Cytokinin Confers Brown Planthopper Resistance by Elevating Jasmonic Acid Pathway in Rice
Abstract
:1. Introduction
2. Results
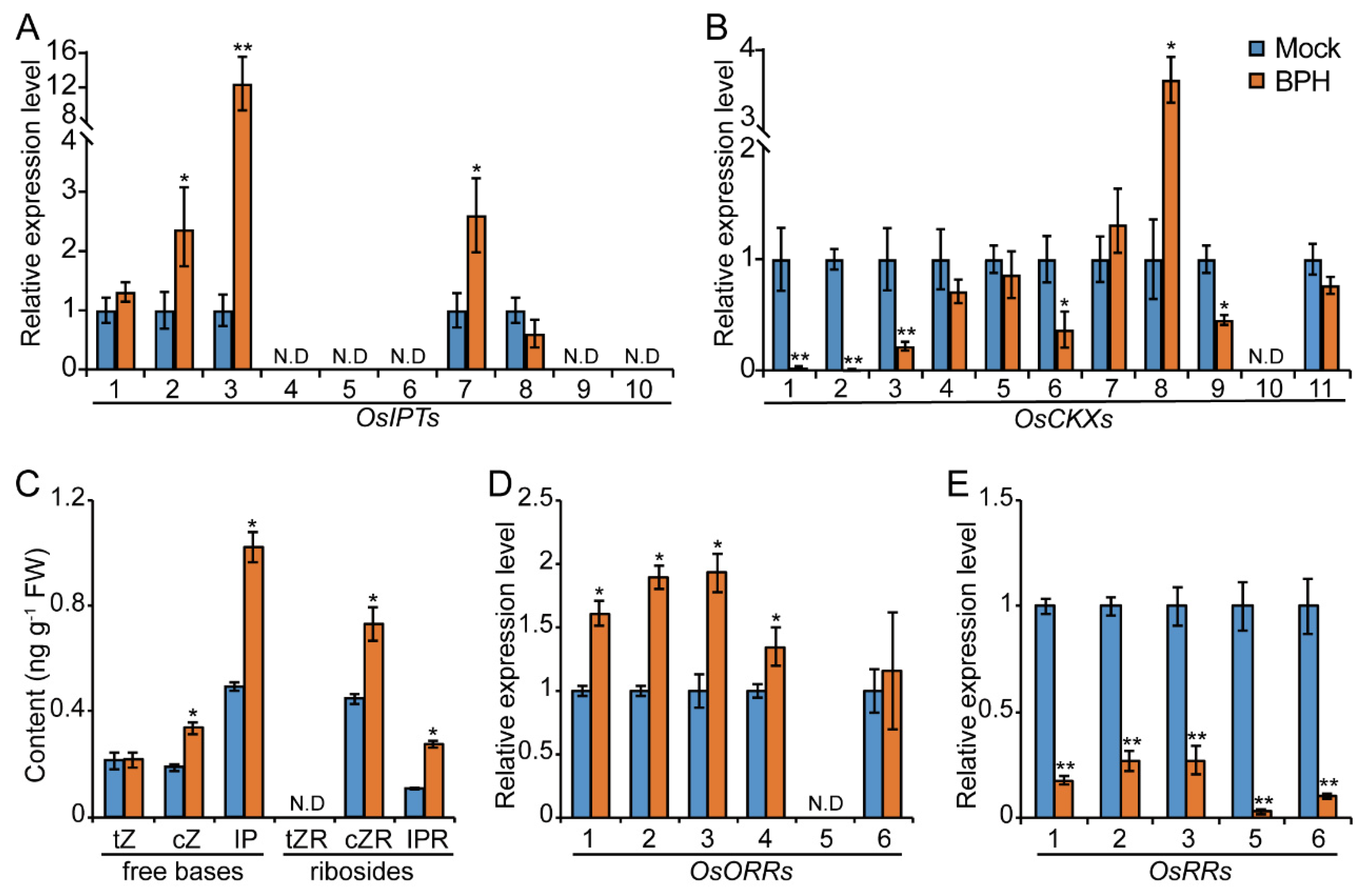
2.1. BPH Infestation Promotes CK Pathway in Rice
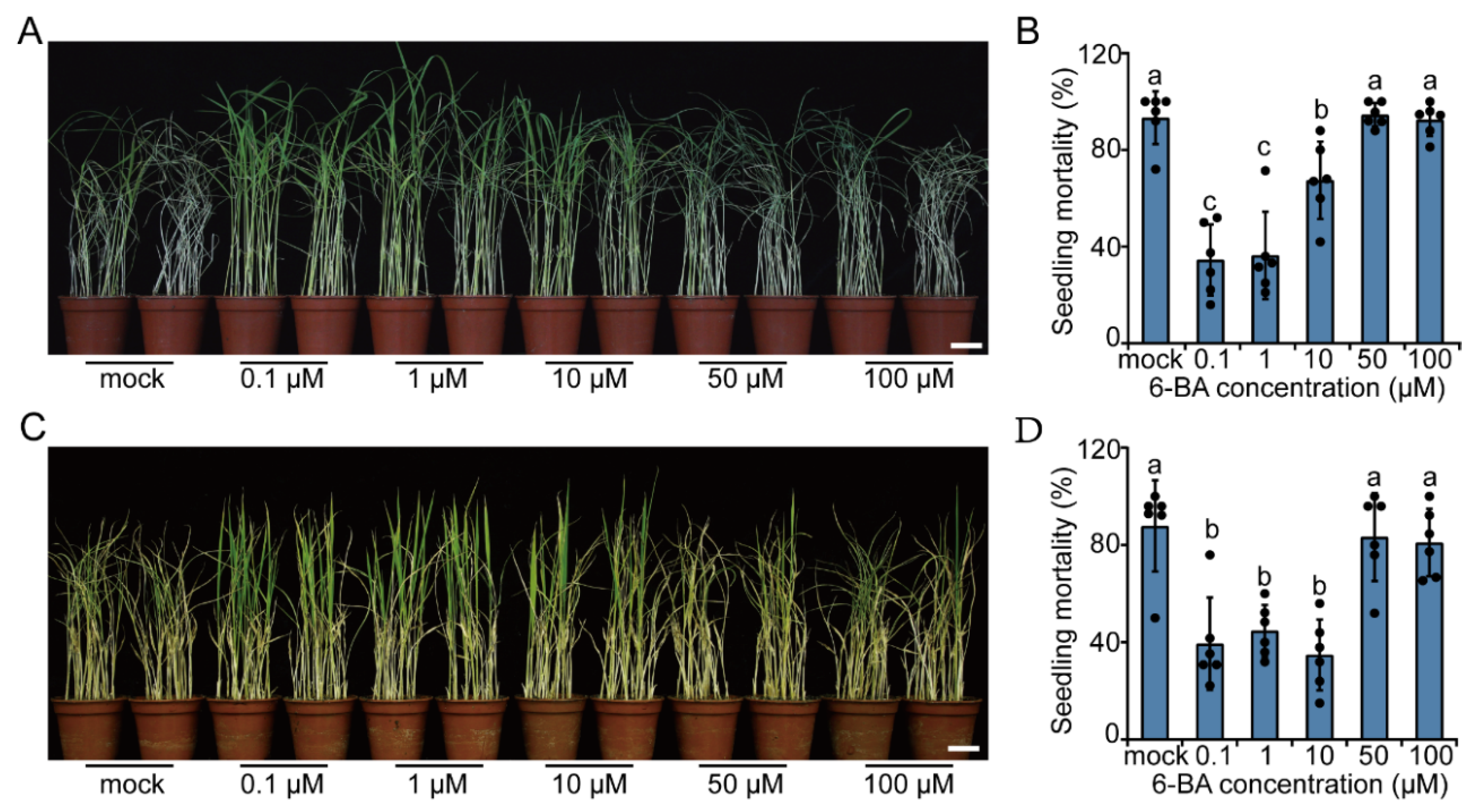
2.2. Exogenous CK Treatment Promotes Resistance to BPH in Rice
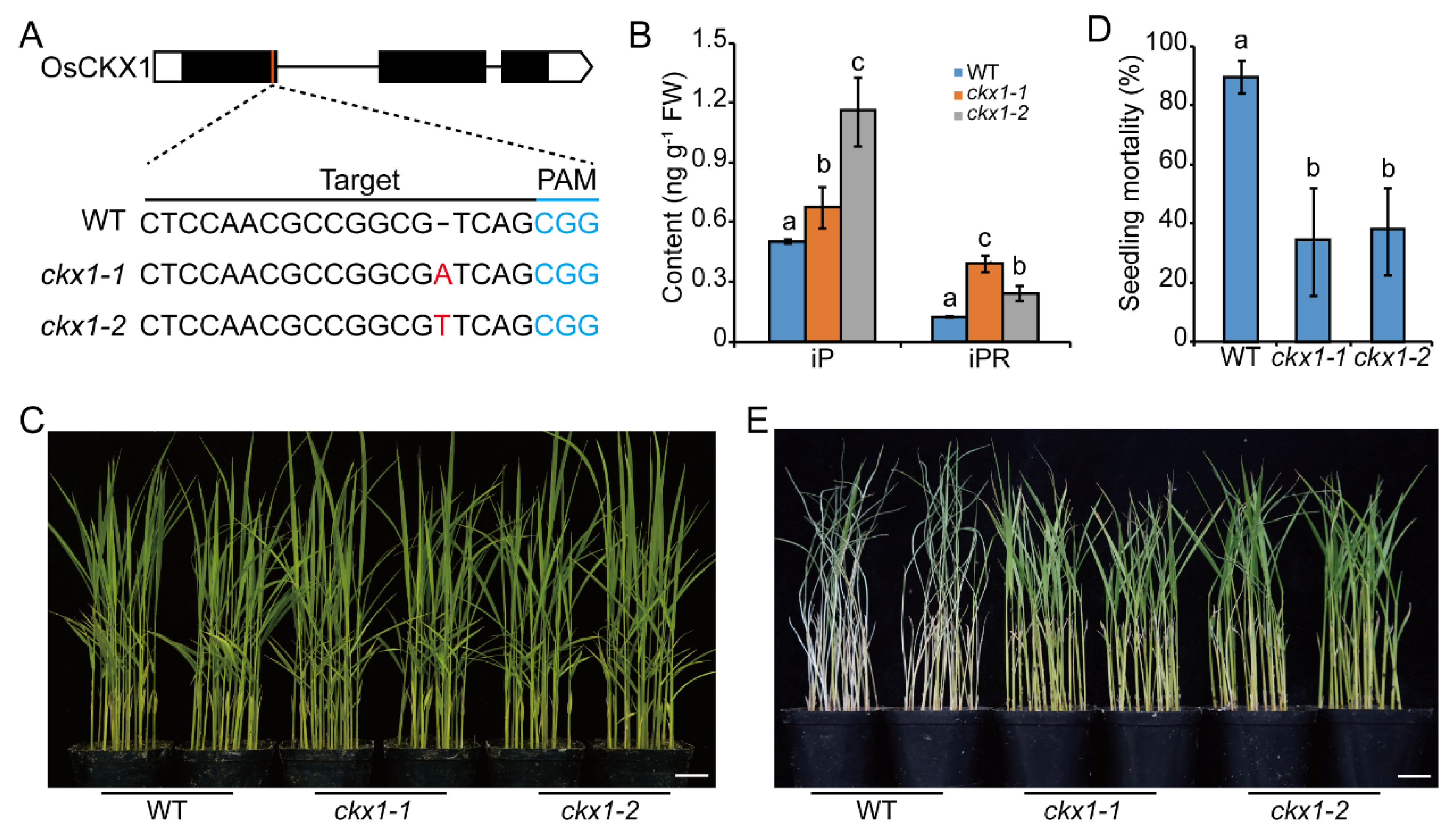
2.3. Knocking out OsCKXs Promotes the Resistance to BPH in Rice
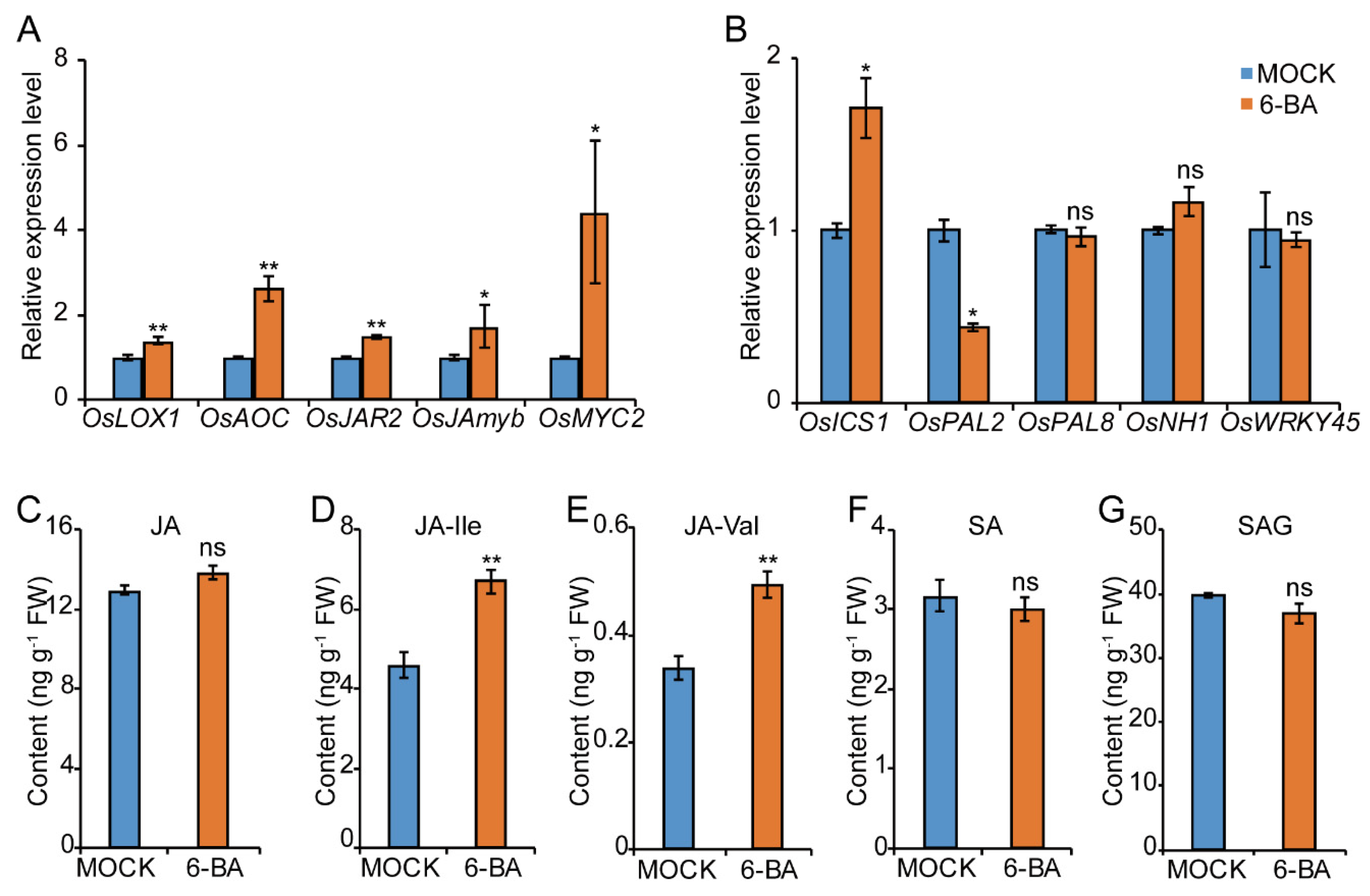
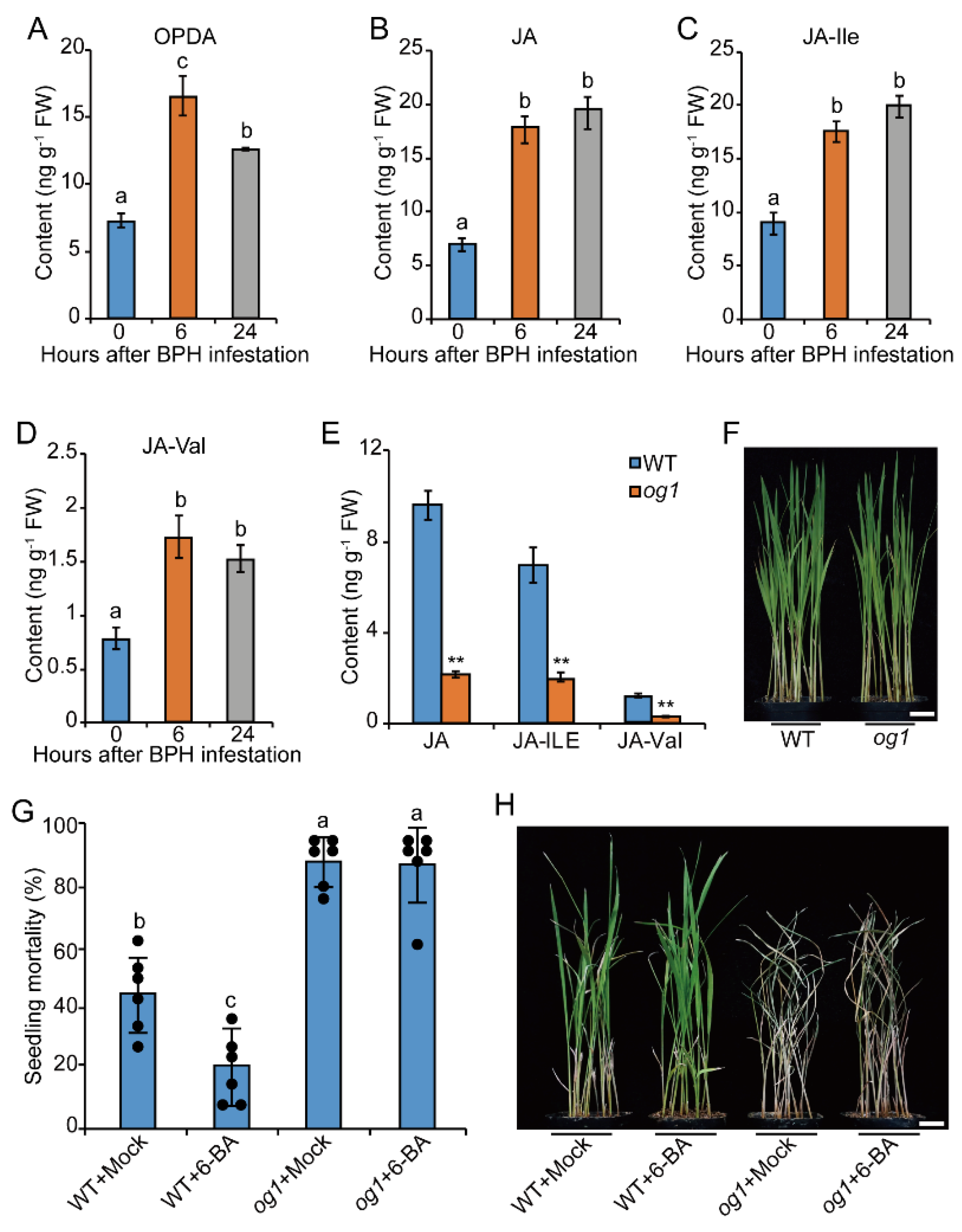
2.4. CKs Promote the JA Rather than the SA Pathway in Response to BPH in Rice
2.5. CK-Mediated BPH Resistance Depends on the JA Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. BPH Maintenance
4.3. Evaluation of Rice Resistance against BPH
4.4. Hormone Treatments
4.5. Phytohormones Measurements
4.6. Lignin Analysis
4.7. RNA Extraction and Quantitative Real-Time RT-PCR (qRT-PCR)
4.8. Plasmid Construction and Plant Transformation
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, L. Development of hybrid rice to ensure food security. Rice Sci. 2014, 21, 1–2. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sōgawa. The rice brown planthopper: Feeding physiology and host plant interactions. Annu. Rev. Entomol. 1982, 27, 49–73. [Google Scholar] [CrossRef]
- Heong, K.; Hardy, B. Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; International Rice Research Institute (IRRI): Los Baños, Philippines, 2009. [Google Scholar]
- Bottrell, D.; Schoenly, K. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, H.; Chen, H.; Liu, Y.; He, J.; Kang, H.; Sun, Z.; Pan, G.; Wang, Q.; Hu, J.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2015, 33, 301–305. [Google Scholar] [CrossRef]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Chen, Y.; Huang, J.; Guo, Q.; Li, Y.; Wang, W.; Qiu, Y.; Guan, W.; Zhang, J.; et al. Balancing selection and wild gene pool contribute to resistance in global rice germplasm against planthopper. J. Integr. Plant Biol. 2021, 63, 1695–1711. [Google Scholar] [CrossRef]
- Pieterse, C.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S. Hormonal modulation of plant immunity. Annu. Rev. Cell. Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.; Humphrey, P.; Whiteman, N. Evolution of jasmonate and salicylate signal crosstalk. Trends. Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Pan, G.; Liu, Y.; Ji, L.; Zhang, X.; He, J.; Huang, J.; Qiu, Z.; Liu, D.; Sun, Z.; Xu, T.; et al. Brassinosteroids mediate susceptibility to brown planthopper by integrating with the salicylic acid and jasmonic acid pathways in rice. J. Exp. Bot. 2018, 69, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, L.; Zhang, Y.; Cao, C.; Liu, F.; Huang, F.; Qiu, Y.; Li, R.; Lou, X. Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J. Exp. Bot. 2015, 66, 6035–6045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Huang, J.; Wang, Z.; Jing, S.; Wang, Y.; Ouyang, Y.; Cai, B.; Xin, X.F.; Liu, X.; Zhang, C.; et al. Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc. Natl. Acad. Sci. USA 2016, 113, 12850–12855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Xu, C.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Ye, M.; Luo, S.; Xie, J.; Li, Y.; Xu, T.; Liu, Y.; Song, Y.; Zhu-Salzman, K.; Zeng, R. Silencing COI1 in rice increases susceptibility to chewing insects and impairs inducible defense. PLoS ONE. 2012, 7, e36214. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zu, H.; Zeng, X.; Baldwin, I.; Lou, Y.; Li, R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol. 2021, 230, 1639–1652. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45, 1028–1036. [Google Scholar] [CrossRef]
- Kieber, J.; Schaller, G. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmulling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, B.; Sheen, J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Xiao, Y.; Xu, F.; Gao, X.; Cao, S.; Zhang, F.; Wang, G.; Sanders, D.; Chu, C. Cytokinin-dependent regulatory module underlies the maintenance of zinc nutrition in rice. New Phytol. 2019, 224, 202–215. [Google Scholar] [CrossRef]
- Takatoshi, K.; Hisami, Y.; Shusei, S.; Tomohiko, K.; Satoshi, T.; Takafumi, Y.; Takeshi, M. The type-a response regulator, ARR15, acts as a negative regulator in the cytokinin-mediated signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 868–874. [Google Scholar]
- To, J.; Haberer, G.; Ferreira, F.; Deruere, J.; Mason, M.; Schaller, G.; Alonso, J.; Ecker, J.; Kieber, J. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 2004, 16, 658–671. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, M.; Meng, Z.; Wang, B.; Chen, M. Research progress on the roles of cytokinin in plant response to stress. Int. J. Mol. Sci. 2020, 21, 6574. [Google Scholar] [CrossRef]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.H.; Hwang, I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev. Cell. 2010, 19, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Shimono, M.; Sugano, S.; Kojima, M.; Liu, X.; Inoue, H.; Sakakibara, H.; Takatsuji, H. Cytokinins act synergistically with salicylic acid to activate defense gene expression in rice. Mol. Plant Microbe Interact. 2013, 26, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Shanks, C.; Rice, J.; Zubo, Y.; Schaller, G.; Hewezi, T.; Kieber, J. The role of cytokinin during infection of Arabidopsis thaliana by the cyst nematode Heterodera schachtii. Mol. Plant Microbe Interact. 2016, 29, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Walters, D.; McRoberts, N. Plants and biotrophs: A pivotal role for cytokinins? Trends Plant Sci. 2006, 11, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Chanclud, E.; Kisiala, A.; Emery, N.; Chalvon, V.; Ducasse, A.; Romiti-Michel, C.; Gravot, A.; Kroj, T.; Morel, J. Cytokinin production by the rice blast fungus is a pivotal requirement for full virulence. PLoS Pathog. 2016, 12, e1005457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smigocki, A.; Neal, J.; Mccanna, I.; Douglass, L. Cytokinin-mediated insect resistance in Nicotiana plants transformed with the ipt gene. Plant Mol. Biol. 1993, 23, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ning, D.; Chen, Y.; Dang, C.; Han, N.; Liu, Y.; Ye, G. Comparing gene expression profiles between Bt and non-Bt rice in response to brown planthopper infestation. Front. Plant Sci. 2015, 6, 1181. [Google Scholar] [CrossRef] [Green Version]
- Hirose, N.; Makita, N.; Kojima, M.; Kamada-Nobusada, T.; Sakakibara, H. Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol. 2007, 48, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Jiang, H.; Zhang, J.; Qian, Y.; Zhu, S.; Cheng, B. Overexpression of type-A rice response regulators, osRR3 and osRR5, results in lower sensitivity to cytokinins. Genet. Mol. Res. 2017, 9, 348–359. [Google Scholar] [CrossRef]
- Kitomi, Y.; Ito, H.; Hobo, T.; Aya, K.; Kitano, H.; Inukai, Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011, 67, 472–484. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, K.; Cui, F.; Wang, D.; Zhao, J.; Zhang, Y.; Yu, N.; Wang, Y.; Zeng, D.; Wang, Y.; et al. Cytokinin oxidase/dehydrogenase OsCKX11 coordinates source and sink relationship in rice by simultaneous regulation of leaf senescence and grain number. Plant Biotechnol. J. 2021, 19, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, R.; Wang, S.; Zhang, J.; Chang, L. Diversified regulation of cytokinin levels and signaling during Botrytis cinerea infection in Arabidopsis. Front. Plant Sci. 2021, 12, 584042. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Duan, E.; Qi, Q.; Zhou, K.; Lin, Q.; Wang, D.; Wang, Y.; Long, W.; Zhao, Z.; et al. OPEN GLUME1: A key enzyme reducing the precursor of JA, participates in carbohydrate transport of lodicules during anthesis in rice. Plant Cell Rep. 2018, 37, 329–346. [Google Scholar] [CrossRef]
- Siddique, S.; Radakovic, Z.; De La Torre, C.; Chronis, D.; Novak, O.; Ramireddy, E.; Holbein, J.; Matera, C.; Hutten, M.; Gutbrod, P.; et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc. Natl. Acad. Sci. USA 2015, 112, 12669–12674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mens, C.; Li, D.; Haaima, L.; Gresshoff, P.; Ferguson, B. Local and systemic effect of cytokinins on soybean nodulation and regulation of their isopentenyl transferase (IPT) biosynthesis genes following rhizobia inoculation. Front Plant Sci. 2018, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M. Role and activity of jasmonates in plants under in vitro conditions. Plant Cell Tissue Organ Cult. 2021, 146, 425–447. [Google Scholar] [CrossRef]
- Yang, J.; He, H.; He, Y.; Zheng, Q.; Li, Q.; Feng, X.; Wang, P.; Qin, G.; Gu, Y.; Wu, P.; et al. TMK1-based auxin signaling regulates abscisic acid responses via phosphorylating ABI1/2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102544118. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.; Ferreira, F.; Epple, P.; To, J.; Hutchison, C.; Schaller, G.; Dangl, J.; Kieber, J. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012, 8, e1002448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, W.; Galuszka, P.; Gasparis, S.; Orczyk, W.; Nadolska-Orczyk, A. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity. J. Exp. Bot. 2010, 61, 1839–1851. [Google Scholar] [CrossRef] [Green Version]
- Crane, K.; Ross, C. Effects of wounding on cytokinin activity in cucumber cotyledons. Plant Physiol. 1987, 82, 1151–1152. [Google Scholar] [CrossRef] [Green Version]
- Gan, S.; Amasino, R. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 1995, 270, 1986–1988. [Google Scholar] [CrossRef]
- Mok, D.; Mok, M. Cytokinin metabolism and action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef]
- Honig, M.; Plihalova, L.; Husickova, A.; Nisler, J.; Dolezal, K. Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, D.; Iqbal, J.; Lehmann, K.; Gase, K.; Saluz, H.; Baldwin, I. Molecular interactions between the specialist herbivore Manduca Sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana Attenuata: V. Microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol. 2003, 131, 1877–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dervinis, C.; Frost, C.; Lawrence, S.; Novak, N.; Davis, J. Cytokinin primes plant responses to wounding and reduces insect performance. J. Plant Growth Regul. 2010, 29, 289–296. [Google Scholar] [CrossRef]
- Mujer, C.; Smigocki, A. Cytokinin- and wound-inducible cytochrome P450 from Nicotiana plumbaginifolia. Physiol. Plant. 2001, 111, 172–181. [Google Scholar] [CrossRef]
- Hammer, D. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol. 1995, 108, 47–57. [Google Scholar]
- Khadr, A.; Wang, Y.; Zhang, R.; Wang, X.; Xu, Z.; Xiong, A. Cytokinin (6-benzylaminopurine) elevates lignification and the expression of genes involved in lignin biosynthesis of carrot. Protoplasma 2020, 257, 1507–1517. [Google Scholar] [CrossRef]
- Ueda, J.; Kato, J. Inhibition of cytokinin-induced plant growth by jasmonic acid and its methyl ester. Physiol. Plant. 1982, 54, 249–252. [Google Scholar] [CrossRef]
- Jang, G.; Chang, S.; Um, T.; Lee, S.; Kim, J.; Choi, Y. Antagonistic interaction between jasmonic acid and cytokinin in xylem development. Sci. Rep. 2017, 7, 10212. [Google Scholar] [CrossRef] [Green Version]
- Schafer, M.; Brutting, C.; Meza-Canales, I.; Grosskinsky, D.; Vankova, R.; Baldwin, I.; Meldau, S. The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J. Exp. Bot. 2015, 66, 4873–4884. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, R.; Hatfield, R. Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J. Agric. Food Chem. 2001, 49, 3133–3139. [Google Scholar] [CrossRef]
- Lam, P.; Tobimatsu, Y.; Takeda, Y.; Suzuki, S.; Yamamura, M.; Umezawa, T.; Lo, C. Disrupting flavone synthase II alters lignin and improves biomass digestibility. Plant Physiol. 2017, 174, 972–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, J.; Guo, D.; Zhang, J.; Huang, Q.; Qin, G.; Zhang, X.; Wan, J.; Gu, H.; Qu, L. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013, 23, 1233–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, D.; Gao, D.; Zhao, W.; Du, H.; Qiu, Z.; Huang, J.; Wen, P.; Wang, Y.; Li, Q.; et al. Cytokinin Confers Brown Planthopper Resistance by Elevating Jasmonic Acid Pathway in Rice. Int. J. Mol. Sci. 2022, 23, 5946. https://doi.org/10.3390/ijms23115946
Zhang X, Liu D, Gao D, Zhao W, Du H, Qiu Z, Huang J, Wen P, Wang Y, Li Q, et al. Cytokinin Confers Brown Planthopper Resistance by Elevating Jasmonic Acid Pathway in Rice. International Journal of Molecular Sciences. 2022; 23(11):5946. https://doi.org/10.3390/ijms23115946
Chicago/Turabian StyleZhang, Xiao, Daoming Liu, Dong Gao, Weining Zhao, Huaying Du, Zeyu Qiu, Jie Huang, Peizheng Wen, Yongsheng Wang, Qi Li, and et al. 2022. "Cytokinin Confers Brown Planthopper Resistance by Elevating Jasmonic Acid Pathway in Rice" International Journal of Molecular Sciences 23, no. 11: 5946. https://doi.org/10.3390/ijms23115946
APA StyleZhang, X., Liu, D., Gao, D., Zhao, W., Du, H., Qiu, Z., Huang, J., Wen, P., Wang, Y., Li, Q., Wang, W., Xu, H., He, J., Liu, Y., & Wan, J. (2022). Cytokinin Confers Brown Planthopper Resistance by Elevating Jasmonic Acid Pathway in Rice. International Journal of Molecular Sciences, 23(11), 5946. https://doi.org/10.3390/ijms23115946





