Transcriptome Analysis Reveals the Role of GA3 in Regulating the Asynchronism of Floral Bud Differentiation and Development in Heterodichogamous Cyclocarya paliurus (Batal.) Iljinskaja
Abstract
:1. Introduction
2. Results
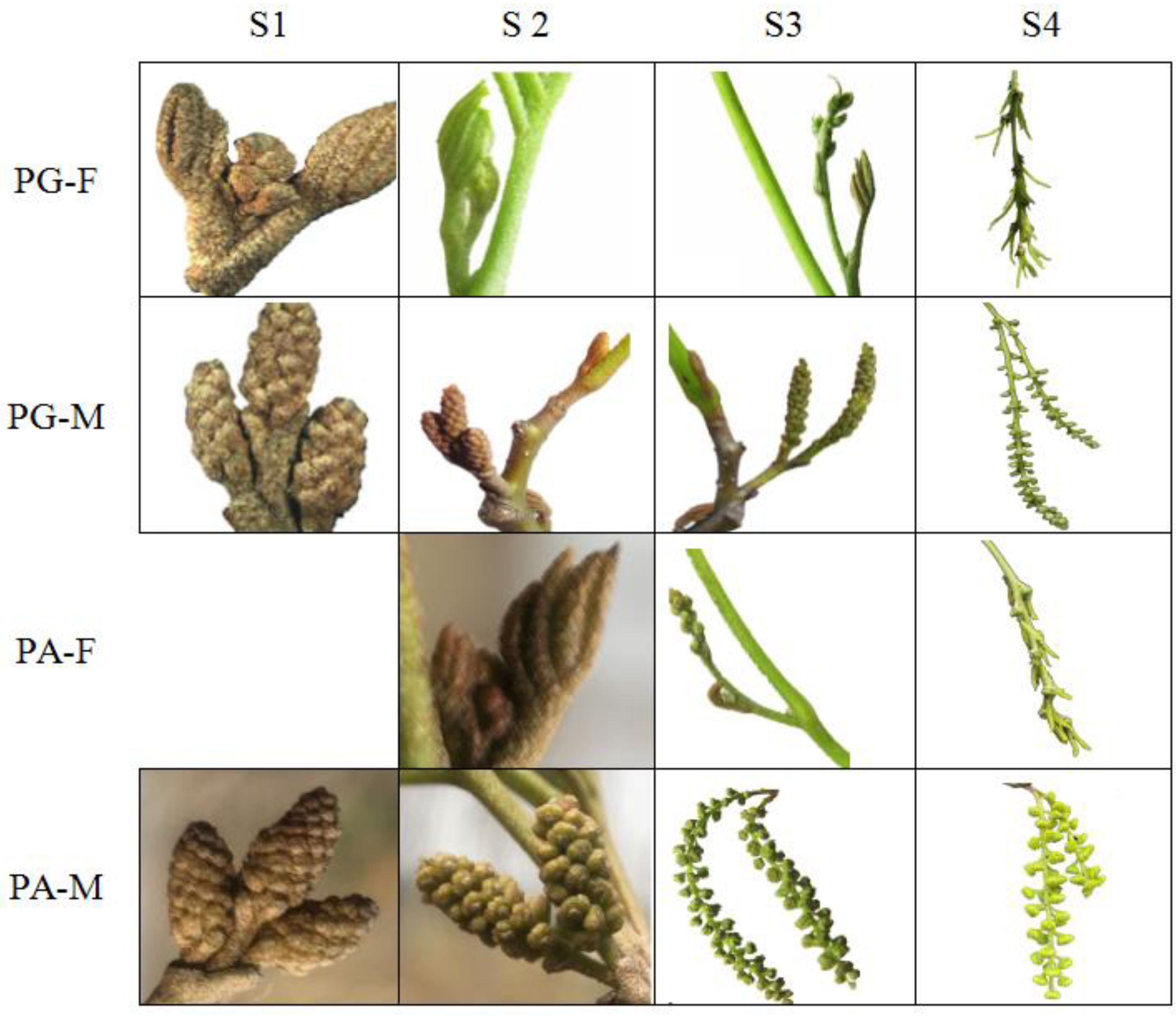
2.1. Phenotypic Characterization
2.2. Sequencing, Assembly, and Annotation of C. paliurus
2.3. Identification of Differentially Expressed Genes (DEGs) during Floral Bud Differentiation and Development
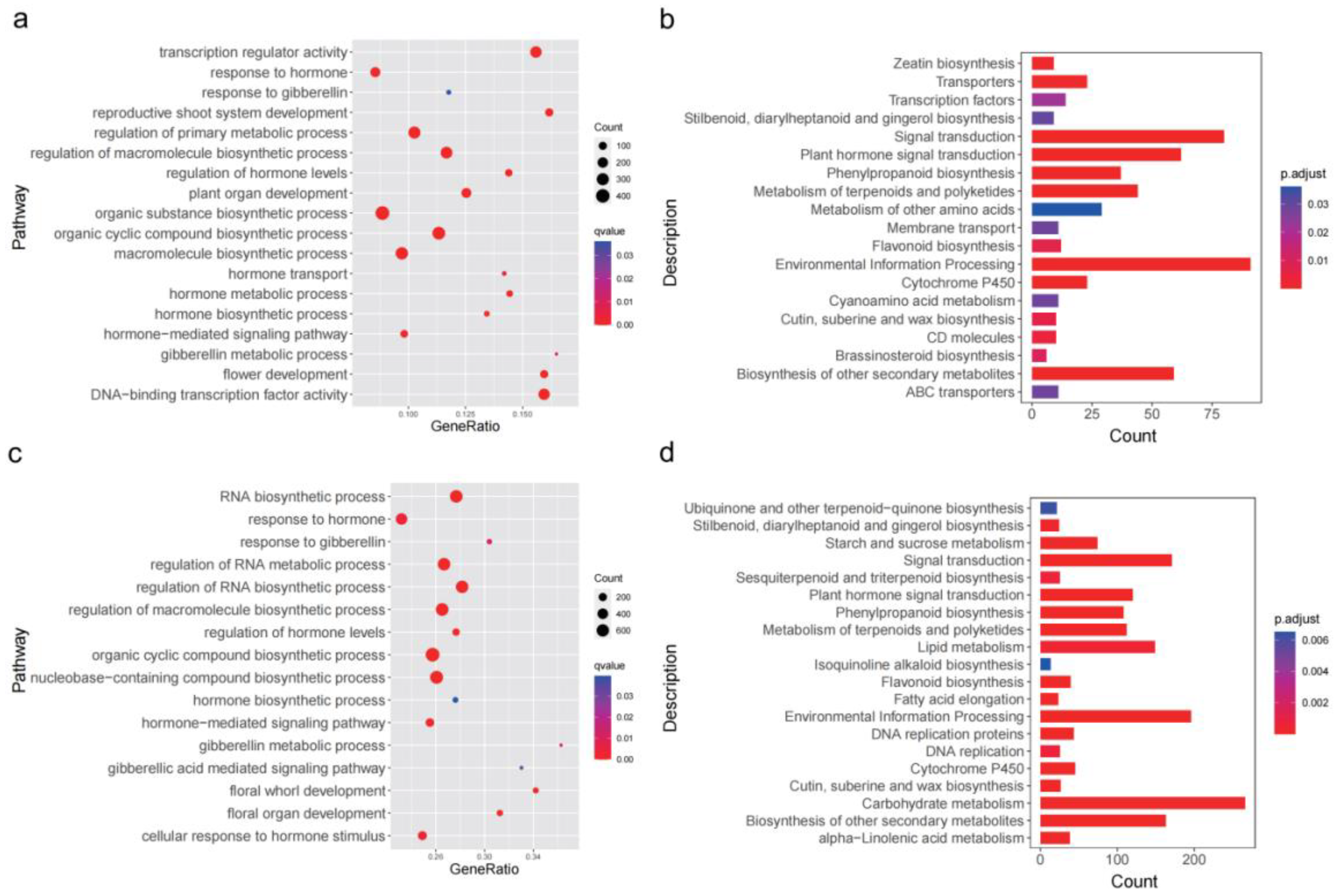
2.4. GO Functional Annotation and KEGG Pathway Enrichment of DEGs
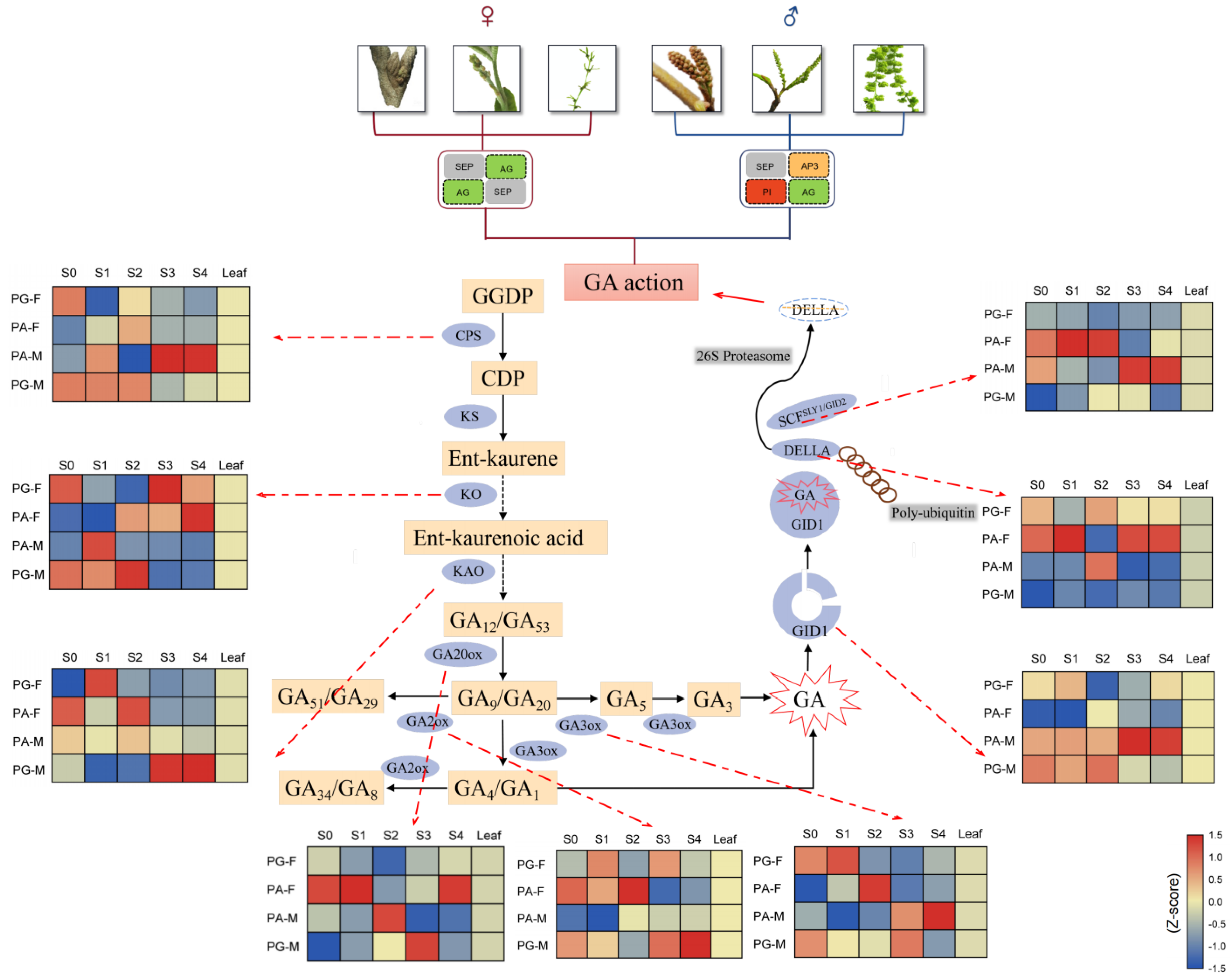
2.5. DEGs Related to Gibberellin in Biosynthesis and Regulatory Pathways
2.6. GA3 Plays a Critical Role in Floral Differentiation and Development
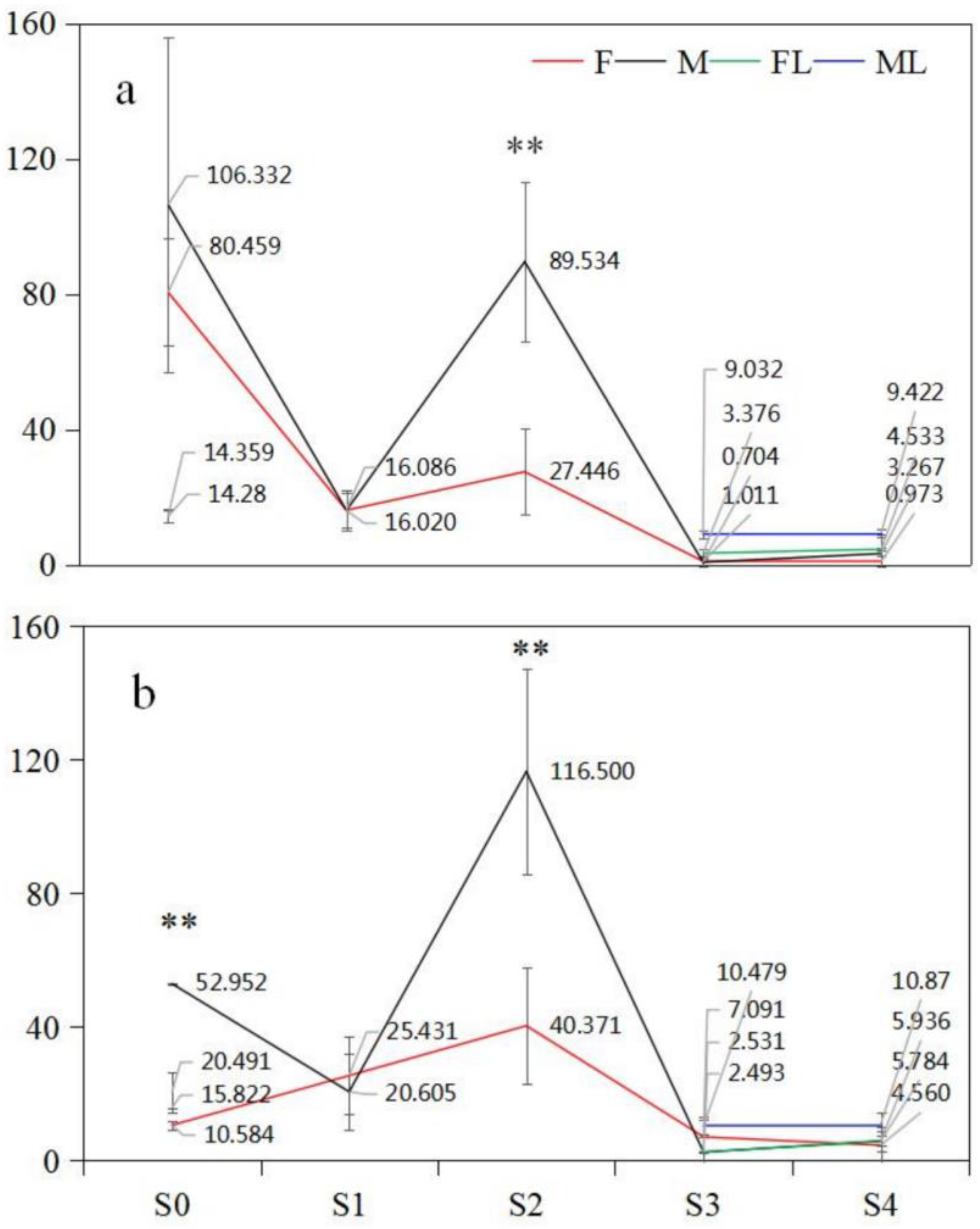
2.7. Dynamic Patterns of GA3 Content in Male and Female Floral Buds
2.7.1. PG Mating Type
2.7.2. PA Mating Type
2.8. Real Time Quantitative PCR Validation of RNA-seq Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. RNA Extraction and Illumina Sequencing
4.3. Assembly and Differentially Expressed Genes (DEGs) Analysis
4.4. Measurement of GAs Content
4.5. Quantitative Real Time PCR (qRT-PCR) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | Protandry |
| PG | Protogyny |
| PA-F | Female floral buds from a protandrous plant |
| PA-M | Male floral buds from a protandrous plant |
| PG-F | Female floral buds from a protogynous plant |
| PG-M | Male floral buds from a protogynous plant |
| GAs | Gibberellins |
| DEGs | Differentially expressed genes |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| FDR | False discovery rate |
| FPKM | Fragments per kilobase per million reads |
References
- Liu, Y.; Fang, S.Z.; Yang, W.X.; Shang, X.L.; Fu, X.X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photochem. Photobiol. B Biol. 2018, 179, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Z.; Fu, X.X. Progress and prospects on silviculture and utilization of Cyclocarya paliurus resources. J. Nanjing For. Univ. 2007, 31, 95–100. [Google Scholar]
- Xie, M.Y.; Li, L. Review in studies on chemical constituents and bioactivities of Cyclocarya paliurus. Zhong Cao Yao 2001, 32, 365–366. [Google Scholar]
- Shang, X.L.; Wu, Z.F.; Yin, Z.Q.; Zhang, J.; Fang, S.Z. Simultaneous determination of flavonoids and triterpenoids in Cyclocarya paliurus leaves using high-performance liquid chromatography. Aer. J. Tradit. Complem. 2015, 12, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.H.; Shen, M.Y.; Nie, S.P.; Liu, X.; Zhang, H.; Xie, M.Y. Analysis of monosaccharide composition of Cyclocarya paliurus polysaccharide with anion exchange chromatography. Carbohyd. Polym. 2013, 98, 976–981. [Google Scholar] [CrossRef]
- Xie, J.H.; Shen, M.Y.; Nie, S.P.; Li, C.; Gong, D.M.; Xie, M.Y. Proximate, amino acid and fatty acid composition in the leaves of Cyclocarya paliurus (Batal.) Iljinskaja. J. Food Agric. Environ. 2013, 11, 377–381. [Google Scholar]
- Fu, X.X.; Feng, L.; Shang, X.L.; Yang, W.X.; Fang, S.Z. Observation of morphological and anatomical characters on staminate and pistillate flower differentiation in Cyclocarya paliurus. J. Nanjing For. Univ. 2011, 35, 17–22. [Google Scholar]
- Fu, X.X.; Feng, L.; Fang, S.Z.; Mao, J. Observation on flowering habits and anatomy of stamen development in Cyclocarya paliurus. J. Nanjing For. Univ. 2010, 34, 67–71. [Google Scholar]
- Webb, D.J.; Lloyd, D.G. The avoidance of interference between the presentation of pollen and stigmas in angiosperms II. Herkogamy. New Zeal. J. Bot. 1986, 24, 163–178. [Google Scholar] [CrossRef]
- Renner, S.S. How common is heterodichogamy? Trends Ecol. Evol. 2001, 16, 595–597. [Google Scholar] [CrossRef]
- Fukuhara, T.; Tokumaru, S. Inflorescence dimorphism, heterodichogamy and thrips pollination in Platycarya strobilacea (Juglandaceae). Ann. Bot. 2014, 113, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Fu, X.X.; Huang, P.; Chen, X.L.; Qu, Y.Q. Heterodichogamy, Pollen Viability, and Seed Set in a Population of Polyploidy Cyclocarya Paliurus (Batal) Iljinskaja (Juglandaceae). Forests 2019, 10, 347. Available online: https://doi:10.3390/f10040347 (accessed on 13 May 2022). [CrossRef] [Green Version]
- Beveridge, C.A. Axillary bud outgrowth: Sending a message. Curr. Opin. Plant Biol. 2006, 9, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mori, H. Control of Outgrowth and Dormancy in Axillary Buds. Plant Physiol. 2001, 127, 1405–1413. [Google Scholar]
- Ni, J.; Shah, F.A.; Liu, W.; Wang, Q.; Wang, D.; Zhao, W.; Lu, W.; Huang, S.; Fu, S.; Wu, L. Comparative transcriptome analysis reveals the regulatory networks of cytokinin in promoting the floral feminization in the oil plant Sapium sebiferum. BMC Plant Biol. 2018, 18, 96. Available online: https://doi:10.1186/s12870-018-1314-5 (accessed on 13 May 2022). [CrossRef] [Green Version]
- Dierck, R.; De, E.K.; De, J.R.; Dhooghe, E.; Van, J.H.; Prinsen, E.; Van, D.S. Change in Auxin and Cytokinin Levels Coincides with Altered Expression of Branching Genes during Axillary Bud Outgrowth in Chrysanthemum. PLoS ONE 2016, 11, e161732. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Zhang, D.; Zhao, C.; Li, Y.; Ma, J.; An, N.; Han, M. Shoot bending promotes flower bud formation by miRNA-mediated regulation in apple (Malus domestica Borkh.). Plant Biotechnol. J. 2016, 14, 749–770. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Liao, J.; Liu, B.; Cao, S.; Azam, M.; Xia, Y.P. Effects of 5-azacytidine and gibberellic acid on flower development of azalea. Pak. J. Agr. Sci. 2016, 53, 1–6. [Google Scholar]
- Li, J.J.; Pan, X.J.; Zhang, W.E. Relationship between Mineral Nutritions, Hormone Content and Flower Bud Differentiation of Juglans sigillata. Acta. Bot. Boreal. Occident. Sin. 2016, 36, 971–978. [Google Scholar]
- Zhu, Z.; Jiang, C.; Shi, Y.; Chen, W.; Chen, N.; Zhao, M.; Wu, W. Variations of Endogenous Hormones in Lateral Buds of Olive Trees (Olea europaea) during Floral Induction and Flower-Bud Differentiation. Sci. Silvae Sin. 2015, 51, 32–39. [Google Scholar]
- Zhang, D.; Ren, L.; Yue, J.H.; Wang, L.; Zhuo, L.H.; Shen, X.H. GA4 and IAA were involved in the morphogenesis and development of flowers in Agapanthus praecox ssp. orientalis. J. Plant Physiol. 2014, 171, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Cecich, R.A. White spruce (Piceaglauca) flowering in response to spray application of gibberellin A4/7. Can. J. For. 2011, 15, 170–174. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, V.S. Sex reversal and fruit formation on male plants of Carica Papaya L by ethrel and chlorflurenol. Proc. Plant Sci. 1984, 93, 635–641. [Google Scholar] [CrossRef]
- Chailakhyan, M.K. Genetic and Hormonal Regulation of Growth, Flowering, and Sex Expression in Plants. Am. J. Bot. 1976, 66, 717–736. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, J.; Wei, L. Studies on the Changes of Endogenous Hormones in the Differentiation Period of Flower Bud in Apple Trees. J. Fruit Sci. 2000, 17, 244–248. [Google Scholar]
- Shi, J.K.; Zhang, W.P.; Fan, W.G.; Wen, X.P. Changes in Endogenous Hormones during the Differentiation of Female Flower Bud of Ginkgo (G. Biloba L). Acta Hortic. Sin. 1999, 26, 194–195. [Google Scholar]
- Randoux, M.; Jeauffre, J.; Thouroude, T.; Vasseur, F.; Foucher, F. Gibberellins regulate the transcription of the continuous flowering regulator, RoKSN, a rose TFL1 homologue. J. Exp. Bot. 2012, 63, 6543–6554. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jia, H.; Wang, J.; Zhang, Z. Flowering and pollination patterns of Magnolia denudata with emphasis on anatomical changes in ovule and seed development. Flora 2010, 205, 259–265. [Google Scholar] [CrossRef]
- Lu, Y.J.; Chen, C.; Wang, R.H.; Ashley, N.E.; Fu, C.X. Effects of domestication on genetic diversity in Chimonanthus praecox: Evidence from chloroplast DNA and amplified fragment length polymorphism data. J. Syst. Evol. 2015, 53, 239–251. [Google Scholar] [CrossRef]
- Lu, N.N.; Li, X.H.; Li, L.; Zhao, Z.G. Variation of nectar production in relation to plant characteristics in protandrous Aconitum gymnandrum. J. Plant Ecol. 2015, 8, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Wang, R.H.; Xie, L.; Long, R.; Zhang, Z.X. Flowering dynamics and dichogamous mechanism in Magnolia grandiflora. J. Beijing For. Univ. 2011, 33, 63–69. [Google Scholar]
- Zhang, R.B.; Dou, Q.L.; Ping, H.E.; Xiao, Y.A.; Liu, Y.; Hu, S.J. Study on the breeding system of the endangered plant Euonymus chloranthoides Yang. Guihaia 2006, 26, 308–312. [Google Scholar]
- Polito, V.S. Microsporogenesis and Anther Differentiation in Juglans regia L.: A Developmental Basis for Heterodichogamy in Walnut. Bot. Gaz. 1988, 149, 30–36. [Google Scholar]
- Zhang, L.J.; Guo, C.; Qin, B.T.; Lu, X.J.; Yang, Y.C.; Qi, Y.H.; Shen, H.L. Phonological Characteristics of Flowering and Pollen Viability of Juglans mandshurica. J. Northeast For. Univ. 2019, 47, 4–8. [Google Scholar]
- Polito, V.S.; Pinney, K. The relationship between phenology of pistillate flower organogenesis and mode of heterodichogamy in Juglans regia L. (Juglandaceae). Sex Plant Reprod. 1997, 10, 36–39. [Google Scholar] [CrossRef]
- Yu, H.; Ito, T.; Zhao, Y.; Peng, J.; Kumar, P.; Meyerowitz, E.M. Floral homeotic genes are targets of gibberellin signaling in flower development. Proc. Natl. Acad. Sci. USA 2004, 101, 7827–7832. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Zhang, X.; Liu, X.; Zhang, L. Evolutionary analysis of MIKCc-type MADS-box genes in gymnosperms and angiosperms. Front Plant Sci. 2017, 8, 895. [Google Scholar] [CrossRef] [Green Version]
- Bai, W.N.; Zeng, Y.F.; Zhang, D.Y. Mating patterns and pollen dispersal in a heterodichogamous tree, Juglans mandshurica (Juglandaceae). New Phytol. 2007, 176, 699–707. [Google Scholar] [CrossRef]
- Thompson, T.E.; Romberg, L.D. Inheritance of heterodichogamy in pecan. Heredity 1985, 76, 456–458. [Google Scholar] [CrossRef]
- Fu, X.; Richards, D.E.; Ait, A.T.; Hynes, L.W.; Harberd, N.P. Gibberellin-Mediated Proteasome-Dependent Degradation of the Barley DELLA Protein SLN1 Repressor. Plant Cell 2003, 14, 3191–3200. [Google Scholar] [CrossRef] [Green Version]
- Murase, K.; Hirano, Y.; Sun, T.; Hakoshima, T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 2008, 456, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Browning, G. Gibberellin structure-activity effects on flower initiation in mature trees and on shoot growth in mature and juvenile Prunus avium. Plant Growth Regul. 1993, 13, 55–63. [Google Scholar] [CrossRef]
- Maria, M.J.P.; Lange, T. Ovary-derived precursor gibberellin A9 is essential for female flower development in cucumber. Development 2016, 143, 4425–4429. [Google Scholar]
- Van, D.W.; Çelikel, F.G.; Pak, C.; Harkema, H. Delay of Iris flower senescence by cytokinins and jasmonates. Physiol. Plantrum. 2013, 148, 105–120. [Google Scholar]
- Chaudhry, N.Y.; Khan, A.S. Improvement of pistillate flowers yield with GA3 in heavy metals treated plants. Plant Growth Regul. 2006, 50, 211–217. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Fan, S.; Du, L.; Shen, Y.; Xing, L.; Li, Y.; Ma, J.; Han, M. Effect of exogenous GA3 and its inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple (Malus domestica Borkh.). Plant Physiol. Bioch. 2016, 107, 178–186. [Google Scholar] [CrossRef]
- Chang, M.Z.; Huang, C.H. Effects of GA3 on promotion of flowering in Kalanchoe spp. Sci. Hortic-Amsterdam. 2018, 238, 7–13. Available online: https://doi:10.1016/j.scienta.2018.04.001 (accessed on 13 May 2022). [CrossRef]
- Shang, M.Y.; Wang, X.T.; Zhang, J.; Qi, X.H.; Amin, P.; Hou, L.P.; Xing, G.M.; Li, G.Z.; Li, M.L. Genetic Regulation of GA Metabolism during Vernalization, Floral Bud Initiation and Development in Pak Choi (Brassica rapa ssp. chinensis Makino). Front. Plant Sci. 2017, 8, 1533. Available online: https://doi:10.3389/fpls.2017.01533 (accessed on 13 May 2022). [CrossRef]
- Wilson, R.N.; Heckman, J.W.; Somerville, C.R. Gibberellin Is Required for Flowering in Arabidopsis thaliana under Short Days. Plant Physiol. 1992, 100, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Groot, S.P.C.; Bruinsma, J.; Karssen, C.M. The role of endogenous gibberellin in seed and fruit development of tomato: Studies with a gibberellin deficient mutant. Physiol. Plantarum. 1987, 71, 184–190. [Google Scholar] [CrossRef]
- Peng, S.; Li, J.; Du, G.; Han, W.; Fu, J.; Diao, S.; Suo, Y.; Yue, Z.; Li, F. Endogenous phytohormone profiles in male and female floral buds of the persimmons (Diospyros kaki Thunb.) during development. Sci. Hortic-Amsterdam. 2017, 218, 213–221. [Google Scholar]
- Fan, L.; Chen, M.; Dong, B.; Wang, N.; Yu, Q.; Wang, X.; Xuan, L.; Wang, Y.; Zhang, S.; Shen, Y. Transcriptomic Analysis of Flower Bud Differentiation in Magnolia sinostellata. Genes-Basel 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Langmeasd, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Regev, A. De novo transcript sequence reconstruction from RNA-Seq using the Trinity platform for reference generation and analysis. Nat. Protocol. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Chen, X.L.; Mao, X.; Huang, P.; Fang, S.Z. Morphological Characterization of Flower Buds Development and Related Gene Expression Profiling at Bud Break Stage in Heterodichogamous Cyclocarya paliurus (Batal.) lljinskaja. Genes 2019, 10, 818. [Google Scholar] [CrossRef] [Green Version]


| GA Type | Floral Bud | Maximum | Minimum | Range * (ng/g FW) | ||
|---|---|---|---|---|---|---|
| Content (ng/g FW) | Occurred Stage | Content (ng/g FW) | Occurred Stage | |||
| GA1 | PA-F | 1.65 | S1 | 0.29 | S2 | 1.36 |
| PA-M | 7.19 | S0 | 0.20 | S3 | 6.99 | |
| PG-F | 3.03 | S0 | 0.00 | S3/S4 | 3.03 | |
| PG-M | 4.05 | S0 | 0.00 | S3/S4 | 4.05 | |
| GA3 | PA-F | 40.37 | S2 | 4.56 | S4 | 35.81 |
| PA-M | 116.50 | S2 | 2.53 | S3 | 113.97 | |
| PG-F | 80.46 | S0 | 0.97 | S4 | 79.49 | |
| PG-M | 106.33 | S2 | 0.70 | S3 | 105.63 | |
| GA4 | PA-F | 0.61 | S4 | 0.00 | S2 | 0.61 |
| PA-M | 1.07 | S2 | 0.00 | S3/S0 | 1.07 | |
| PG-F | 0.78 | S2 | 0.00 | S1/S0 | 0.78 | |
| PG-M | 0.97 | S0 | 0.00 | S1/S2 | 0.97 | |
| GA7 | PA-F | 0.00 | S0/S1/S2/S3/S4 | 0.00 | S0/S1/S2/S3/S4 | 0.00 |
| PA-M | 0.33 | S4 | 0.00 | S0/S2/S3 | 0.33 | |
| PG-F | 3.27 | S0 | 0.00 | S2/S4 | 3.27 | |
| PG-M | 0.70 | S2 | 0.00 | S1/S2/S3 | 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Chen, X.; Mao, X.; Huang, P.; Fu, X. Transcriptome Analysis Reveals the Role of GA3 in Regulating the Asynchronism of Floral Bud Differentiation and Development in Heterodichogamous Cyclocarya paliurus (Batal.) Iljinskaja. Int. J. Mol. Sci. 2022, 23, 6763. https://doi.org/10.3390/ijms23126763
Qu Y, Chen X, Mao X, Huang P, Fu X. Transcriptome Analysis Reveals the Role of GA3 in Regulating the Asynchronism of Floral Bud Differentiation and Development in Heterodichogamous Cyclocarya paliurus (Batal.) Iljinskaja. International Journal of Molecular Sciences. 2022; 23(12):6763. https://doi.org/10.3390/ijms23126763
Chicago/Turabian StyleQu, Yinquan, Xiaolin Chen, Xia Mao, Peng Huang, and Xiangxiang Fu. 2022. "Transcriptome Analysis Reveals the Role of GA3 in Regulating the Asynchronism of Floral Bud Differentiation and Development in Heterodichogamous Cyclocarya paliurus (Batal.) Iljinskaja" International Journal of Molecular Sciences 23, no. 12: 6763. https://doi.org/10.3390/ijms23126763
APA StyleQu, Y., Chen, X., Mao, X., Huang, P., & Fu, X. (2022). Transcriptome Analysis Reveals the Role of GA3 in Regulating the Asynchronism of Floral Bud Differentiation and Development in Heterodichogamous Cyclocarya paliurus (Batal.) Iljinskaja. International Journal of Molecular Sciences, 23(12), 6763. https://doi.org/10.3390/ijms23126763






