New Developments in Carbonic Anhydrase IX-Targeted Fluorescence and Nuclear Imaging Agents
Abstract
:1. Introduction
1.1. CAIX Is a Biomarker for the Detection of Solid Tumors
1.2. Fluorescence Imaging and Nuclear Imaging of CAIX
2. Antibody-Based Imaging Agents
3. Peptide-Based Compounds
4. Small-Molecule-Based Compounds
4.1. Benzenesulfonamide (BSA)
4.2. Acetazolamide (AAZ)
4.3. Saccharin
4.4. Imidazothiadiazole Sulfonamide (IS)
4.5. Multivalent CAIX Ligands
4.6. Dual-Motif CAIX Inhibitor: XYIMSR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.H.; Griffiths, J.R. How and Why Are Cancers Acidic? Carbonic Anhydrase IX and the Homeostatic Control of Tumour Extracellular pH. Cancers 2020, 12, 1616. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swietach, P.; Wigfield, S.; Cobden, P.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J. Biol. Chem. 2008, 283, 20473–20483. [Google Scholar] [CrossRef] [Green Version]
- Pastorek, J.; Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer Biol. 2015, 31, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Stillebroer, A.B.; Mulders, P.F.; Boerman, O.C.; Oyen, W.J.; Oosterwijk, E. Carbonic anhydrase IX in renal cell carcinoma: Implications for prognosis, diagnosis, and therapy. Eur. Urol. 2010, 58, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Svastova, E.; Hulikova, A.; Rafajova, M.; Zat'ovicova, M.; Gibadulinova, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.; Sevick-Muraca, E.M. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br. J. Radiol. 2015, 88, 20140547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uemura, H.; Nakagawa, Y.; Yoshida, K.; Saga, S.; Yoshikawa, K.; Hirao, Y.; Oosterwijk, E. MN/CA IX/G250 as a potential target for immunotherapy of renal cell carcinomas. Br. J. Cancer 1999, 81, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Oosterwijk, E.; Bander, N.H.; Divgi, C.R.; Welt, S.; Wakka, J.C.; Finn, R.D.; Carswell, E.A.; Larson, S.M.; Warnaar, S.O.; Fleuren, G.J.; et al. Antibody localization in human renal cell carcinoma: A phase I study of monoclonal antibody G250. J. Clin. Oncol. 1993, 11, 738–750. [Google Scholar] [CrossRef]
- Divgi, C.R.; Pandit-Taskar, N.; Jungbluth, A.A.; Reuter, V.E.; Gonen, M.; Ruan, S.; Pierre, C.; Nagel, A.; Pryma, D.A.; Humm, J.; et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: A phase I trial. Lancet Oncol. 2007, 8, 304–310. [Google Scholar] [CrossRef]
- Divgi, C.R.; Uzzo, R.G.; Gatsonis, C.; Bartz, R.; Treutner, S.; Yu, J.Q.; Chen, D.; Carrasquillo, J.A.; Larson, S.; Bevan, P.; et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: Results from the REDECT trial. J. Clin. Oncol. 2013, 31, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muselaers, C.H.; Boerman, O.C.; Oosterwijk, E.; Langenhuijsen, J.F.; Oyen, W.J.; Mulders, P.F. Indium-111-labeled girentuximab immunoSPECT as a diagnostic tool in clear cell renal cell carcinoma. Eur. Urol. 2013, 63, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, A.H.; Buijs, W.C.; Oosterwijk, E.; Boerman, O.C.; Mala, C.; De Mulder, P.H.; Corstens, F.H.; Mulders, P.F.; Oyen, W.J. Targeting of metastatic renal cell carcinoma with the chimeric monoclonal antibody G250 labeled with (131)I or (111)In: An intrapatient comparison. Clin. Cancer Res. 2003, 9, 3953S–3960S. [Google Scholar] [PubMed]
- Merkx, R.I.J.; Lobeek, D.; Konijnenberg, M.; Jimenez-Franco, L.D.; Kluge, A.; Oosterwijk, E.; Mulders, P.F.A.; Rijpkema, M. Phase I study to assess safety, biodistribution and radiation dosimetry for (89)Zr-girentuximab in patients with renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Muselaers, C.H.; Stillebroer, A.B.; Rijpkema, M.; Franssen, G.M.; Oosterwijk, E.; Mulders, P.F.; Oyen, W.J.; Boerman, O.C. Optical Imaging of Renal Cell Carcinoma with Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab. J. Nucl. Med. 2014, 55, 1035–1040. [Google Scholar] [CrossRef] [Green Version]
- Muselaers, C.H.; Rijpkema, M.; Bos, D.L.; Langenhuijsen, J.F.; Oyen, W.J.; Mulders, P.F.; Oosterwijk, E.; Boerman, O.C. Radionuclide and Fluorescence Imaging of Clear Cell Renal Cell Carcinoma Using Dual Labeled Anti-Carbonic Anhydrase IX Antibody G250. J. Urol. 2015, 194, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Hekman, M.C.; Rijpkema, M.; Muselaers, C.H.; Oosterwijk, E.; Hulsbergen-Van de Kaa, C.A.; Boerman, O.C.; Oyen, W.J.; Langenhuijsen, J.F.; Mulders, P.F. Tumor-targeted Dual-modality Imaging to Improve Intraoperative Visualization of Clear Cell Renal Cell Carcinoma: A First in Man Study. Theranostics 2018, 8, 2161–2170. [Google Scholar] [CrossRef]
- Hoeben, B.A.; Kaanders, J.H.; Franssen, G.M.; Troost, E.G.; Rijken, P.F.; Oosterwijk, E.; van Dongen, G.A.; Oyen, W.J.; Boerman, O.C.; Bussink, J. PET of hypoxia with 89Zr-labeled cG250-F(ab')2 in head and neck tumors. J. Nucl. Med. 2010, 51, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Huizing, F.J.; Hoeben, B.A.W.; Franssen, G.; Lok, J.; Heskamp, S.; Oosterwijk, E.; Boerman, O.C.; Bussink, J. Preclinical validation of (111)In-girentuximab-F(ab')2 as a tracer to image hypoxia related marker CAIX expression in head and neck cancer xenografts. Radiother. Oncol. 2017, 124, 521–525. [Google Scholar] [CrossRef]
- Huizing, F.J.; Hoeben, B.A.W.; Franssen, G.M.; Boerman, O.C.; Heskamp, S.; Bussink, J. Quantitative Imaging of the Hypoxia-Related Marker CAIX in Head and Neck Squamous Cell Carcinoma Xenograft Models. Mol. Pharm. 2019, 16, 701–708. [Google Scholar] [CrossRef]
- Huizing, F.J.; Hoeben, B.A.W.; Lok, J.; Boerman, O.C.; Heskamp, S.; Bussink, J. Imaging carbonic anhydrase IX as a method for monitoring hypoxia-related radioresistance in preclinical head and neck cancer models. Phys. Imaging Radiat. Oncol. 2021, 19, 145–150. [Google Scholar] [CrossRef] [PubMed]
- van Lith, S.A.M.; Huizing, F.J.; Franssen, G.M.; Hoeben, B.A.W.; Lok, J.; Doulkeridou, S.; Boerman, O.C.; Gotthardt, M.; van Bergen En Henegouwen, P.M.P.; Bussink, J.; et al. Novel VHH-Based Tracers with Variable Plasma Half-Lives for Imaging of CAIX-Expressing Hypoxic Tumor Cells. Mol. Pharm. 2022. [Google Scholar] [CrossRef] [PubMed]
- Huizing, F.J.; Garousi, J.; Lok, J.; Franssen, G.; Hoeben, B.A.W.; Frejd, F.Y.; Boerman, O.C.; Bussink, J.; Tolmachev, V.; Heskamp, S. CAIX-targeting radiotracers for hypoxia imaging in head and neck cancer models. Sci. Rep. 2019, 9, 18898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garousi, J.; Huizing, F.J.; Vorobyeva, A.; Mitran, B.; Andersson, K.G.; Leitao, C.D.; Frejd, F.Y.; Lofblom, J.; Bussink, J.; Orlova, A.; et al. Comparative evaluation of affibody- and antibody fragments-based CAIX imaging probes in mice bearing renal cell carcinoma xenografts. Sci. Rep. 2019, 9, 14907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Brussel, A.S.; Adams, A.; Oliveira, S.; Dorresteijn, B.; El Khattabi, M.; Vermeulen, J.F.; van der Wall, E.; Mali, W.P.; Derksen, P.W.; van Diest, P.J.; et al. Hypoxia-Targeting Fluorescent Nanobodies for Optical Molecular Imaging of Pre-Invasive Breast Cancer. Mol. Imaging Biol. 2016, 18, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Garousi, J.; Honarvar, H.; Andersson, K.G.; Mitran, B.; Orlova, A.; Buijs, J.; Lofblom, J.; Frejd, F.Y.; Tolmachev, V. Comparative Evaluation of Affibody Molecules for Radionuclide Imaging of in Vivo Expression of Carbonic Anhydrase IX. Mol. Pharm. 2016, 13, 3676–3687. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Garcia-Boy, R.; Rana, S.; Kramer, S.; Hebling, U.; Mier, W.; Altmann, A.; Markert, A.; Debus, J.; Haberkorn, U. A new peptide ligand for targeting human carbonic anhydrase IX, identified through the phage display technology. PLoS ONE 2010, 5, e15962. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Ehemann, V.; Rana, S.; Kramer, S.; Rahbari, N.N.; Debus, J.; Haberkorn, U. Binding of the phage display derived peptide CaIX-P1 on human colorectal carcinoma cells correlates with the expression of carbonic anhydrase IX. Int. J. Mol. Sci. 2012, 13, 13030–13048. [Google Scholar] [CrossRef]
- Rana, S.; Nissen, F.; Marr, A.; Markert, A.; Altmann, A.; Mier, W.; Debus, J.; Haberkorn, U.; Askoxylakis, V. Optimization of a novel peptide ligand targeting human carbonic anhydrase IX. PLoS ONE 2012, 7, e38279. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Nissen, F.; Lindner, T.; Altmann, A.; Mier, W.; Debus, J.; Haberkorn, U.; Askoxylakis, V. Screening of a novel peptide targeting the proteoglycan-like region of human carbonic anhydrase IX. Mol Imaging 2013, 12, 7290. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Li, X.; Cheng, D.; Zhang, L. Fluorine-18 click radiosynthesis and microPET/CT evaluation of a small peptide—A potential PET probe for carbonic anhydrase IX. Bioorg. Med. Chem. 2019, 27, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Angapelly, S.; Sri Ramya, P.V.; Angeli, A.; Supuran, C.T.; Arifuddin, M. Sulfocoumarin-, Coumarin-, 4-Sulfamoylphenyl-Bearing Indazole-3-carboxamide Hybrids: Synthesis and Selective Inhibition of Tumor-Associated Carbonic Anhydrase Isozymes IX and XII. ChemMedChem 2017, 12, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Aggarwal, M.; Maresca, A.; Scozzafava, A.; McKenna, R.; Masini, E.; Supuran, C.T. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J. Med. Chem. 2012, 55, 1721–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresca, A.; Carta, F.; Vullo, D.; Supuran, C.T. Dithiocarbamates strongly inhibit the beta-class carbonic anhydrases from Mycobacterium tuberculosis. J. Enzyme Inhib. Med. Chem. 2013, 28, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg. Med. Chem. Lett. 2008, 18, 1583–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, L.; Lieuwes, N.G.; Maresca, A.; Thiry, A.; Supuran, C.T.; Scozzafava, A.; Wouters, B.G.; Lambin, P. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother. Oncol. 2009, 92, 423–428. [Google Scholar] [CrossRef]
- Akurathi, V.; Dubois, L.; Lieuwes, N.G.; Chitneni, S.K.; Cleynhens, B.J.; Vullo, D.; Supuran, C.T.; Verbruggen, A.M.; Lambin, P.; Bormans, G.M. Synthesis and biological evaluation of a 99mTc-labelled sulfonamide conjugate for in vivo visualization of carbonic anhydrase IX expression in tumor hypoxia. Nucl. Med. Biol. 2010, 37, 557–564. [Google Scholar] [CrossRef]
- Lu, G.; Hillier, S.M.; Maresca, K.P.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. Synthesis and SAR of novel Re/99mTc-labeled benzenesulfonamide carbonic anhydrase IX inhibitors for molecular imaging of tumor hypoxia. J. Med. Chem. 2013, 56, 510–520. [Google Scholar] [CrossRef]
- Nakai, M.; Pan, J.; Lin, K.S.; Thompson, J.R.; Nocentini, A.; Supuran, C.T.; Nakabayashi, Y.; Storr, T. Evaluation of (99m)Tc-sulfonamide and sulfocoumarin derivatives for imaging carbonic anhydrase IX expression. J. Inorg. Biochem. 2018, 185, 63–70. [Google Scholar] [CrossRef]
- Pan, J.; Lau, J.; Mesak, F.; Hundal, N.; Pourghiasian, M.; Liu, Z.; Benard, F.; Dedhar, S.; Supuran, C.T.; Lin, K.S. Synthesis and evaluation of 18F-labeled carbonic anhydrase IX inhibitors for imaging with positron emission tomography. J. Enzyme Inhib. Med. Chem. 2014, 29, 249–255. [Google Scholar] [CrossRef]
- Lau, J.; Liu, Z.; Lin, K.S.; Pan, J.; Zhang, Z.; Vullo, D.; Supuran, C.T.; Perrin, D.M.; Benard, F. Trimeric Radiofluorinated Sulfonamide Derivatives to Achieve In Vivo Selectivity for Carbonic Anhydrase IX-Targeted PET Imaging. J. Nucl. Med. 2015, 56, 1434–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Lau, J.; Zhang, C.; Colpo, N.; Nocentini, A.; Supuran, C.T.; Benard, F.; Lin, K.S. Design, synthesis and evaluation of (18)F-labeled cationic carbonic anhydrase IX inhibitors for PET imaging. J. Enzyme Inhib. Med. Chem. 2017, 32, 722–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlskog, J.K.; Dumelin, C.E.; Trussel, S.; Marlind, J.; Neri, D. In vivo targeting of tumor-associated carbonic anhydrases using acetazolamide derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 4851–4856. [Google Scholar] [CrossRef] [PubMed]
- Groves, K.; Bao, B.; Zhang, J.; Handy, E.; Kennedy, P.; Cuneo, G.; Supuran, C.T.; Yared, W.; Peterson, J.D.; Rajopadhye, M. Synthesis and evaluation of near-infrared fluorescent sulfonamide derivatives for imaging of hypoxia-induced carbonic anhydrase IX expression in tumors. Bioorg. Med. Chem. Lett. 2012, 22, 653–657. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Chu, H.; Liu, X.; Leamon, C.P.; Low, P.S. Carbonic Anhydrase IX-Targeted Near-Infrared Dye for Fluorescence Imaging of Hypoxic Tumors. Bioconjug. Chem. 2018, 29, 3320–3331. [Google Scholar] [CrossRef]
- Krall, N.; Pretto, F.; Mattarella, M.; Muller, C.; Neri, D. A 99mTc-Labeled Ligand of Carbonic Anhydrase IX Selectively Targets Renal Cell Carcinoma In Vivo. J. Nucl. Med. 2016, 57, 943–949. [Google Scholar] [CrossRef] [Green Version]
- More, K.N.; Lee, J.Y.; Kim, D.Y.; Cho, N.C.; Pyo, A.; Yun, M.; Kim, H.S.; Kim, H.; Ko, K.; Park, J.H.; et al. Acetazolamide-based [(18)F]-PET tracer: In vivo validation of carbonic anhydrase IX as a sole target for imaging of CA-IX expressing hypoxic solid tumors. Bioorg. Med. Chem. Lett. 2018, 28, 915–921. [Google Scholar] [CrossRef]
- Chen, K.T.; Nguyen, K.; Ieritano, C.; Gao, F.; Seimbille, Y. A Flexible Synthesis of (68)Ga-Labeled Carbonic Anhydrase IX (CAIX)-Targeted Molecules via CBT/1,2-Aminothiol Click Reaction. Molecules 2018, 24, 23. [Google Scholar] [CrossRef] [Green Version]
- Shin, U.C.; Choi, J.S.; Beak, Y.J.; Lee, M.W.; Kim, H.S.; Choi, D.W.; Kim, D.G.; Kim, S.W. Development of a (68) Ga-labelled PET tracer for carbonic anhydrase IX-overexpressed tumors using the artificial sweetener saccharin. J. Labelled Comp. Radiopharm. 2021, 64, 129–139. [Google Scholar] [CrossRef]
- Iikuni, S.; Okada, Y.; Shimizu, Y.; Watanabe, H.; Ono, M. Synthesis and evaluation of indium-111-labeled imidazothiadiazole sulfonamide derivative for single photon emission computed tomography imaging targeting carbonic anhydrase-IX. Bioorg. Med. Chem. Lett. 2020, 30, 127255. [Google Scholar] [CrossRef]
- Iikuni, S.; Okada, Y.; Shimizu, Y.; Watanabe, H.; Ono, M. Modulation of the Pharmacokinetics of a Radioligand Targeting Carbonic Anhydrase-IX with Albumin-Binding Moieties. Mol. Pharm. 2021, 18, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Krall, N.; Pretto, F.; Neri, D. A bivalent small molecule-drug conjugate directed against carbonic anhydrase IX can elicit complete tumour regression in mice. Chem. Sci. 2014, 5, 3640–3644. [Google Scholar] [CrossRef]
- Lv, P.C.; Roy, J.; Putt, K.S.; Low, P.S. Evaluation of a Carbonic Anhydrase IX-Targeted Near-Infrared Dye for Fluorescence-Guided Surgery of Hypoxic Tumors. Mol. Pharm. 2016, 13, 1618–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, P.C.; Putt, K.S.; Low, P.S. Evaluation of Nonpeptidic Ligand Conjugates for SPECT Imaging of Hypoxic and Carbonic Anhydrase IX-Expressing Cancers. Bioconjug. Chem. 2016, 27, 1762–1769. [Google Scholar] [CrossRef] [Green Version]
- Iikuni, S.; Ono, M.; Watanabe, H.; Shimizu, Y.; Sano, K.; Saji, H. Cancer radiotheranostics targeting carbonic anhydrase-IX with (111)In- and (90)Y-labeled ureidosulfonamide scaffold for SPECT imaging and radionuclide-based therapy. Theranostics 2018, 8, 2992–3006. [Google Scholar] [CrossRef]
- Iikuni, S.; Watanabe, H.; Shimizu, Y.; Nakamoto, Y.; Ono, M. PET imaging and pharmacological therapy targeting carbonic anhydrase-IX high-expressing tumors using US2 platform based on bivalent ureidosulfonamide. PLoS ONE 2020, 15, e0243327. [Google Scholar] [CrossRef]
- Nakashima, K.; Iikuni, S.; Okada, Y.; Watanabe, H.; Shimizu, Y.; Nakamoto, Y.; Ono, M. Synthesis and evaluation of (68)Ga-labeled imidazothiadiazole sulfonamide derivatives for PET imaging of carbonic anhydrase-IX. Nucl. Med. Biol. 2021, 93, 46–53. [Google Scholar] [CrossRef]
- Iikuni, S.; Tanimura, K.; Watanabe, H.; Shimizu, Y.; Saji, H.; Ono, M. Development of the (99m)Tc-Hydroxamamide Complex as a Probe Targeting Carbonic Anhydrase IX. Mol. Pharm. 2019, 16, 1489–1497. [Google Scholar] [CrossRef]
- Iikuni, S.; Kitano, A.; Watanabe, H.; Shimizu, Y.; Ono, M. Synthesis and evaluation of novel technetium-99m-hydroxamamide complex based on imidazothiadiazole sulfonamide targeting carbonic anhydrase-IX for tumor imaging. Bioorg. Med. Chem. Lett. 2020, 30, 127596. [Google Scholar] [CrossRef]
- Lau, J.; Zhang, Z.; Jenni, S.; Kuo, H.T.; Liu, Z.; Vullo, D.; Supuran, C.T.; Lin, K.S.; Benard, F. PET Imaging of Carbonic Anhydrase IX Expression of HT-29 Tumor Xenograft Mice with (68)Ga-Labeled Benzenesulfonamides. Mol. Pharm. 2016, 13, 1137–1146. [Google Scholar] [CrossRef]
- Yang, X.; Minn, I.; Rowe, S.P.; Banerjee, S.R.; Gorin, M.A.; Brummet, M.; Lee, H.S.; Koo, S.M.; Sysa-Shah, P.; Mease, R.C.; et al. Imaging of carbonic anhydrase IX with an 111In-labeled dual-motif inhibitor. Oncotarget 2015, 6, 33733–33742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhu, H.; Yang, X.; Li, N.; Huang, H.; Liu, T.; Guo, X.; Xu, X.; Xia, L.; Deng, C.; et al. Targeting CAIX with [(64)Cu]XYIMSR-06 Small Molecular Radiotracer Enables Noninvasive PET Imaging of Malignant Glioma in U87 MG Tumor Cell Xenograft Mice. Mol. Pharm. 2019, 16, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, K.; An, Y.; Meng, H.; Gao, Y.; Xiong, Z.; Yan, H.; Wang, Q.; Cai, X.; Yang, X.; et al. In vivo three-dimensional evaluation of tumour hypoxia in nasopharyngeal carcinomas using FMT-CT and MSOT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1027–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchi, A.; Hulikova, A.; Pastorek, J.; Pastorekova, S.; Scozzafava, A.; Winum, J.Y.; Montero, J.L.; Supuran, C.T. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J. Med. Chem. 2005, 48, 4834–4841. [Google Scholar] [CrossRef]
- Turkbey, B.; Lindenberg, M.L.; Adler, S.; Kurdziel, K.A.; McKinney, Y.L.; Weaver, J.; Vocke, C.D.; Anver, M.; Bratslavsky, G.; Eclarinal, P.; et al. PET/CT imaging of renal cell carcinoma with (18)F-VM4-037: A phase II pilot study. Abdom. Radiol. 2016, 41, 109–118. [Google Scholar] [CrossRef]
- Krall, N.; Pretto, F.; Decurtins, W.; Bernardes, G.J.; Supuran, C.T.; Neri, D. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew. Chem. Int. Ed. Engl. 2014, 53, 4231–4235. [Google Scholar] [CrossRef]
- Bao, B.; Groves, K.; Zhang, J.; Handy, E.; Kennedy, P.; Cuneo, G.; Supuran, C.T.; Yared, W.; Rajopadhye, M.; Peterson, J.D. In vivo imaging and quantification of carbonic anhydrase IX expression as an endogenous biomarker of tumor hypoxia. PLoS ONE 2012, 7, e50860. [Google Scholar] [CrossRef] [Green Version]
- Kulterer, O.C.; Pfaff, S.; Wadsak, W.; Garstka, N.; Remzi, M.; Vraka, C.; Nics, L.; Mitterhauser, M.; Bootz, F.; Cazzamalli, S.; et al. A Microdosing Study with (99m)Tc-PHC-102 for the SPECT/CT Imaging of Primary and Metastatic Lesions in Renal Cell Carcinoma Patients. J. Nucl. Med. 2021, 62, 360–365. [Google Scholar] [CrossRef]
- Mahon, B.P.; Hendon, A.M.; Driscoll, J.M.; Rankin, G.M.; Poulsen, S.A.; Supuran, C.T.; McKenna, R. Saccharin: A lead compound for structure-based drug design of carbonic anhydrase IX inhibitors. Bioorg. Med. Chem. 2015, 23, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Wichert, M.; Krall, N.; Decurtins, W.; Franzini, R.M.; Pretto, F.; Schneider, P.; Neri, D.; Scheuermann, J. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat. Chem. 2015, 7, 241–249. [Google Scholar] [CrossRef]
- De Silva, R.A.; Gorin, M.A.; Mease, R.C.; Minn, I.; Lisok, A.; Plyku, D.; Nimmagadda, S.; Allaf, M.E.; Yang, X.; Sgouros, G.; et al. Process validation, current good manufacturing practice production, dosimetry, and toxicity studies of the carbonic anhydrase IX imaging agent [(111) In]In-XYIMSR-01 for phase I regulatory approval. J. Labelled Comp. Radiopharm. 2021, 64, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Minn, I.; Koo, S.M.; Lee, H.S.; Brummet, M.; Rowe, S.P.; Gorin, M.A.; Sysa-Shah, P.; Lewis, W.D.; Ahn, H.H.; Wang, Y.; et al. [64Cu]XYIMSR-06: A dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. Oncotarget 2016, 7, 56471–56479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, S.S.; Wu, C.T.; Liao, T.Z.; Lin, K.L.; Peng, C.L.; Shih, Y.H.; Weng, M.F.; Chen, C.T.; Yeh, C.H.; Wang, Y.C.; et al. novel (111)indium-labeled dual carbonic anhydrase 9-targeted probe as a potential SPECT imaging radiotracer for detection of hypoxic colorectal cancer cells. Eur. J. Pharm. Biopharm. 2021, 168, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Dudutiene, V.; Matuliene, J.; Smirnov, A.; Timm, D.D.; Zubriene, A.; Baranauskiene, L.; Morkunaite, V.; Smirnoviene, J.; Michailoviene, V.; Juozapaitiene, V.; et al. Discovery and characterization of novel selective inhibitors of carbonic anhydrase IX. J. Med. Chem. 2014, 57, 9435–9446. [Google Scholar] [CrossRef] [PubMed]
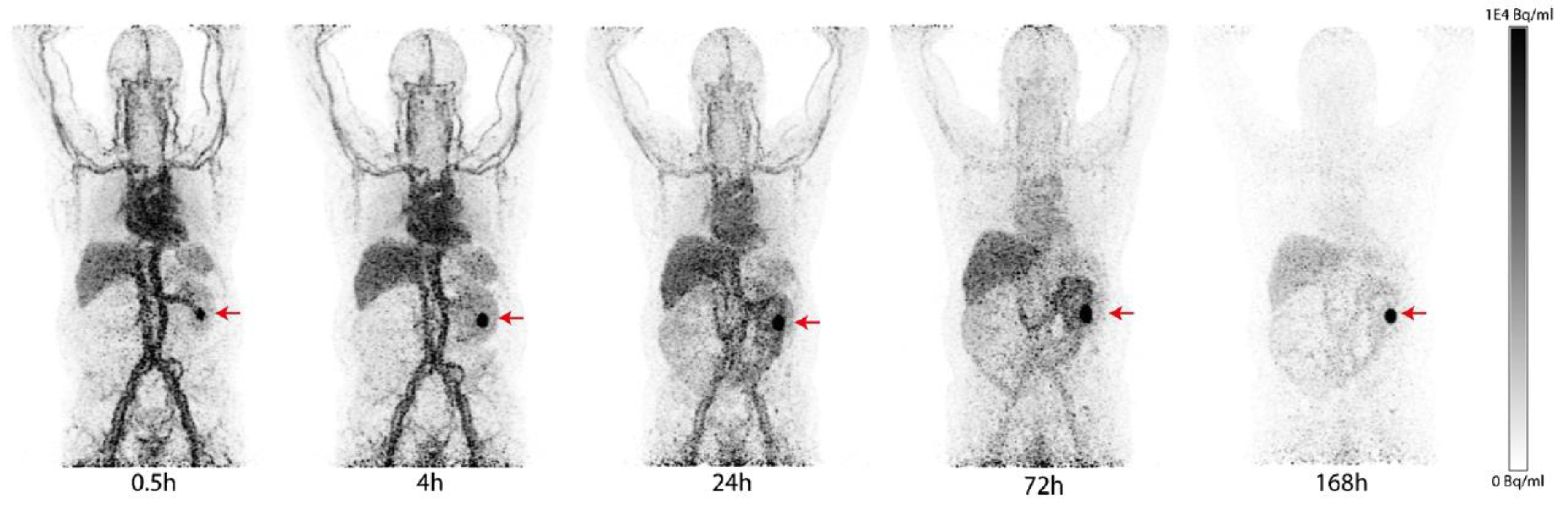
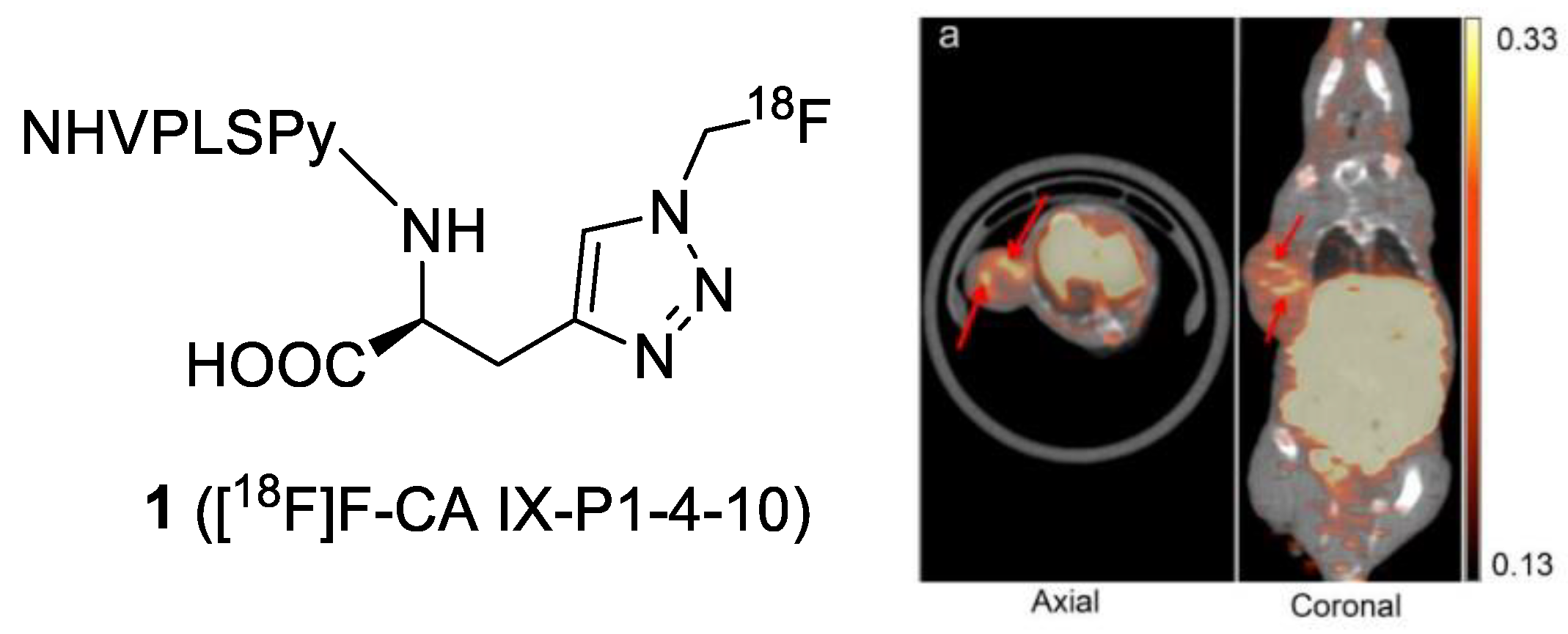
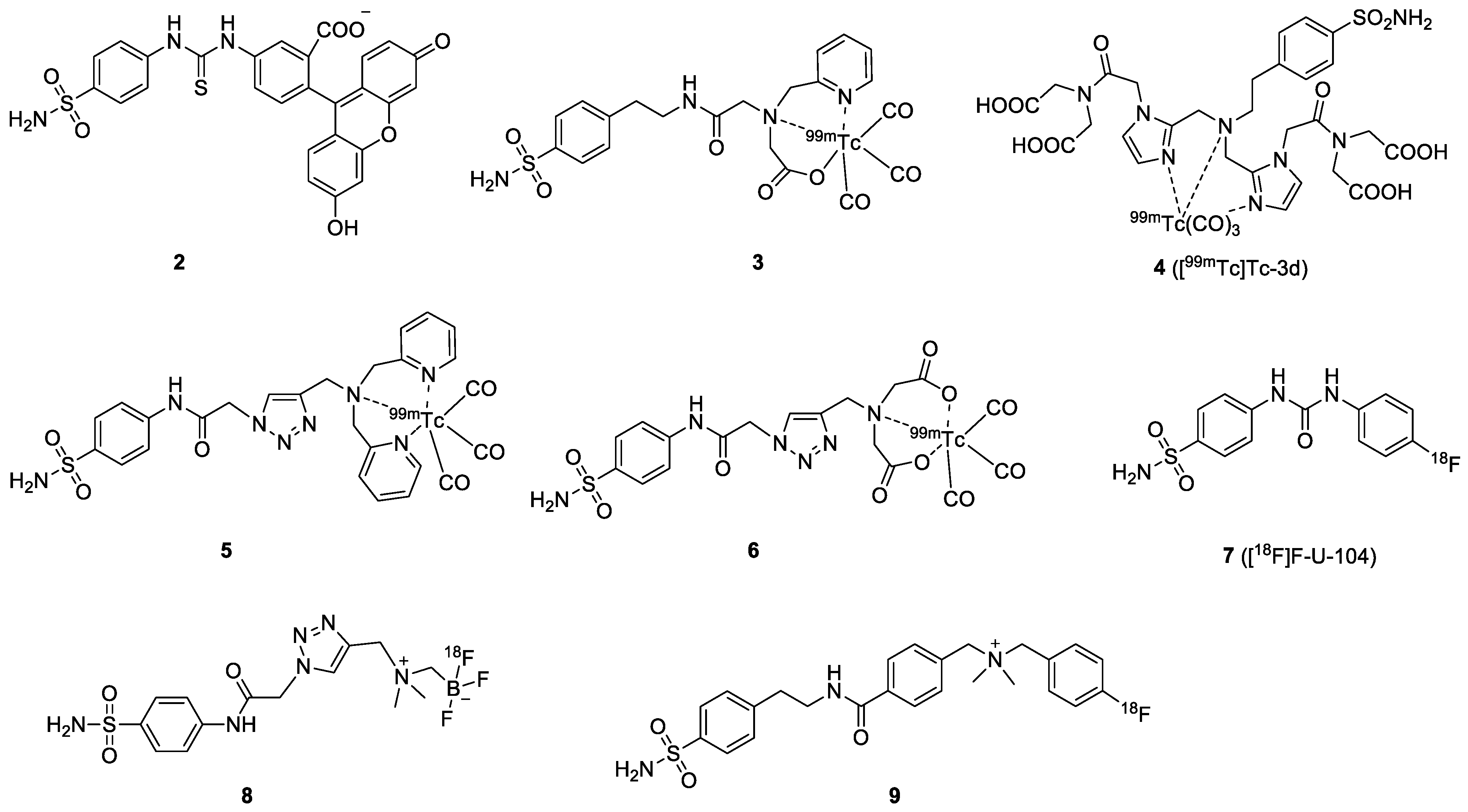
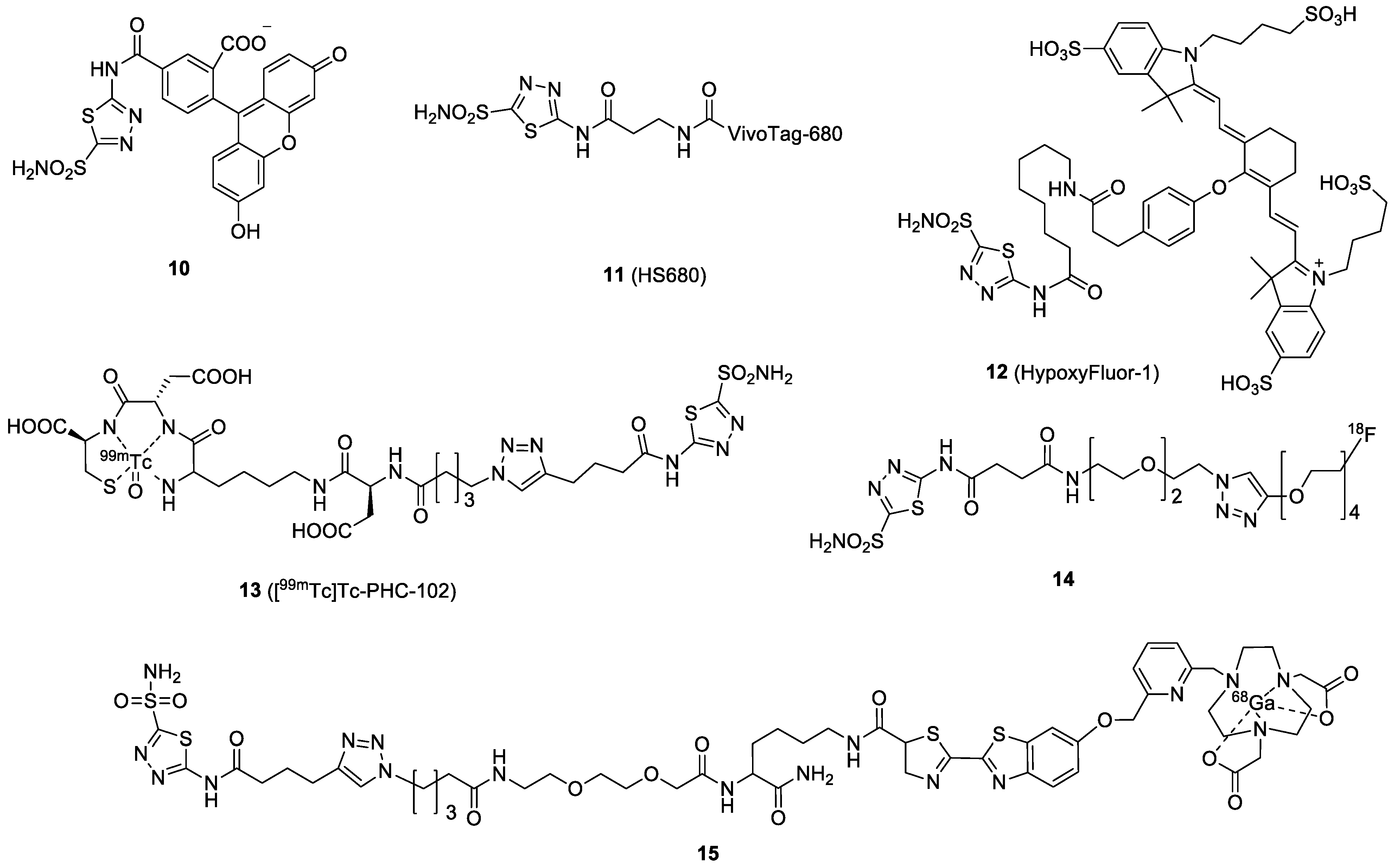

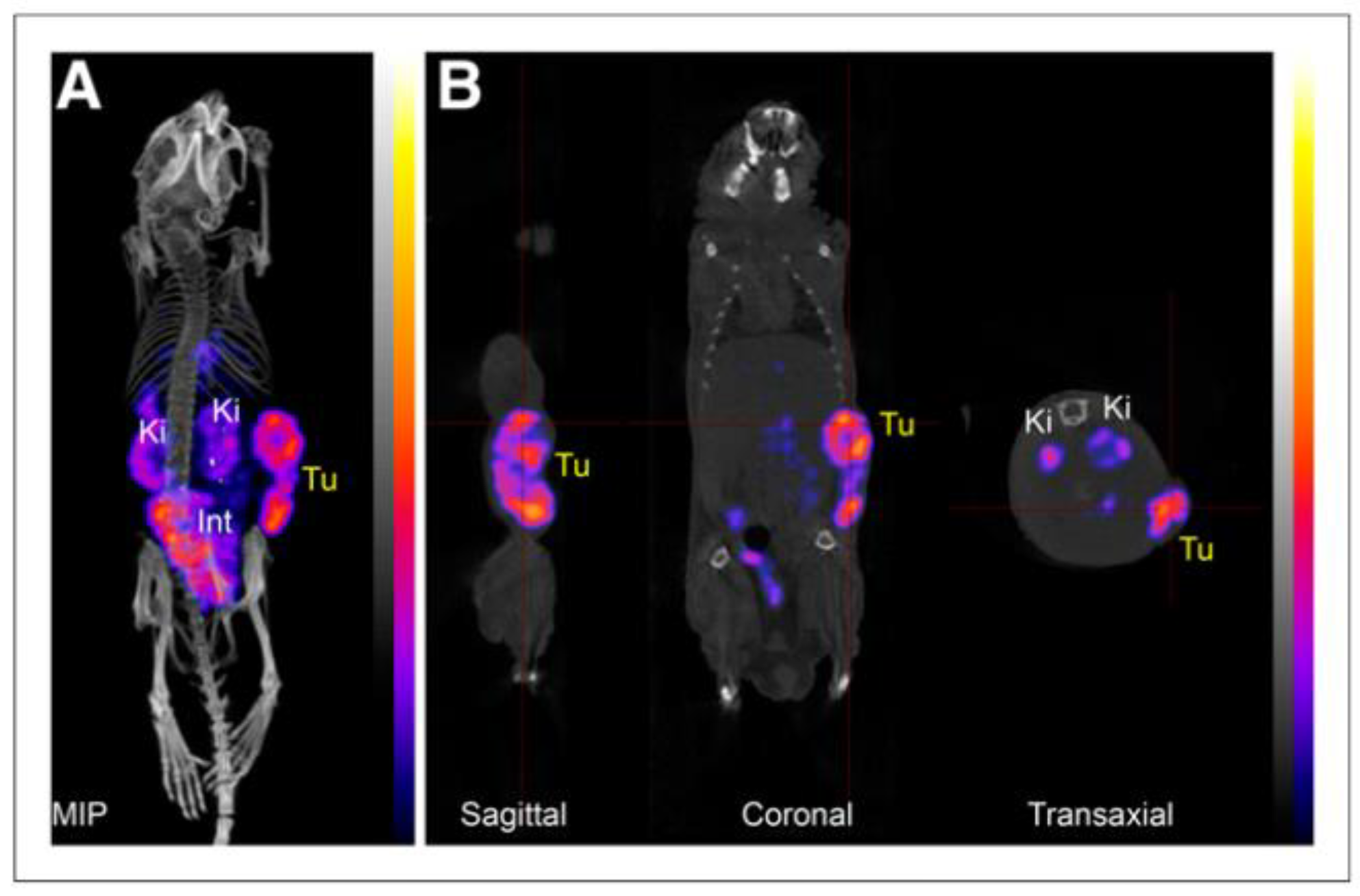
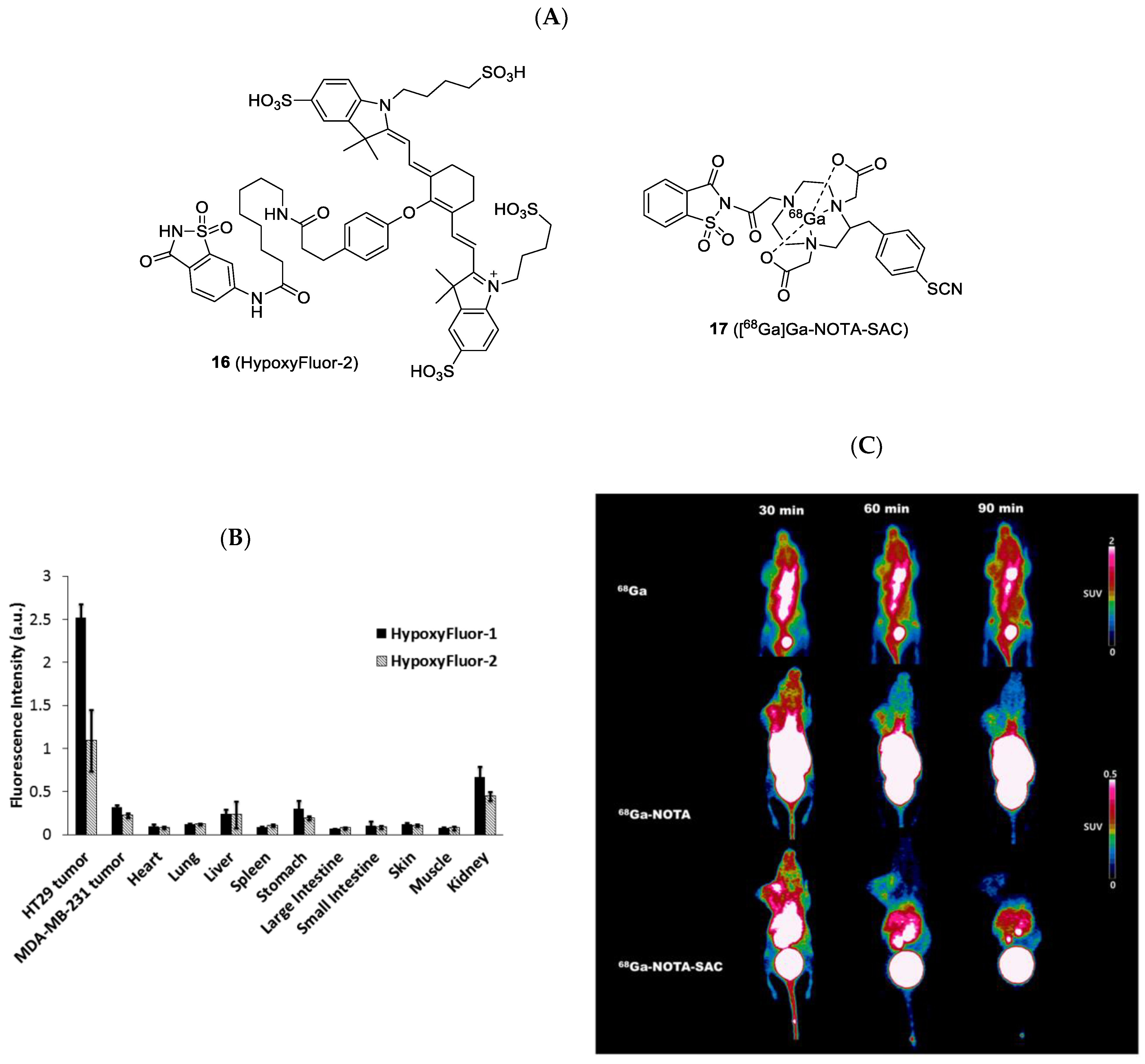

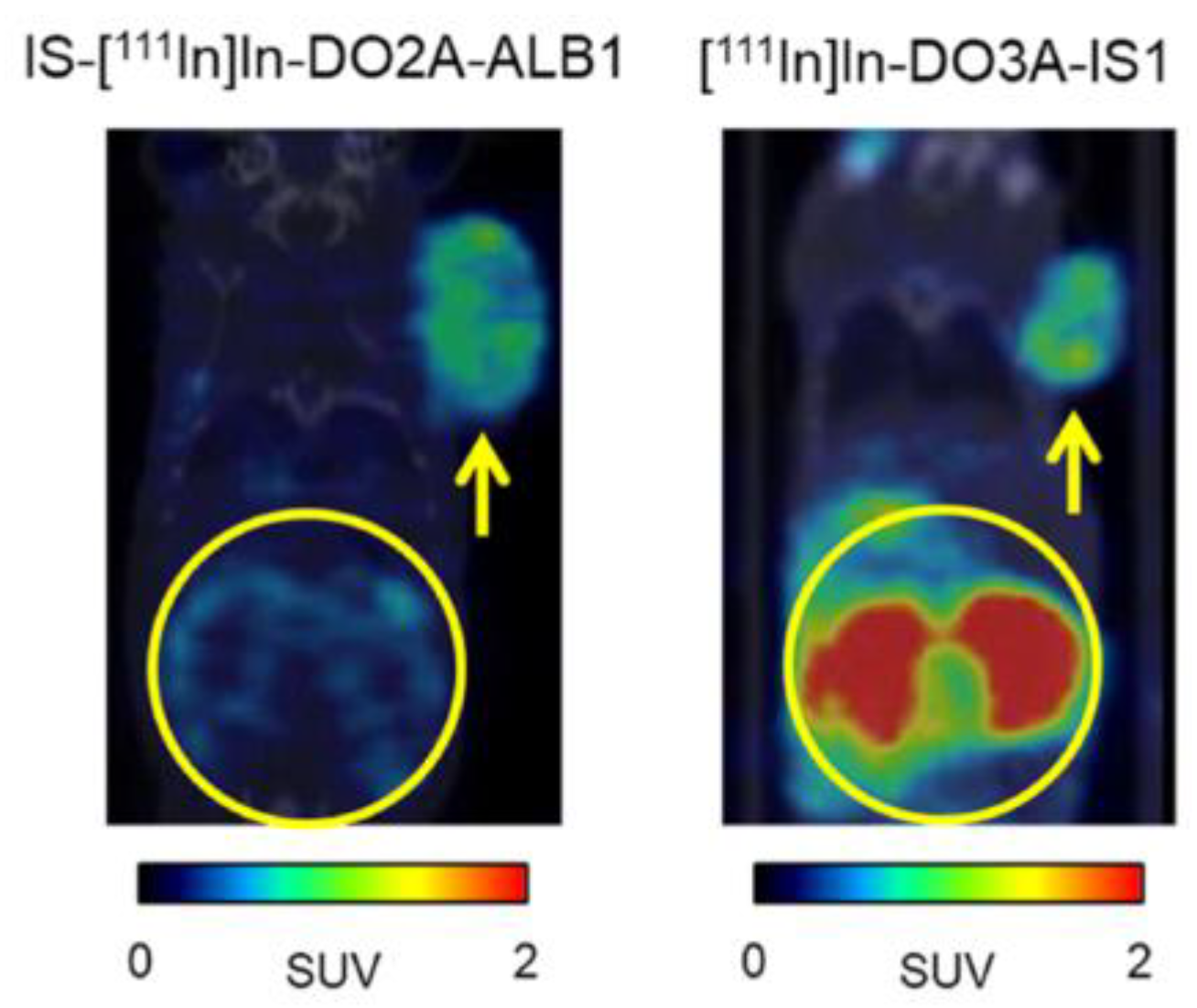

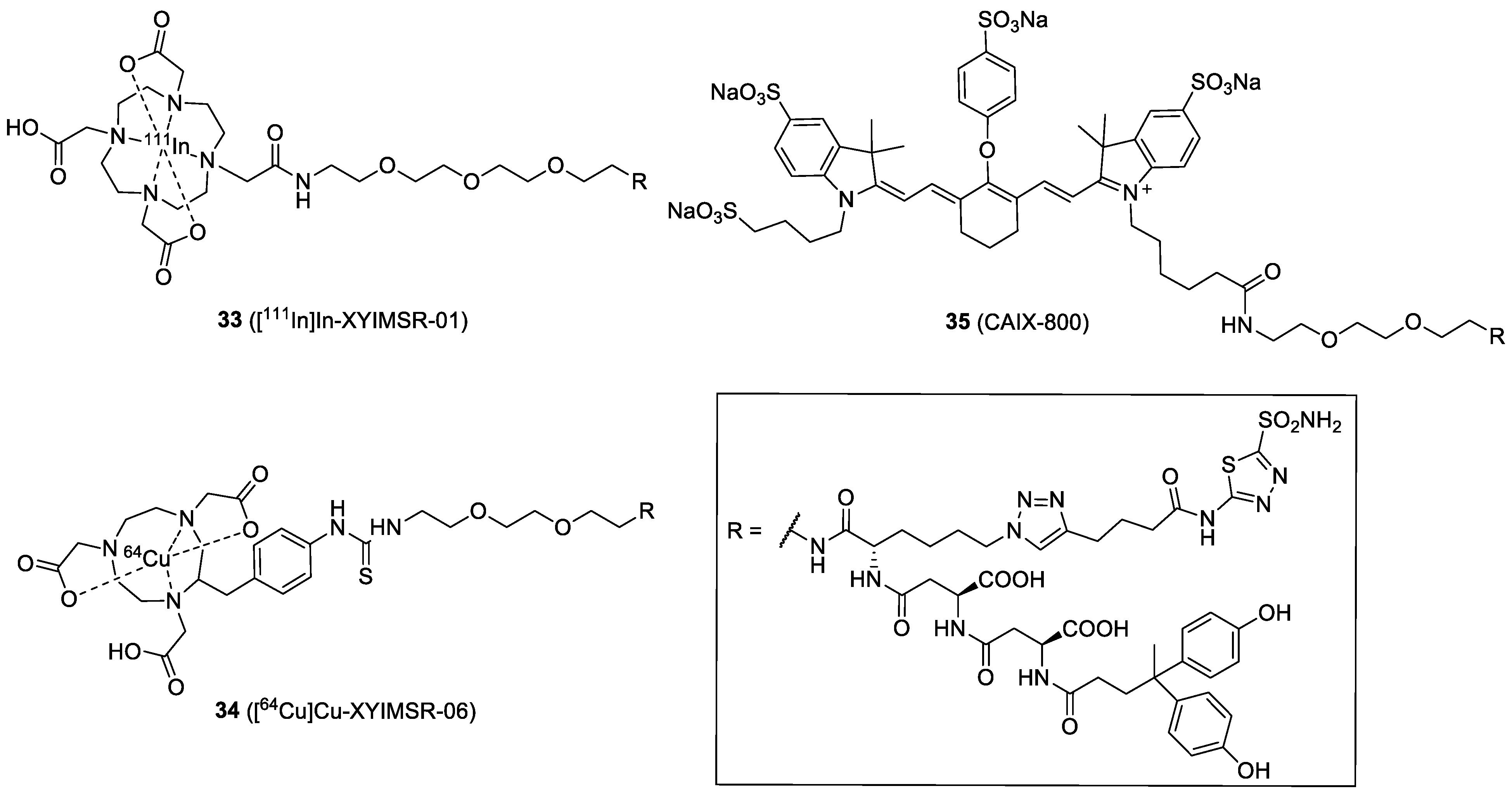

| Compound | Tracer Type | Tumor Model | Tumor Uptake (% ID/g) | T/B a | T/M b | Time p.i. | Year |
|---|---|---|---|---|---|---|---|
| [125I]I-girentuximab-IRDye800CW | antibody | SKRC-52 | 6.9 | - c | - c | 72 h | 2014 [15] |
| [111In]In-DTPA-G250-IRDye800CW | antibody | SKRC-52 | 58.5 | - c | - c | 168 h | 2015 [16] |
| [89Zr]Zr-DFO-cG250-F(ab’)2 | F(ab’)2 | SCCNij-3 | 3.71 | 0.9 | 6.8 | 4 h | 2010 [18] |
| [111In]In-DTPA-cG250-F(ab’)2 | F(ab’)2 | SCCNij-153 | 4.1 | 30.8 | 12.1 | 24 h | 2017 [19] |
| [111In]In-DTPA-cG250-F(ab’)2 | F(ab’)2 | FaDu | 5.61 at 24 h PI (before treatment) 1.92 at 24 h PI (after treatment) | - c | - c | 24 h | 2021 [21] |
| [111In]In-DTPA-VHH-B9 | nanobody | SCCNij-153 | 0.51 | 8.1 | 19.6 | 4 h | 2022 [22] |
| [111In]In-DOTA-ZCAIX:2 | affibody | SCCNij-153 | 0.32 | 4.9 | 3.3 | 4 h | 2019 [23] |
| [111In]In-DOTA-ZCAIX:2 | affibody | SKRC-52 | 15 | 63 | 102 | 4 h | 2019 [24] |
| Agent | Imaging Modality | Fluorophores/Radioisotopes | Binding Affinity | Selectivity CAII/CAIX | Model | Tumor Uptake | Tumor Visualization | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | PET | 18F | N/A | N/A | HT-29 | SUVmean = 0.38 ± 0.03 at 1 h | Y | [31] |
| 2 | FL | FITC | Ki = 24 nM | NA | HT-29 | N/A | Y | [36] |
| 3 | SPECT | 99mTc | Ki = 58 nM | 0.86 | HT-29 | 0.2 ± 0.1% ID/g at 0.5 h | Y | [37] |
| 4 | SPECT | 99mTc | IC50 = 9 nM | N/A | HeLa | N/A | N/A | [38] |
| 5 | SPECT | 99mTc | Ki = 0.22 µM a | 0.3 | HT-29 | 0.07 ± 0.03% ID/g at 1 h | N/A | [39] |
| 6 | SPECT | 99mTc | Ki = 0.037 µM b | 1.2 | HT-29 | 0.14 ± 0.10% ID/g at 1h | Y | [39] |
| 7 | PET | 18F | Ki = 45 nM | 2.1 | HT-29 | 0.83 ± 0.06% ID/g at 1 h | Y | [40] |
| 8 | PET | 18F | Ki = 6.6 nM | 9.0 | HT-29 | 0.64 ± 0.08% ID/g at 1 h | Y | [41] |
| 9 | PET | 18F | Ki = 0.22 µM | 0.3 | HT-29 | 0.41 ± 0.06% ID/g at 1 h | Y | [42] |
| 10 | FL | FITC | N/A | N/A | N/A | N/A | N/A | [43] |
| 11 | FL | VivoTag-680 | Ki = 7.5 nM | 33.1 | HT-29 | 10% ID/g at 24 h | Y | [44] |
| 12 | FL | S0456 | Kd∼10 nM. | N/A | HT-29 | FL intensity: 2.5 (a.u.) at 4 h | Y | [45] |
| 13 | SPECT | 99mTc | N/A | N/A | SKRC-52 | 22% IA/g at 3 h | Y | [46] |
| 14 | PET | 18F | N/A | N/A | 4T1 HT-29 | 4T1: 0.2% ID/g at 30 min HT-29: 0.2% ID/g at 15 min | Y | [47] |
| 15 | PET | 68Ga | N/A | N/A | N/A | N/A | N/A | [48] |
| 16 | FL | S0456 | N/A | N/A | HT-29 | FL intensity: 1.1 (a.u.) at 4 h | Y | [45] |
| 17 | PET | 68Ga | N/A | N/A | U87MG | 1.5% ID/g at 30 min | Y | [49] |
| 18 | SPECT | 111In | 118 ± 21% initial dose/mg protein | N/A | HT-29 | 8.71 ± 1.41% ID/g at 24 h | Y | [50] |
| 19 | SPECT | 111In | IC50 = 574 nM | N/A | HT-29 | 12.32% ID/g at 48 h | Y | [51] |
| 20 | FL | IRDye750 | ka2 = 1.36×10−6 RU−1 s−1 | N/A | SKRC-52 | 5.3 ± 0.6% ID/g at 24 h | Y | [52] |
| 21 | FL | S0456 | Kd = 45 nM | N/A | HT-29 | Epi-fluorescence: 4.3 × 107 at 4 h | Y | [53] |
| 22 | SPECT | 99mTc | Ki = 57 nM | N/A | HT-29 | 5% ID/g at 4 h | Y | [54] |
| 23 | SPECT | 111In | 125% initial dose/mg | N/A | HT-29 | 4.57 ± 0.21% ID/g at 1 h | Y | [55] |
| 25 | PET | 68Ga | 60.4 % initial dose/mg | N/A | HT-29 | 3.81% ID/g at 1 h | Y | [56] |
| 26 | PET | 68Ga | 859 ± 71.7 % initial dose/mg | N/A | HT-29 | 0.71 ± 0.06% ID/g at 1 h | N | [57] |
| 27 | SPECT | 99mTc | IC50 = 38.2 nM | N/A | HT-29 | 3.44 ± 0.50% ID/g at 1 h | N | [58] |
| 28 | SPECT | 99mTc | IC50 = 211.6 nM | N/A | HT-29 | 1.82% ID/g at 1 h | N/A | [59] |
| 29 | PET | 18F | Ki = 8.5 nM | 1.0 | HT-29 | 0.33 ± 0.07% ID/g at 1 h | Y | [41] |
| 30 | PET | 68Ga | Ki = 10.8 nM | 12.7 | HT-29 | 0.81 ± 0.15% ID/g at 1 h | Y | [60] |
| 31 | PET | 68Ga | Ki = 25.4 nM | 1.6 | HT-29 | 1.93 ± 0.26% ID/g at 1 h | Y | [60] |
| 32 | PET | 68Ga | Ki = 7.7 nM | 0.93 | HT-29 | 2.30 ± 0.53% ID/g at 1 h | Y | [60] |
| 33 | SPECT | 111In | IC50 = 108.2 nM | N/A | SKRC-52 | 34.00 ± 15.16% ID/g at 8 h | Y | [61] |
| 34 | PET | 64Cu | Kd = 4.22 nM | N/A | U87 MG | 3.13 ± 0.26% ID/g at 4 h | Y | [62] |
| 35 | FMT-CT/MSOT | IRDye 800CW | N/A | N/A | NPC | N/A | Y | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-T.; Seimbille, Y. New Developments in Carbonic Anhydrase IX-Targeted Fluorescence and Nuclear Imaging Agents. Int. J. Mol. Sci. 2022, 23, 6125. https://doi.org/10.3390/ijms23116125
Chen K-T, Seimbille Y. New Developments in Carbonic Anhydrase IX-Targeted Fluorescence and Nuclear Imaging Agents. International Journal of Molecular Sciences. 2022; 23(11):6125. https://doi.org/10.3390/ijms23116125
Chicago/Turabian StyleChen, Kuo-Ting, and Yann Seimbille. 2022. "New Developments in Carbonic Anhydrase IX-Targeted Fluorescence and Nuclear Imaging Agents" International Journal of Molecular Sciences 23, no. 11: 6125. https://doi.org/10.3390/ijms23116125
APA StyleChen, K.-T., & Seimbille, Y. (2022). New Developments in Carbonic Anhydrase IX-Targeted Fluorescence and Nuclear Imaging Agents. International Journal of Molecular Sciences, 23(11), 6125. https://doi.org/10.3390/ijms23116125







