future Perspectives
6.1. Halophytes Can Be Used to Identify Rhizosphere Bacteria
6.2. Methods to Improve Symbiont Bacteria Utilization
6.3. Challenges in Applying Symbiotic Bacteria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.A.; Akram, W.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A. Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ. Sci. Pollut. Res. Int. 2018, 25, 23236–23250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Pu, L.J.; Han, M.F.; Zhu, M.; Zhang, R.S.; Xiang, Y.Z. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Munns, R. A Leaf Elongation Assay Detects an Unknown Growth Inhibitor in Xylem Sap from Wheat and Barley. Funct. Plant Biol. 1992, 19, 127–135. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Bird, P.R.; Jackson, T.T.; Kearney, G.A.; Saul, G.R.; Waller, R.A.; Whipp, G. The effect of improved pastures and grazing management on soil water storage on a basaltic plains site in south-west Victoria. Aust. J. Exp. Agric. 2004, 44, 559–569. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Ma, D. Construction and Engineering Application of Salt-Discharging Model for Local Saline-Alkali Soil with Compact Structure in the Yellow River Delta. Appl. Environ. Soil Sci. 2020, 2020, 2906747. [Google Scholar] [CrossRef]
- Xue, B.X.; Zhao, Z.Q.; Wei, L.; Li, T.Y.; Kang, X.F. Study of the comprehensive landscaping treatment technique of saline-alkali land in Daqing based on the network of the grading of trapezoidal terrace ditches. Desalination Water Treat. 2014, 52, 1183–1192. [Google Scholar] [CrossRef]
- Banin, A.; Fish, A. Secondary desertification due to salinization of intensively irrigated lands: The Israeli experience. Environ. Monit. Assess. 1995, 37, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Wu, D.; Sun, P.; Lu, P.-Z.; Chen, Y.-Y.; Guo, J.-M.; Liu, M.; Wang, L.; Zhang, C.-J. Effect and Approach of Enteromorpha prolifera Biochar to Improve Coastal Saline Soil. Huan Jing Ke Xue 2020, 41, 1941–1949. [Google Scholar] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.; Ozturk, M.; Fujita, M. Potential Use of Halophytes to Remediate Saline Soils. BioMed Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Príncipe, A.; Alvarez, F.; Castro, M.G.; Zachi, L.; Fischer, S.E.; Mori, G.B.; Jofré, E. Biocontrol and PGPR Features in Native Strains Isolated from Saline Soils of Argentina. Curr. Microbiol. 2007, 55, 314–322. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Wu, G.H.; Tian, C.Y. Isolation of Endophytic Plant Growth-Promoting Bacteria Associated with the Halophyte Salicornia europaea and Evaluation of their Promoting Activity Under Salt Stress. Curr. Microbiol. 2016, 73, 574–581. [Google Scholar] [CrossRef]
- Jha, B.; Gontia, I.; Hartmann, A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, Y.; Hao, C.; Tan, Y. Effect of Salt Solution on Characteristics of Soil Infiltration. Agric. Sci. Technol. 2012, 357–360, 438. [Google Scholar]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Patel, T.; Saraf, M. Biosynthesis of phytohormones from novel rhizobacterial isolates and their in vitro plant growth-promoting efficacy. J. Plant Interact. 2017, 12, 480–487. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef]
- Cardinale, M.; Ratering, S.; Suarez, C.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S. Paradox of plant growth promotion potential of rhizobacteria and their actual promotion effect on growth of barley (Hordeum vulgare L.) under salt stress. Microbiol. Res. 2015, 181, 22–32. [Google Scholar] [CrossRef]
- Suarez, C.; Cardinale, M.; Ratering, S.; Steffens, D.; Jung, S.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S. Plant growth-promoting effects of Hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) under salt stress. Appl. Soil Ecol. 2015, 95, 23–30. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Bouket, A.C.; Cherif-Silini, H.; Eshelli, M.; Rabhi, N.E.H.; Belbahri, L. Mitigation of NaCl Stress in Wheat by Rhizosphere Engineering Using Salt Habitat Adapted PGPR Halotolerant Bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Hamdia, A.B.E.; Shaddad, M.A.K.; Doaa, M.M. Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 2004, 44, 165–174. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.V.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef]
- Li, H.Q.; Jiang, X.W. Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russ. J. Plant Physiol. 2017, 64, 235–241. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib Un, N.; Ullah, I.; do Amaral Junior, A.T.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Abdelkader, A.F.; Esawy, M.A. Case study of a biological control: Geobacillus caldoxylosilyticus (IRD) contributes to alleviate salt stress in maize (Zea mays L.) plants. Acta Physiol. Plant 2011, 33, 2289–2299. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M. Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol. 2009, 55, 1302–1309. [Google Scholar] [CrossRef]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fert. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Sultana, S.; Paul, S.C.; Parveen, S.; Alam, S.; Rahman, N.; Jannat, B.; Hoque, S.; Rahman, M.T.; Karim, M. Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 2020, 66, 144–160. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B.; Patel, S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant 2011, 33, 797–802. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. Paddy plants inoculated with PGPR show better growth physiology and nutrient content under saline conditions. Chil. J. Agric. Res. 2013, 73, 213–219. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.Z.; Guan, C.F. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant 2020, 42, 42. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Kim, Y.J.; Choi, E.S.; Koh, S.C.; Lee, S.W.; Kim, Y.J.; Yang, D.C. Paenibacillus yonginensis DCY84(T) induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015, 172, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fert. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, P.; Tiwari, R.; Meena, K.K.; Yandigeri, M.; Singh, D.P.; Arora, D.K. Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biol. Fert. Soils 2011, 47, 907–916. [Google Scholar] [CrossRef]
- Pourbabaee, A.A.; Bahmani, E.; Alikhani, H.A.; Emami, S. Promotion of Wheat Growth under Salt Stress by Halotolerant Bacteria Containing ACC deaminase. J. Agric. Sci. Technol. 2016, 18, 855–864. [Google Scholar]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2013, 2, 6. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Nawaz, S. Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann. Microbiol. 2013, 63, 225–232. [Google Scholar] [CrossRef]
- Rajput, L.; Imran, A.; Mubeen, F.; Hafeez, F.Y. Salt-Tolerant Pgpr Strain Planococcus Rifietoensis Promotes the Growth and Yield of Wheat (Triticum Aestivum L.) Cultivated in Saline Soil. Pak. J. Bot. 2013, 45, 1955–1962. [Google Scholar]
- Nawaz, A.; Shahbaz, M.; Assadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of Salt Tolerant PGPR in Growth and Yield Augmentation of Wheat (Triticum aestivum L.) under Saline Conditions. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Jha, P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomonas maltophilia SBP-9 Augments Resistance against Biotic and Abiotic Stress in Wheat Plants. Front. Microbiol. 2017, 8, 01945. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. Analysis of fatty acid composition of PGPR Klebsiella sp SBP-8 and its role in ameliorating salt stress in wheat. Symbiosis 2017, 73, 213–222. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The Multifarious PGPR Serratia marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Real, F.; Pouchelet, M.; Rabinovitch, M. Leishmania (L.) amazonensis: Fusion between parasitophorous vacuoles in infected bone-marrow derived mouse macrophages. Exp. Parasitol. 2008, 119, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of Salt Stress by Enterobacter sp EJ01 in Tomato and Arabidopsis Is Accompanied by Up-Regulation of Conserved Salinity Responsive Factors in Plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Andres-Barrao, C.; Alzubaidy, H.; Jalal, R.; Mariappan, K.G.; de Zelicourt, A.; Bokhari, A.; Artyukh, O.; Alwutayd, K.; Rawat, A.; Shekhawat, K.; et al. Coordinated bacterial and plant sulfur metabolism in Enterobacter sp. SA187-induced plant salt stress tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107417118. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved Salinity Tolerance of Arachis hypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Paulucci, N.S.; Gallarato, L.A.; Reguera, Y.B.; Vicario, J.C.; Cesari, A.B.; de Lema, M.B.G.; Dardanelli, M.S. Arachis hypogaea PGPR isolated from Argentine soil modifies its lipids components in response to temperature and salinity. Microbiol. Res. 2015, 173, 1–9. [Google Scholar] [CrossRef]
- Alexander, A.; Singh, V.K.; Mishra, A. Halotolerant PGPR Stenotrophomonas maltophilia BJ01 Induces Salt Tolerance by Modulating Physiology and Biochemical Activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 568289. [Google Scholar] [CrossRef] [PubMed]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Arbuscular Mycorrhizal Fungi (AMF) on Salt Stress Tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 598–608. [Google Scholar] [CrossRef]
- Han, Q.Q.; Wu, Y.N.; Gao, H.J.; Xu, R.; Pare, P.W.; Shi, H.Z.; Zhao, Q.; Li, H.R.; Khan, S.A.; Wang, Y.Q.; et al. Improved salt tolerance of medicinal plant Codonopsis pilosula by Bacillus amyloliquefaciens GB. Acta Physiol. Plant 2017, 39, 35. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria With Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kore, T.; Lembisana, V.; Devi, H.; Kabir, J. Packaging, storage and value addition of aonla, an underutilized fruit, in India. Fruits 2013, 68, 255–266. [Google Scholar] [CrossRef]
- Yao, L.X.; Wu, Z.S.; Zheng, Y.Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Naz, R.; Bano, A. Molecular and Physiological Responses of Sunflower (Helianthus Annuus L.) to Pgpr and Sa under Salt Stress. Pak. J. Bot. 2015, 47, 35–42. [Google Scholar]
- Aisha Waheed, Q. Osmoadaptation and plant growth promotion by salt tolerant bacteria under salt stress. Afr. J. Microbiol. Res. 2011, 5, 3546–3554. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.W.; Wang, T.T.; Ding, P.; Xing, K.; Jiang, J.H. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 2017, 416, 117–132. [Google Scholar] [CrossRef]
- Salah, I.B.; Albacete, A.; Messedi, D.; Gandour, M.; Andujar, C.M.; Zribi, K.; Martinez, V.; Abdelly, C.; Perez-Alfocea, F. Hormonal responses of nodulated Medicago ciliaris lines differing in salt tolerance. Environ. Exp. Bot. 2013, 86, 35–43. [Google Scholar] [CrossRef]
- Bharti, N.; Barnawal, D.; Awasthi, A.; Yadav, A.; Kalra, A. Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Mentha arvensis. Acta Physiol. Plant 2014, 36, 45–60. [Google Scholar] [CrossRef]
- Khalilpour, M.; Mozafari, V.; Abbaszadeh-Dahaji, P. Tolerance to salinity and drought stresses in pistachio (Pistacia vera L.) seedlings inoculated with indigenous stress-tolerant PGPR isolates. Sci. Hortic. 2021, 289. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J. Plant Physiol. 2014, 171, 884–894. [Google Scholar] [CrossRef]
- Wang, Q.; Dodd, I.C.; Belimov, A.A.; Jiang, F. Rhizosphere bacteria containing 1-aminocyclopropane-1- carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct. Plant Biol. 2016, 43, 161–172. [Google Scholar] [CrossRef]
- Hussein, K.A.; Joo, J.H. Plant Growth-Promoting Rhizobacteria Improved Salinity Tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 2018, 28, 938–945. [Google Scholar] [CrossRef]
- Desale, P.; Patel, B.; Singh, S.; Malhotra, A.; Nawani, N. Plant growth promoting properties of Halobacillus sp. and Halomonas sp. in presence of salinity and heavy metals. J. Basic Microbiol. 2014, 54, 781–791. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Berg, G.; Lindstrom, K.; Rasanen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Cordero, I.; Balaguer, L.; Rincon, A.; Pueyo, J.J. Inoculation of tomato plants with selected PGPR represents a feasible alternative to chemical fertilization under salt stress. J. Plant Nutr. Soil Sci. 2018, 181, 694–703. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Halo, B.A.; Khan, A.L.; Waqas, M.; Al-Harrasi, A.; Hussain, J.; Ali, L.; Adnan, M.; Lee, I.J. Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. J. Plant Interact. 2015, 10, 117–125. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Damodharan, K.; Yang, S.H.; Suh, J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Panwar, M.; Tewari, R.; Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 2016, 22, 445–459. [Google Scholar] [CrossRef]
- Ahamd, M.; Zahir, Z.A.; Nadeem, S.M.; Nazli, F.; Jamil, M.; Jamshaid, M.U. Physiological Response of Mung Bean to Rhizobium and Pseudomonas Based Biofertilizers under Salinity Stress. Pak. J. Agric. Sci. 2014, 51, 557–564. [Google Scholar]
- Yuan, Z.; Druzhinina, I.S.; Labbé, J.; Redman, R.; Qin, Y.; Rodriguez, R.; Zhang, C.; Tuskan, G.; Lin, F. Specialized Microbiome of a Halophyte and its Role in Helping Non-Host Plants to Withstand Salinity. Sci. Rep. 2016, 6, 32467. [Google Scholar] [CrossRef]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2015, 80, 23–36. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, U. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Hosseini, H.M.; Alikhani, H.A.; Mohammadi, L. Bacterial Biosynthesis of 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase and Indole-3-Acetic Acid (IAA) as Endophytic Preferential Selection Traits by Rice Plant Seedlings. J. Plant Growth Regul. 2014, 33, 654–670. [Google Scholar] [CrossRef]
- Singh, R.P.; Shelke, G.M.; Kumar, A.; Jha, P.N. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Water deficit stress mitigation by calcium chloride in Catharanthus roseus: Effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf. B Biointerfaces 2007, 60, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Upadhyaya, C.P.; Strasser, R.J.; Yu, J.W.; Park, S.W. Evaluation of abiotic stress tolerance in transgenic potato plants with reduced expression of PSII manganese stabilizing protein. Plant Sci. 2013, 198, 7–16. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, V.; Gupta, V.K.; Shukla, R.; Prabha, R.; Sarma, B.; Patel, J.S. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci. Rep. 2020, 10, 4818. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 2013, 8, e26741. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2014, 38, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Gond, S.K.; Torres, M.S.; Bergen, M.S.; Helsel, Z.; White, J.F. Induction of salt tolerance and up-regulation of aquaporin genes in tropical corn by rhizobacterium Pantoea agglomerans. Lett. Appl. Microbiol. 2015, 60, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Beattie, G.A. Microbiomes: Curating communities from plants. Nature 2015, 528, 340–341. [Google Scholar] [CrossRef][Green Version]
- Pii, Y.; Penn, A.; Terzano, R.; Crecchio, C.; Mimmo, T.; Cesco, S. Plant-microorganism-soil interactions influence the Fe availability in the rhizosphere of cucumber plants. Plant Physiol. Biochem. 2015, 87, 45–52. [Google Scholar] [CrossRef]
- Rueda-Puente, E.; Castellanos, T.; Troyo-Dieguez, E.; de Leon-Alvarez, J.L.D.; Murillo-Amador, B. Effects of a nitrogen-fixing indigenous bacterium (Klebsiella pneumoniae) on the growth and development of the halophyte Salicornia bigelovii as a new crop for saline environments. J. Agron. Crop Sci. 2003, 189, 323–332. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant Rhizobacteria Promote Growth and Enhance Salinity Tolerance in Peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Tchakounté, G.V.T.; Berger, B.; Patz, S.; Becker, M.; Fankem, H.; Taffouo, V.D.; Ruppel, S. Selected Rhizosphere Bacteria Help Tomato Plants Cope with Combined Phosphorus and Salt Stresses. Microorganisms 2020, 8, 1844. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-Ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and Characterization of Phosphorus Solubilizing Bacteria with Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef]
- Alkhatib, R.; Alkhatib, B.; Abdo, N.; Al-Eitan, L.; Creamer, R. Physio-biochemical and ultrastructural impact of (Fe3O4) nanoparticles on tobacco. BMC Plant Biol. 2019, 19, 253. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.W.; Li, G.X.; Yu, X.H.; Zheng, S.J. Plant Fe status affects the composition of siderophore-secreting microbes in the rhizosphere. Ann. Bot. 2010, 105, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Gururani, M.A.; Chun, S.C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef]
- Kirankumar, R.; Jagadeesh, K.S.; Krishnaraj, P.U.; Patil, M.S. Enhanced growth promotion of tomato and nutrient uptake by plant growth promoting rhizobacterial isolates in presence of tobacco mosaic virus pathogen. Karnataka J. Agric. Sci. 2008, 21, 309–311. [Google Scholar]
- Sgroy, V.; Cassan, F.; Masciarelli, O.; Del Papa, M.F.; Lagares, A.; Luna, V. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl. Microbiol. Biotechnol. 2009, 85, 371–381. [Google Scholar] [CrossRef]
- Teng, S.; Liu, Y.; Zhao, L. Isolation, identification and characterization of ACC deaminase-containing endophytic bacteria from halophyte Suaeda salsa. Wei Sheng Wu Xue Bao 2010, 50, 1503–1509. [Google Scholar]
- Kataoka, R.; Güneri, E.; Turgay, O.C.; Yaprak, A.E.; Sevilir, B.; Başköse, I. Sodium-resistant plant growth-promoting rhizobacteria isolated from a halophyte, Salsola grandis, in saline-alkaline soils of Turkey. Eurasian J. Soil Sci. 2017, 6, 216–225. [Google Scholar] [CrossRef][Green Version]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Shruthi, K.S.; Mathew, M.K. Vesicular trafficking and salinity responses in plants. IUBMB Life 2015, 67, 677–686. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Che-Othman, M.H.; Millar, A.H.; Taylor, N.L. Analysis of the sodium chloride-dependent respiratory kinetics of wheat mitochondria reveals differential effects on phosphorylating and non-phosphorylating electron transport pathways. Plant Cell Environ. 2016, 39, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Halfter, U.; Ishitani, M.; Zhu, J.K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. How calcium enhances plant salt tolerance. Science 1998, 280, 1906–1907. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.; Rubio, F. High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 2006, 57, 1149–1160. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) with Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-Producing Plant Growth-Promoting Rhizobacteria Under Salinity Condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Dodd, I.C.; Perez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.; Pare, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT. Mol. Plant Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; Naveed, M.; Zahir, Z.A.; Jacobsen, S.-E. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Atouei, M.T.; Pourbabaee, A.A.; Shorafa, M. Alleviation of Salinity Stress on Some Growth Parameters of Wheat by Exopolysaccharide-Producing Bacteria. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 2725–2733. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Biol. 2010, 3, a001438. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kucharova, Z. Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fertil. Soils 2009, 45, 563–571. [Google Scholar] [CrossRef]
- Bottini, R.; Cassan, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Gulati, A.; Nayyar, H. Indigenous salt-tolerant rhizobacterium Pantoea dispersa (PSB3) reduces sodium uptake and mitigates the effects of salt stress on growth and yield of chickpea. Acta Physiol. Plant. 2016, 38, 278. [Google Scholar] [CrossRef]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Prittesh, P.; Avnika, P.; Kinjal, P.; Jinal, H.N.; Sakthivel, K.; Amaresan, N. Amelioration effect of salt-tolerant plant growth-promoting bacteria on growth and physiological properties of rice (Oryza sativa) under salt-stressed conditions. Arch. Microbiol. 2020, 202, 2419–2428. [Google Scholar] [CrossRef]
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ. Appl. Soil Ecol. 2017, 119, 26–34. [Google Scholar] [CrossRef]
- Li, X.Z.; Sun, P.; Zhang, Y.N.; Jin, C.; Guan, C.F. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2022, 41, 549–569. [Google Scholar] [CrossRef]
- Gusain, Y.S.; Singh, U.S.; Sharma, A.K. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr. J. Biotechnol. 2015, 14, 764–773. [Google Scholar]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Morcillo, R.J.L.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Abbasi, S.; Sadeghi, A.; Safaie, N. Streptomyces alleviate drought stress in tomato plants and modulate the expression of transcription factors ERF1 and WRKY70 genes. Sci. Hortic. 2020, 265, 109206. [Google Scholar] [CrossRef]
- Luo, S.; Xu, T.; Chen, L.; Chen, J.; Rao, C.; Xiao, X.; Wan, Y.; Zeng, G.; Long, F.; Liu, C.; et al. Endophyte-assisted promotion of biomass production and metal-uptake of energy crop sweet sorghum by plant-growth-promoting endophyte Bacillus sp. SLS18. Appl. Microbiol. Biotechnol. 2012, 93, 1745–1753. [Google Scholar] [CrossRef]
- Rajini, S.B.; Nandhini, M.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Prakash, H.S. Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of Sorghum bicolor. Plant Pathol. 2020, 69, 642–654. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Joe, M.M.; Islam, M.R.; Sa, T. ACC deaminase containing diazotrophic endophytic bacteria ameliorate salt stress in Catharanthus roseus through reduced ethylene levels and induction of antioxidative defense systems. Symbiosis 2012, 56, 77–86. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Srivastava, A.K.; Tiwari, R.K. Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum). Braz. J. Microbiol. 2020, 51, 229–241. [Google Scholar] [CrossRef]
- Pan, D.; Mionetto, A.; Tiscornia, S.; Bettucci, L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015, 31, 137–143. [Google Scholar] [CrossRef]
- Weilharter, A.; Mitter, B.; Shin, M.V.; Chain, P.S.G.; Nowak, J.; Sessitsch, A. Complete Genome Sequence of the Plant Growth-Promoting Endophyte Burkholderia phytofirmans Strain PsJN. J. Bacteriol. 2011, 193, 3383–3384. [Google Scholar] [CrossRef]
- Megías, E.; Megías, M.; Ollero, F.J.; Hungria, M. Draft Genome Sequence of Pantoea ananatis Strain AMG521, a Rice Plant Growth-Promoting Bacterial Endophyte Isolated from the Guadalquivir Marshes in Southern Spain. Genome Announc. 2016, 4, e01681-15. [Google Scholar] [CrossRef]
- Beracochea, M.; Taule, C.; Battistoni, F. Draft Genome Sequence of Kosakonia radicincitans UYSO10, an Endophytic Plant Growth-Promoting Bacterium of Sugarcane (Saccharum officinarum). Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Bertalan, M.; Albano, R.; de Pádua, V.; Rouws, L.; Rojas, C.; Hemerly, A.; Teixeira, K.; Schwab, S.; Araujo, J.; Oliveira, A.; et al. Complete genome sequence of the sugarcane nitrogen-fixing endophyte Gluconacetobacter diazotrophicus Pal. BMC Genom. 2009, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.Z.; Yaseen, M.; Naveed, M.; Wang, X.; Fatima, K.; Saeed, Q.; Mustafa, A. Polymer-Paraburkholderia phytofirmans PsJN Coated Diammonium Phosphate Enhanced Microbial Survival, Phosphorous Use Efficiency, and Production of Wheat. Agronomy 2020, 10, 1344. [Google Scholar] [CrossRef]
- de Los Santos Villalobos, S.; Robles, R.I.; Parra Cota, F.I.; Larsen, J.; Lozano, P.; Tiedje, J.M. Bacillus cabrialesii sp. nov., an endophytic plant growth promoting bacterium isolated from wheat (Triticum turgidum subsp. durum) in the Yaqui Valley, Mexico. Int. J. Syst. Evol. Microbiol. 2019, 69, 3939–3945. [Google Scholar] [CrossRef]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-Mediated Effects Predominate in Paraburkholderia phytofirmans Growth Promotion and Salt Stress Tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef]
- Zúñiga, A.; Poupin, M.J.; Donoso, R.; Ledger, T.; Guiliani, N.; Gutiérrez, R.A.; González, B. Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN. Mol. Plant Microbe Interact. 2013, 26, 546–553. [Google Scholar] [CrossRef]
- Dhole, A.; Shelat, H.; Vyas, R.; Jhala, Y.; Bhange, M. Endophytic occupation of legume root nodules by nifH-positive non-rhizobial bacteria, and their efficacy in the groundnut (Arachis hypogaea). Ann. Microbiol. 2016, 66, 1397–1407. [Google Scholar] [CrossRef]
- Li, C.H.; Zhao, M.W.; Tang, C.M.; Li, S.P. Population dynamics and identification of endophytic bacteria antagonistic toward plant-pathogenic fungi in cotton root. Microb. Ecol. 2010, 59, 344–356. [Google Scholar] [CrossRef]
- Li, C.-H.; Shi, L.; Han, Q.; Hu, H.-L.; Zhao, M.-W.; Tang, C.-M.; Li, S.-P. Biocontrol of verticillium wilt and colonization of cotton plants by an endophytic bacterial isolate. J. Appl. Microbiol. 2012, 113, 641–651. [Google Scholar] [CrossRef]
- Afzal, I.; Iqrar, I.; Shinwari, Z.K.; Yasmin, A. Plant growth-promoting potential of endophytic bacteria isolated from roots of wild Dodonaea viscosa L. Plant Growth Regul. 2016, 81, 399–408. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Roles of Plant Endosphere Microbes in Agriculture-A Review. J. Plant Growth Regul. 2022, 41, 1411–1428. [Google Scholar] [CrossRef]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Nawangsih, A.A.; Damayanti, I.K.A.; Wiyono, S.; Kartika, J.G. Selection and Characterization of Endophytic Bacteria as Biocontrol Agents of Tomato Bacterial Wilt Disease. HAYATI J. Biosci. 2011, 18, 66–70. [Google Scholar] [CrossRef]
- Shinjo, R.; Uesaka, K.; Ihara, K.; Sakazaki, S.; Yano, K.; Kondo, M.; Tanaka, A. Draft Genome Sequence of Burkholderia vietnamiensis Strain RS1, a Nitrogen-Fixing Endophyte Isolated from Sweet Potato. Microbiol. Resour. Announc. 2018, 7, e00820-18. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Kim, K.; Krishnamoorthy, R.; Sundaram, S.; Sa, T. Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110. Plant Growth Regul. 2014, 76, 327–332. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Issa, A.; Esmaeel, Q.; Sanchez, L.; Courteaux, B.; Guise, J.-F.; Gibon, Y.; Ballias, P.; Clément, C.; Jacquard, C.; Vaillant-Gaveau, N.; et al. Impacts of Paraburkholderia phytofirmans Strain PsJN on Tomato (Lycopersicon esculentum L.) Under High Temperature. Front. Plant Sci. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar] [CrossRef]
- Bu, N.; Li, X.M.; Li, Y.Y.; Ma, C.Y.; Ma, L.J.; Zhang, C. Effects of Na2CO3 stress on photosynthesis and antioxidative enzymes in endophyte infected and non-infected rice. Ecotox. Environ. Safe 2012, 78, 35–40. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Mirlohi, A. Neotyphodium endophytes trigger salt resistance in tall and meadow fescues. J. Plant Nutr. Soil. Sci. 2010, 173, 952–957. [Google Scholar] [CrossRef]
- Shukla, K.P.; Sharma, S.; Singh, N.K.; Singh, V.; Tiwari, K.; Singh, S. Nature and role of root exudates: Efficacy in bioremediation. Afr. J. Biotechnol. 2011, 10, 9717–9724. [Google Scholar]
- Ramesh, A.; Sharma, S.K.; Joshi, O.P.; Khan, I.R. Phytase, phosphatase activity and p-nutrition of soybean as influenced by inoculation of bacillus. Indian J. Microbiol. 2011, 51, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.P.; Belesky, D.P. Adaptations of Endophyte-Infected Cool-Season Grasses to Environmental Stresses: Mechanisms of Drought and Mineral Stress Tolerance. Crop Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-Tolerant Halophyte Rhizosphere Bacteria Stimulate Growth of Alfalfa in Salty Soil. Front. Microbiol. 2019, 10, 1849. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, J.; Hu, B. Regulation of microbial siderophore transport and its application in environmental remediation. Sheng Wu Gong Cheng Xue Bao 2019, 35, 2189–2200. [Google Scholar]
- Carrion, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Saad, M.M.; Abo-Koura, H.A.; Bishara, M.M.; Gomaa, I.M. Microencapsulation: Toward the Reduction of the Salinity Stress Effect on Wheat Plants Using NPK Rhizobacteria. Biotechnol. J. Int. 2020, 1–18. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, Z.; Liu, H.; Liu, Y.; Zhou, Y.; Meng, C.; Ma, S.; Xie, Z.; Li, Y.; Zhang, C.-S. Patterns in the Microbial Community of Salt-Tolerant Plants and the Functional Genes Associated with Salt Stress Alleviation. Microbiol. Spectr. 2021, 9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zou, H.; Wang, B.; Yuan, F. Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops. Int. J. Mol. Sci. 2022, 23, 7036. https://doi.org/10.3390/ijms23137036
Gao Y, Zou H, Wang B, Yuan F. Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops. International Journal of Molecular Sciences. 2022; 23(13):7036. https://doi.org/10.3390/ijms23137036
Chicago/Turabian StyleGao, Yaru, Hong Zou, Baoshan Wang, and Fang Yuan. 2022. "Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops" International Journal of Molecular Sciences 23, no. 13: 7036. https://doi.org/10.3390/ijms23137036
APA StyleGao, Y., Zou, H., Wang, B., & Yuan, F. (2022). Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops. International Journal of Molecular Sciences, 23(13), 7036. https://doi.org/10.3390/ijms23137036






