Early Life Stage Folic Acid Deficiency Delays the Neurobehavioral Development and Cognitive Function of Rat Offspring by Hindering De Novo Telomere Synthesis
Abstract
1. Introduction
2. Results
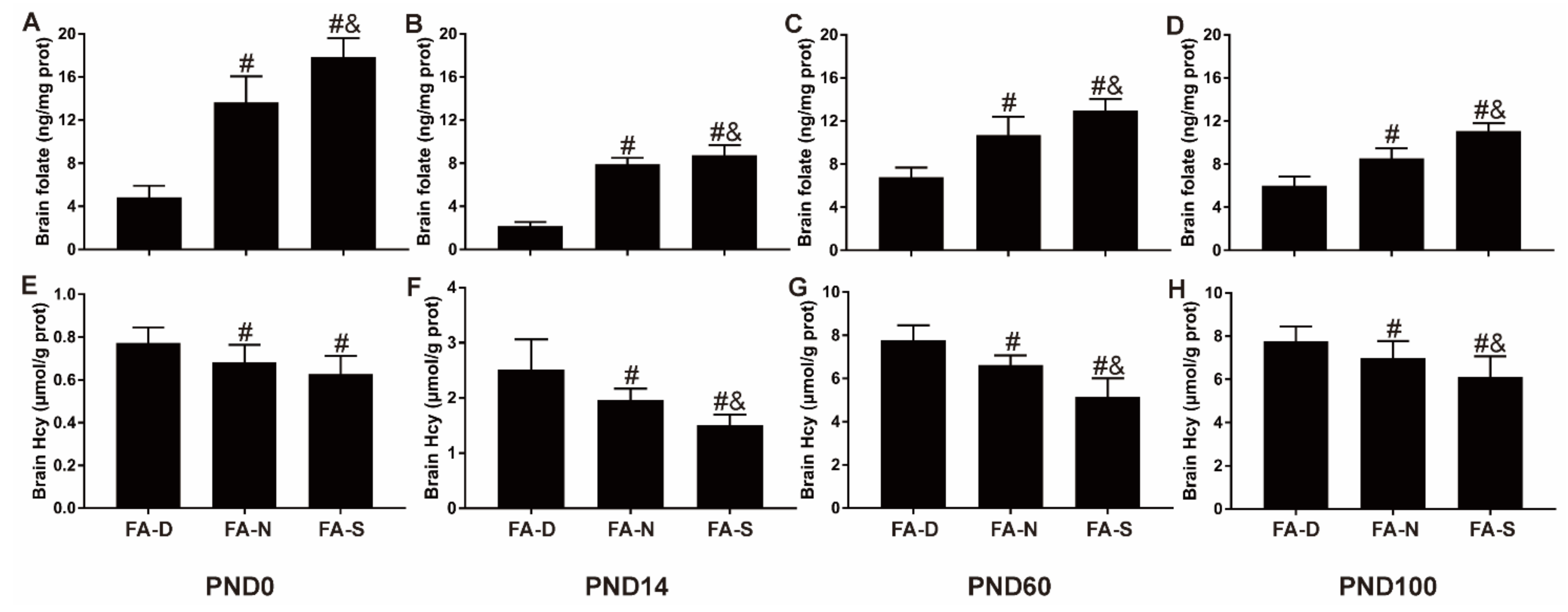
2.1. Early Life Folic Acid Deficiency Decreased the Level of Folate and Increased the Level of Hcy in the Brain Tissue of Offspring
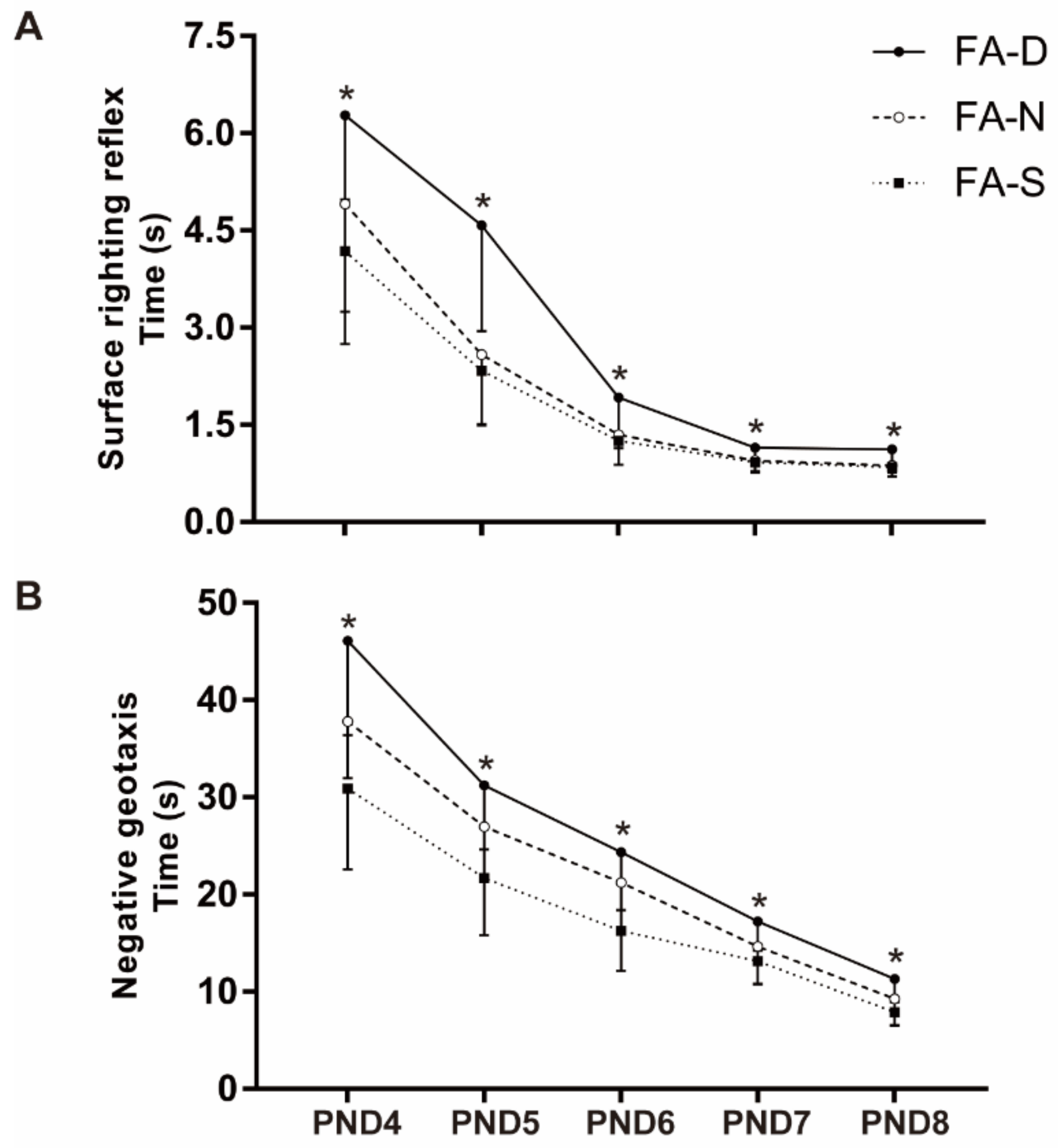
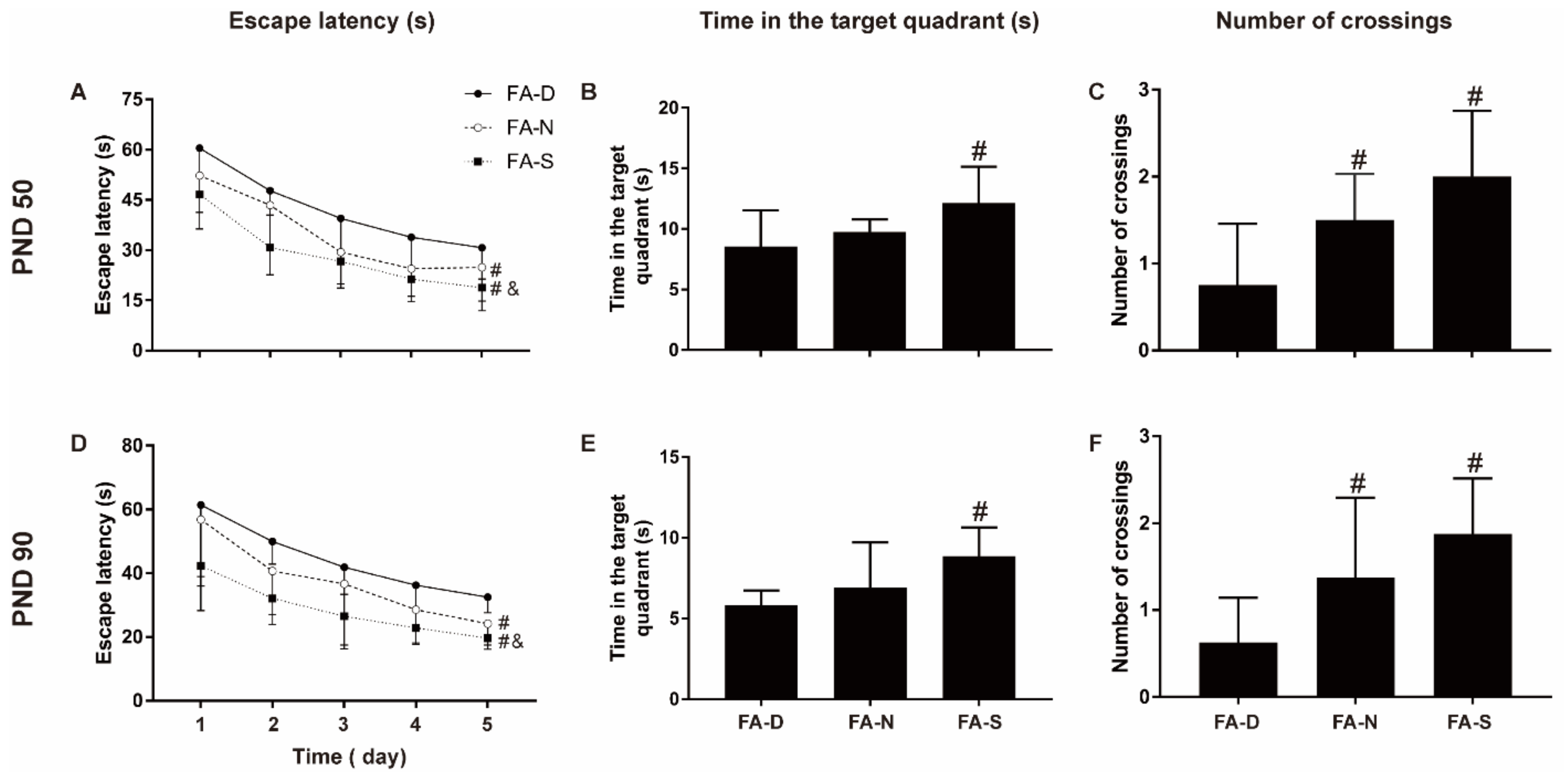
2.2. Early Life Folic Acid Deficiency Delayed the Neurodevelopment and Spatial Learning/Memory Abilities of the Offspring
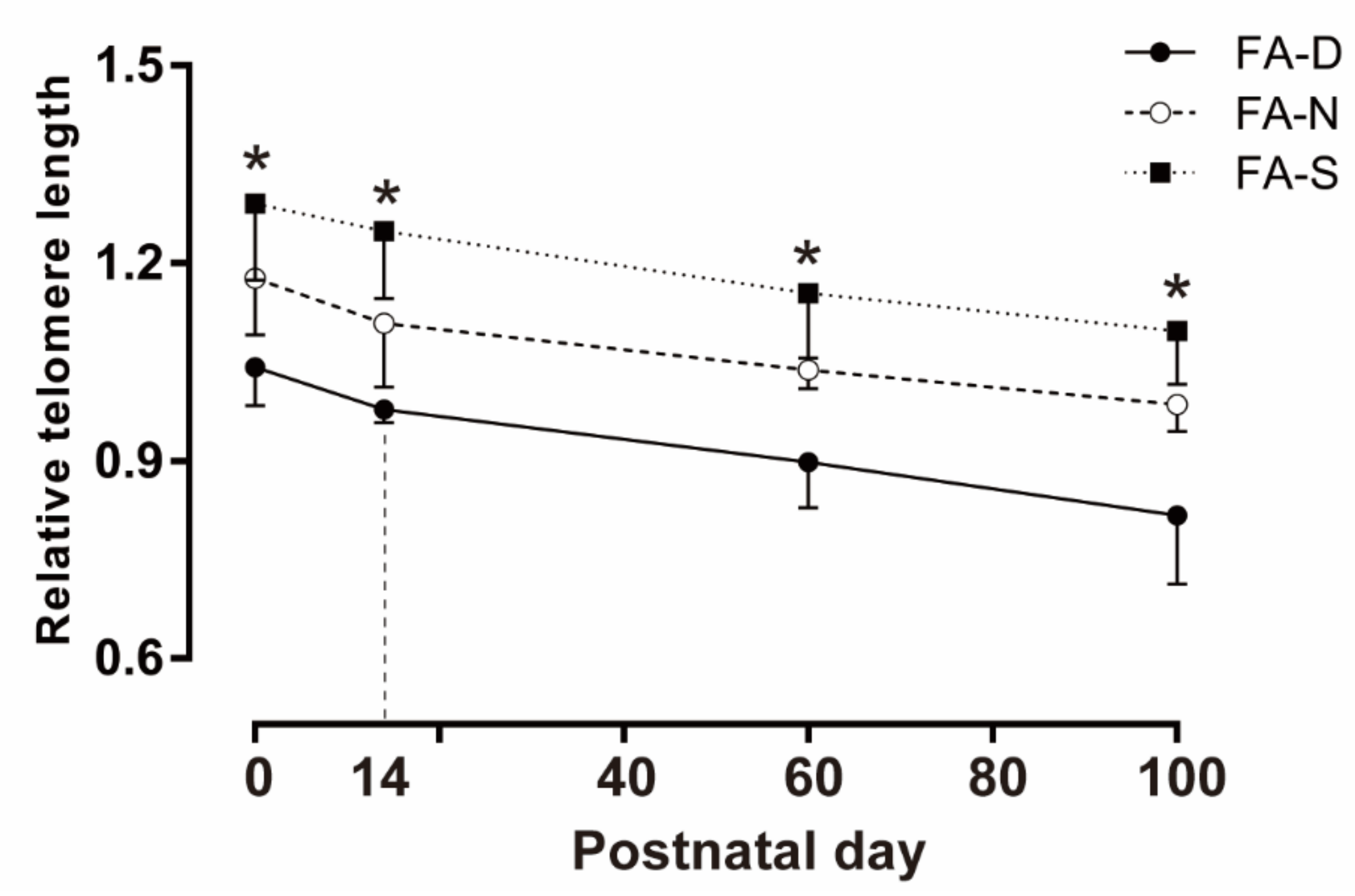
2.3. Early Life Folic Acid Deficiency Hindered De Novo Telomere Synthesis in the Brain of Offspring
2.4. Early Life Folic Acid Deficiency Increased Uracil Misincorporation in Telomeres in Brain Tissue of Offspring
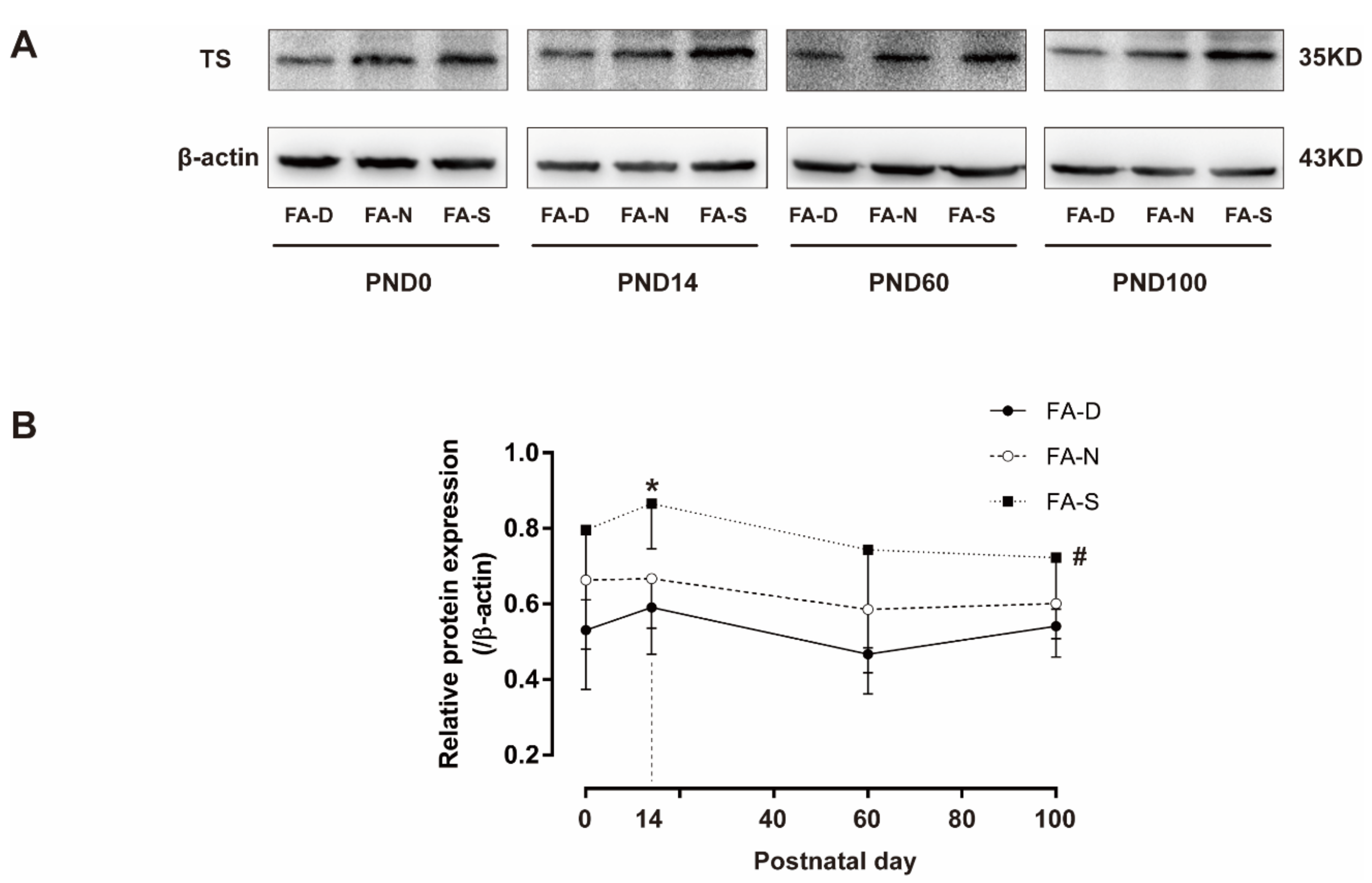
2.5. Early Life Folic Acid Deficiency Inhibited the Expression of Thymidylate Synthase in the Brain Tissue of Offspring
3. Discussion
4. Materials and Methods
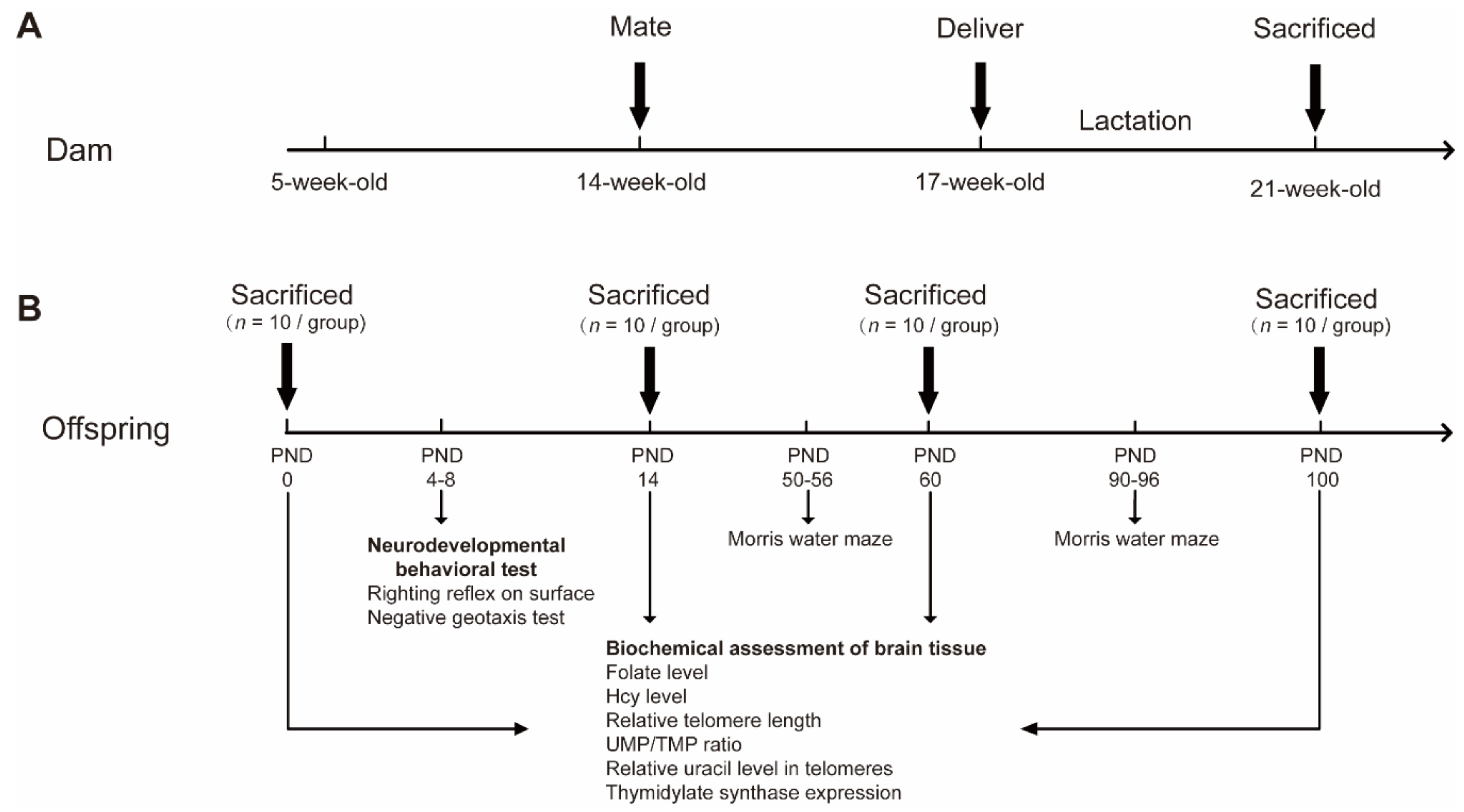
4.1. Animals and Dietary Treatment
4.2. Folate and Homocysteine (Hcy) Assay
4.3. Neurobehavioral Tests
4.3.1. Righting Reflex on the Surface
4.3.2. Negative Geotaxis Test
4.3.3. Morris Water Maze Test
4.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.4.1. Relative Telomere Length Detected by Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.4.2. Uracil Misincorporation in Telomeres Detected by qPCR
4.5. dUMP and dTMP in Brain Tissue Detected by High-Performance Liquid Chromatography (HPLC)
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.-Y.; Liu, S.-M.; Zhang, Y.-Z. Maternal Folic Acid Supplementation Mediates Offspring Health via DNA Methylation. Reprod. Sci. 2020, 27, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Lavriša, Z.; Hribar, M.; Hristov, H.; Kvarantan, N.; Seljak, B.K.; Gregorič, M.; Blaznik, U.; Gregorič, N.; Zaletel, K.; et al. Dietary Intake of Folate and Assessment of the Folate Deficiency Prevalence in Slovenia Using Serum Biomarkers. Nutrients 2021, 13, 3860. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Solehdin, F. Folic acid for the prevention of congenital anomalies. Eur. J. Pediatr. 1998, 157, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Picciano, M.F. Folate and human reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef]
- Carlsson, C.M. Homocysteine Lowering with Folic Acid and Vitamin B Supplements: Effects on cardiovascular disease in older adults. Drugs Aging 2006, 23, 491–502. [Google Scholar] [CrossRef]
- Duthie, S.J. Folate and cancer: How DNA damage, repair and methylation impact on colon carcinogenesis. J. Inherit. Metab. Dis. 2011, 34, 101–109. [Google Scholar] [CrossRef]
- Araújo, J.R.; Martel, F.; Borges, N.; Keating, E. Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res. Rev. 2015, 22, 9–19. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Li, S.; Yan, J.; Wilson, J.X.; Huang, G. Maternal Folic Acid Supplementation During Pregnancy Improves Neurobehavioral Development in Rat Offspring. Mol. Neurobiol. 2018, 55, 2676–2684. [Google Scholar] [CrossRef]
- Burgos-Barragan, G.; Wit, N.; Meiser, J.; Dingler, F.A.; Pietzke, M.; Mulderrig, L.; Pontel, L.; Rosado, I.; Brewer, T.F.; Cordell, R.; et al. Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature 2017, 548, 549–554. [Google Scholar] [CrossRef]
- Caffrey, A.; McNulty, H.; Irwin, R.E.; Walsh, C.P.; Pentieva, K. Maternal folate nutrition and offspring health: Evidence and current controversies. Proc. Nutr. Soc. 2019, 78, 208–220. [Google Scholar] [CrossRef]
- Morscher, R.J.; Ducker, G.; Li, S.H.-J.; Mayer, J.A.; Gitai, Z.; Sperl, W.; Rabinowitz, J.D. Mitochondrial translation requires folate-dependent tRNA methylation. Nature 2018, 554, 128–132. [Google Scholar] [CrossRef]
- Beaudin, A.E.; Abarinov, E.V.; Noden, D.M.; Perry, C.A.; Chu, S.; Stabler, S.P.; Allen, R.H.; Stover, P.J. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am. J. Clin. Nutr. 2011, 93, 789–798. [Google Scholar] [CrossRef]
- Chon, J.; Stover, P.J.; Field, M.S. Targeting nuclear thymidylate biosynthesis. Mol. Asp. Med. 2017, 53, 48–56. [Google Scholar] [CrossRef]
- Sapienza, P.J.; Falk, B.T.; Lee, A.L. Bacterial Thymidylate Synthase Binds Two Molecules of Substrate and Cofactor without Cooperativity. J. Am. Chem. Soc. 2015, 137, 14260–14263. [Google Scholar] [CrossRef]
- Carreras, C.W.; Santi, D.V. The Catalytic Mechanism and Structure of Thymidylate Synthase. Annu. Rev. Biochem. 1995, 64, 721–762. [Google Scholar] [CrossRef]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef]
- Fenech, M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat. Res. Fundam. Mol. Mech. Mutagenes. 2012, 733, 21–33. [Google Scholar] [CrossRef]
- Knock, E.; Deng, L.; Krupenko, N.; Mohan, R.D.; Wu, Q.; Leclerc, D.; Gupta, S.; Elmore, C.L.; Kruger, W.; Tini, M.; et al. Susceptibility to intestinal tumorigenesis in folate-deficient mice may be influenced by variation in one-carbon metabolism and DNA repair. J. Nutr. Biochem. 2011, 22, 1022–1029. [Google Scholar] [CrossRef]
- Zhang, P.; Dilley, C.; Mattson, M.P. DNA damage responses in neural cells: Focus on the telomere. Neuroscience 2007, 145, 1439–1448. [Google Scholar] [CrossRef]
- González-Giraldo, Y.; Forero, D.; Echeverria, V.; Gonzalez, Y.; Avila-Rodríguez, M.; Garcia-Segura, L.M.; Barreto, G.E. Neuroprotective effects of the catalytic subunit of telomerase: A potential therapeutic target in the central nervous system. Ageing Res. Rev. 2016, 28, 37–45. [Google Scholar] [CrossRef]
- Paul, L.; Cattaneo, M.; D’Angelo, A.; Sampietro, F.; Fermo, I.; Razzari, C.; Fontana, G.; Eugene, N.; Jacques, P.F.; Selhub, J. Telomere Length in Peripheral Blood Mononuclear Cells Is Associated with Folate Status in Men. J. Nutr. 2009, 139, 1273–1278. [Google Scholar] [CrossRef]
- Bull, C.F.; Mayrhofer, G.; O’Callaghan, N.J.; Au, A.Y.; Pickett, H.A.; Low, G.K.M.; Zeegers, D.; Hande, M.P.; Fenech, M.F. Folate Deficiency Induces Dysfunctional Long and Short Telomeres; Both States Are Associated with Hypomethylation and DNA Damage in Human WIL2-NS Cells. Cancer Prev. Res. 2014, 7, 128–138. [Google Scholar] [CrossRef]
- Lintas, C. Linking genetics to epigenetics: The role of folate and folate-related pathways in neurodevelopmental disorders. Clin. Genet. 2019, 95, 241–252. [Google Scholar] [CrossRef]
- Zou, R.; El Marroun, H.; Cecil, C.; Jaddoe, V.W.V.; Hillegers, M.; Tiemeier, H.; White, T. Maternal folate levels during pregnancy and offspring brain development in late childhood. Clin. Nutr. 2021, 40, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Tomàs, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, T.; Sun, L.; Li, W.; Zhang, C.; Yu, L.; Guan, Y. Gestational B-vitamin supplementation alleviates PM2.5-induced autism-like behavior and hippocampal neurodevelopmental impairment in mice offspring. Ecotoxicol. Environ. Saf. 2019, 185, 109686. [Google Scholar] [CrossRef]
- Cai, H.; Lin, L.; Wang, G.; Berman, Z.; Yang, X.; Cheng, X. Folic acid rescues corticosteroid-induced vertebral malformations in chick embryos through targeting TGF-β signaling. J. Cell. Physiol. 2020, 235, 8626–8639. [Google Scholar] [CrossRef]
- Cai, C.; Xiao, R.; Van Halm-Lutterodt, N.; Zhen, J.; Huang, X.; Xu, Y.; Chen, S.; Yuan, L. Association of MTHFR, SLC19A1 Genetic Polymorphism, Serum Folate, Vitamin B12 and Hcy Status with Cognitive Functions in Chinese Adults. Nutrients 2016, 8, 665. [Google Scholar] [CrossRef]
- Morris, M.S. The Role of B Vitamins in Preventing and Treating Cognitive Impairment and Decline. Adv. Nutr. Int. Rev. J. 2012, 3, 801–812. [Google Scholar] [CrossRef]
- Kaiyawet, N.; Rungrotmongkol, T.; Hannongbua, S. Effect of Halogen Substitutions on dUMP to Stability of Thymidylate Synthase/dUMP/mTHF Ternary Complex Using Molecular Dynamics Simulation. J. Chem. Inf. Model. 2013, 53, 1315–1323. [Google Scholar] [CrossRef]
- Thomas, P.; Fenech, M. Chapter 13 Methylenetetrahydrofolate Reductase, Common Polymorphisms, and Relation to Disease. Folic Acid and Folates. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2008; Volume 79, pp. 375–392. [Google Scholar] [CrossRef]
- Nazki, F.H.; Sameer, A.S.; Ganaie, B.A. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene 2014, 533, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Herbert, V. In vitro DNA synthesis by megaloblastic bone marrow: Effect of folates and cobalamins on thymidine incorporation and de novo thymidylate synthesis. Am. J. Hematol. 1989, 31, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Praveen, G.; Shalini, T.; Sivaprasad, M.; Reddy, G.B. Relative telomere length and mitochondrial DNA copy number variation with age: Association with plasma folate and vitamin B12. Mitochondrion 2020, 51, 79–87. [Google Scholar] [CrossRef]
- Rolyan, H.; Scheffold, A.; Heinrich, A.; Begus-Nahrmann, Y.; Langkopf, B.H.; Hölter, S.M.; Vogt-Weisenhorn, D.M.; Liss, B.; Wurst, W.; Lie, D.C.; et al. Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain 2011, 134, 2044–2056. [Google Scholar] [CrossRef]
- Anitha, A.; Thanseem, I.; Vasu, M.M.; Viswambharan, V.; Poovathinal, S.A. Telomeres in neurological disorders. Adv. Clin. Chem. 2019, 90, 81–132. [Google Scholar] [CrossRef]
- Entringer, S.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Buss, C.; Shahbaba, B.; Gillen, D.L.; Venkataramanan, R.; Simhan, H.N.; Wadhwa, P.D. Maternal Folate Concentration in Early Pregnancy and Newborn Telomere Length. Ann. Nutr. Metab. 2015, 66, 202–208. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, D.; Zhang, D.; Zhao, J.; Li, W.; Sun, Y.; Chen, Y.; Liu, H.; Wilson, J.X.; Qian, Z.; et al. Folic Acid Inhibits Aging-Induced Telomere Attrition and Apoptosis in Astrocytes In Vivo and In Vitro. Cereb. Cortex 2022, 32, 286–297. [Google Scholar] [CrossRef]
- Li, W.; Ma, Y.; Li, Z.; Lv, X.; Wang, X.; Zhou, D.; Luo, S.; Wilson, J.X.; Huang, G. Folic Acid Decreases Astrocyte Apoptosis by Preventing Oxidative Stress-Induced Telomere Attrition. Int. J. Mol. Sci. 2019, 21, 62. [Google Scholar] [CrossRef]
- Gomes, S.; Lopes, C.; Pinto, E. Folate and folic acid in the periconceptional period: Recommendations from official health organizations in thirty-six countries worldwide and WHO. Public Health Nutr. 2016, 19, 176–189. [Google Scholar] [CrossRef]
- Šlamberová, R.; Pometlová, M.; Charousová, P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 82–88. [Google Scholar] [CrossRef]
- Lee, S.M.; Jeon, S.; Jeong, H.J.; Kim, B.-N.; Kim, Y. Dibutyl phthalate exposure during gestation and lactation in C57BL/6 mice: Maternal behavior and neurodevelopment in pups. Environ. Res. 2020, 182, 109025. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Shoeb, M.; Kodali, V.; Farris, B.Y.; Bishop, L.M.; Meighan, T.G.; Salmen, R.; Eye, T.; Friend, S.; Schwegler-Berry, D.; Roberts, J.R.; et al. Oxidative stress, DNA methylation, and telomere length changes in peripheral blood mononuclear cells after pulmonary exposure to metal-rich welding nanoparticles. NanoImpact 2017, 5, 61–69. [Google Scholar] [CrossRef]
- Antonini, J.M.; Kodali, V.; Meighan, T.G.; Roach, K.A.; Roberts, J.R.; Salmen, R.; Boyce, G.R.; Zeidler-Erdely, P.C.; Kashon, M.; Erdely, A.; et al. Effect of Age, High-Fat Diet, and Rat Strain on Serum Biomarkers and Telomere Length and Global DNA Methylation in Peripheral Blood Mononuclear Cells. Sci. Rep. 2019, 9, 1996. [Google Scholar] [CrossRef]
- Sarno, A.; Lundbæk, M.; Liabakk, N.B.; Aas, P.A.; Mjelle, R.; Hagen, L.; Sousa, M.M.L.; Krokan, H.E.; Kavli, B. Uracil–DNA glycosylase UNG1 isoform variant supports class switch recombination and repairs nuclear genomic uracil. Nucleic Acids Res. 2019, 47, 4569–4585. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, X.; Zhang, J.; Guan, Z.; Xu, L.; Wang, J.; Zhang, T.; Niu, B. Raltitrexed’s effect on the development of neural tube defects in mice is associated with DNA damage, apoptosis, and proliferation. Mol. Cell. Biochem. 2015, 398, 223–231. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Western blotting. Methods 2006, 38, 283–293. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Li, Z.; Sun, Y.; Yan, J.; Huang, G.; Li, W. Early Life Stage Folic Acid Deficiency Delays the Neurobehavioral Development and Cognitive Function of Rat Offspring by Hindering De Novo Telomere Synthesis. Int. J. Mol. Sci. 2022, 23, 6948. https://doi.org/10.3390/ijms23136948
Zhou D, Li Z, Sun Y, Yan J, Huang G, Li W. Early Life Stage Folic Acid Deficiency Delays the Neurobehavioral Development and Cognitive Function of Rat Offspring by Hindering De Novo Telomere Synthesis. International Journal of Molecular Sciences. 2022; 23(13):6948. https://doi.org/10.3390/ijms23136948
Chicago/Turabian StyleZhou, Dezheng, Zhenshu Li, Yue Sun, Jing Yan, Guowei Huang, and Wen Li. 2022. "Early Life Stage Folic Acid Deficiency Delays the Neurobehavioral Development and Cognitive Function of Rat Offspring by Hindering De Novo Telomere Synthesis" International Journal of Molecular Sciences 23, no. 13: 6948. https://doi.org/10.3390/ijms23136948
APA StyleZhou, D., Li, Z., Sun, Y., Yan, J., Huang, G., & Li, W. (2022). Early Life Stage Folic Acid Deficiency Delays the Neurobehavioral Development and Cognitive Function of Rat Offspring by Hindering De Novo Telomere Synthesis. International Journal of Molecular Sciences, 23(13), 6948. https://doi.org/10.3390/ijms23136948




