Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models
Abstract
:1. Introduction
2. Results and Discussion
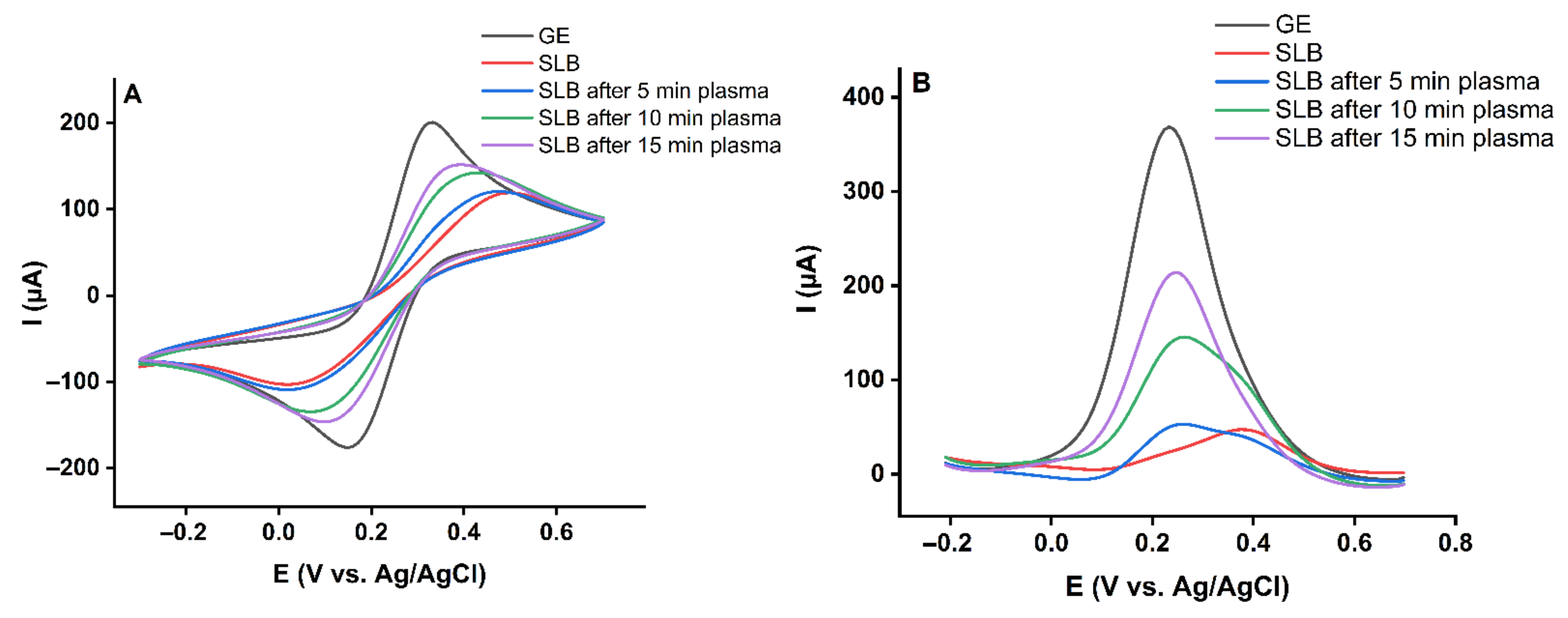
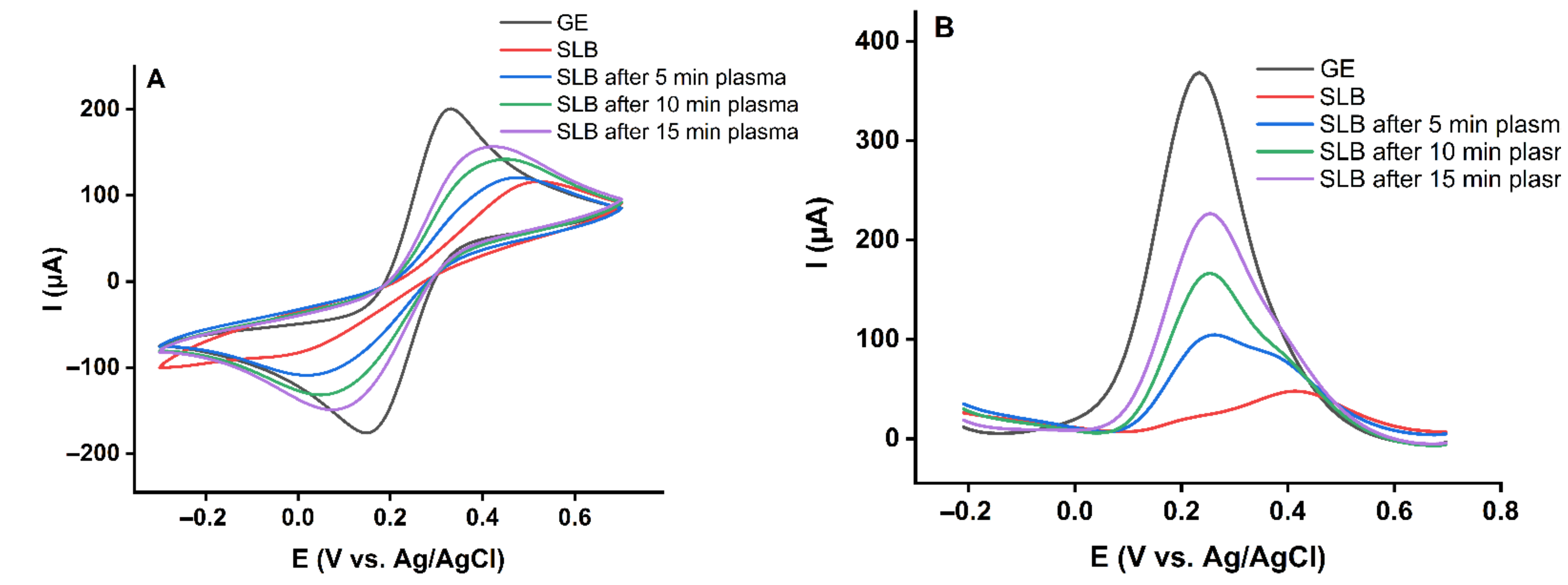
2.1. Effect of Plasma-Derived RONS on the Permeability of the Asymmetric Model Lipid Bilayer
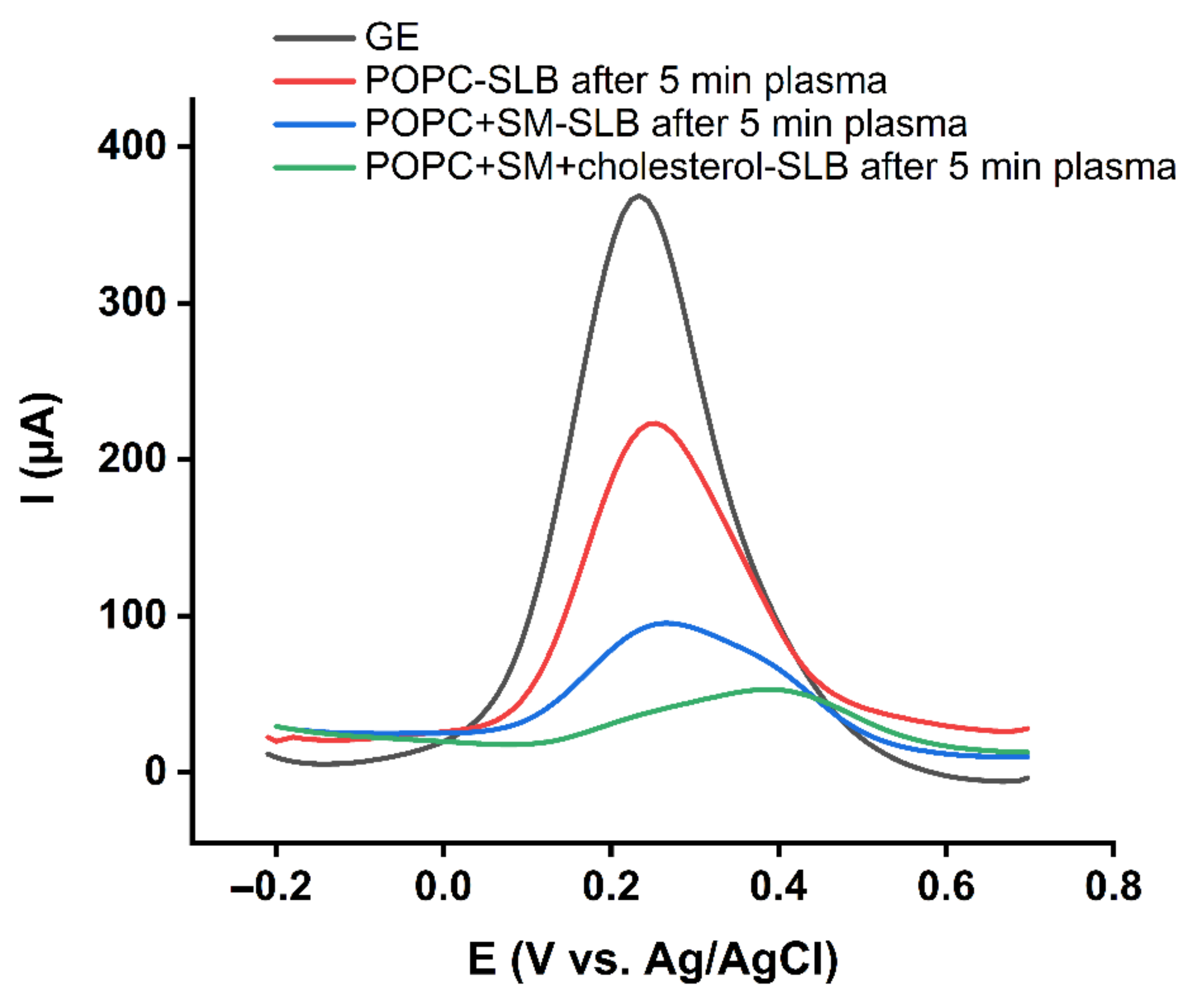
2.2. Effect of Cholesterol on the Barrier Properties of the Model Lipid Bilayer
2.3. Effect of Lipid Structure on the Barrier Properties of the Model Lipid Bilayers
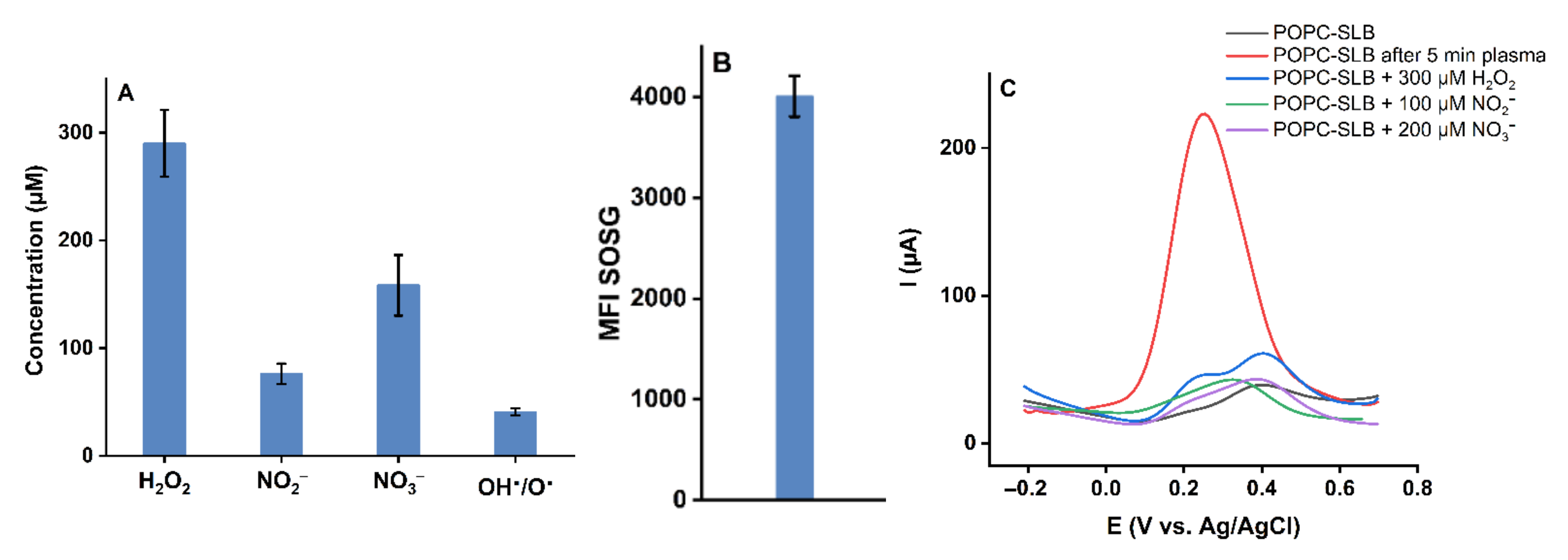
2.4. Effect of Long-Lived Species on the Model Lipid Bilayer
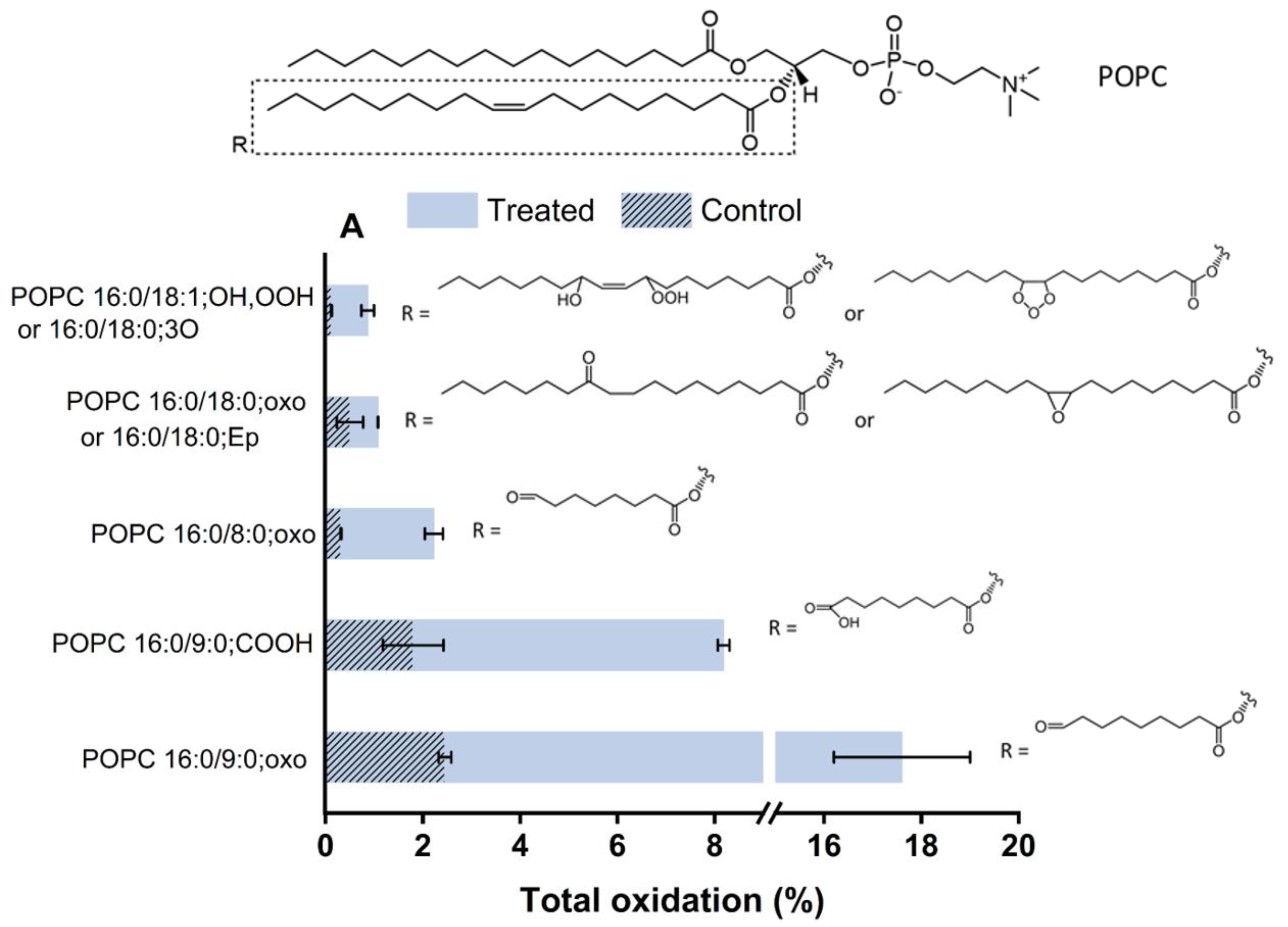
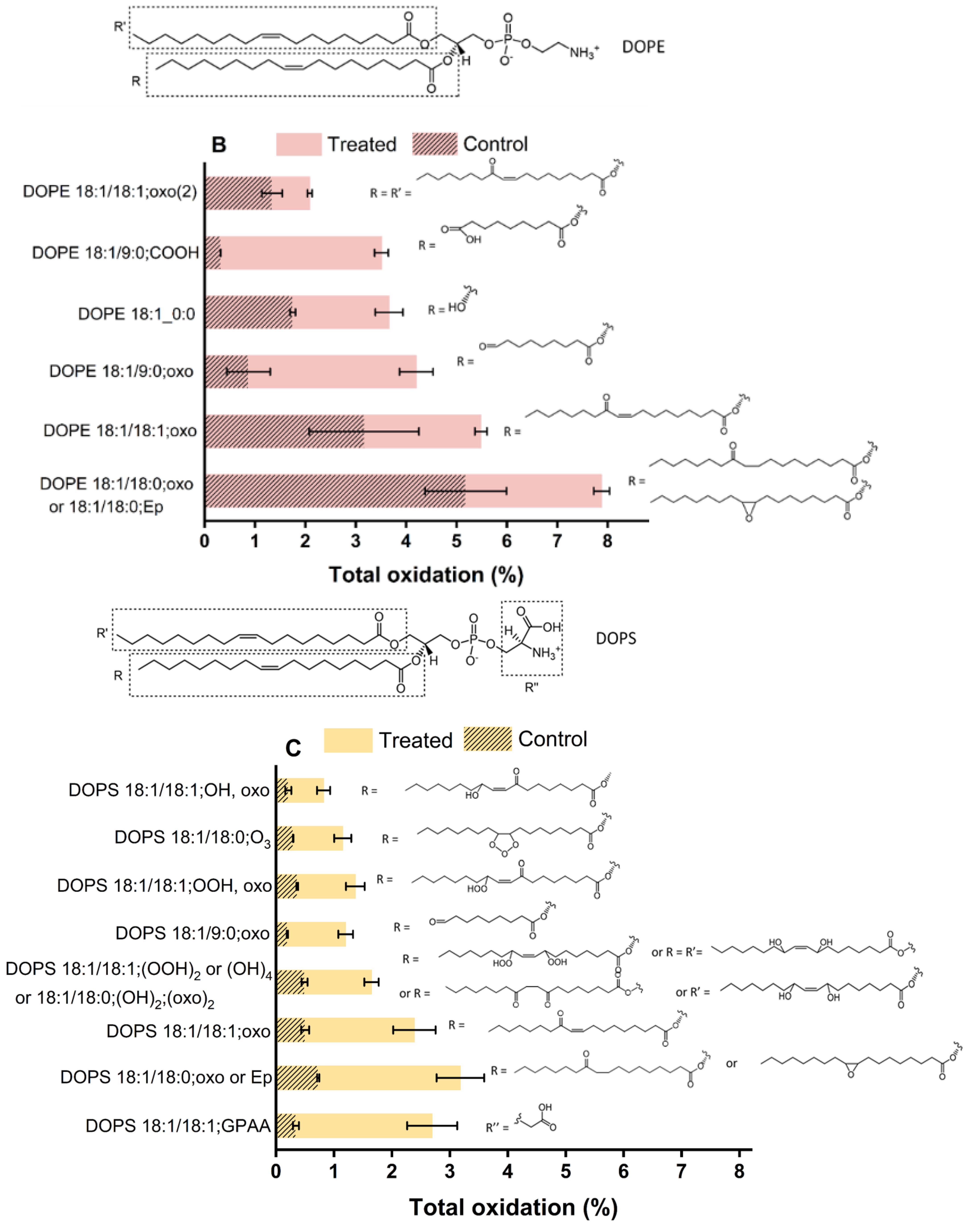
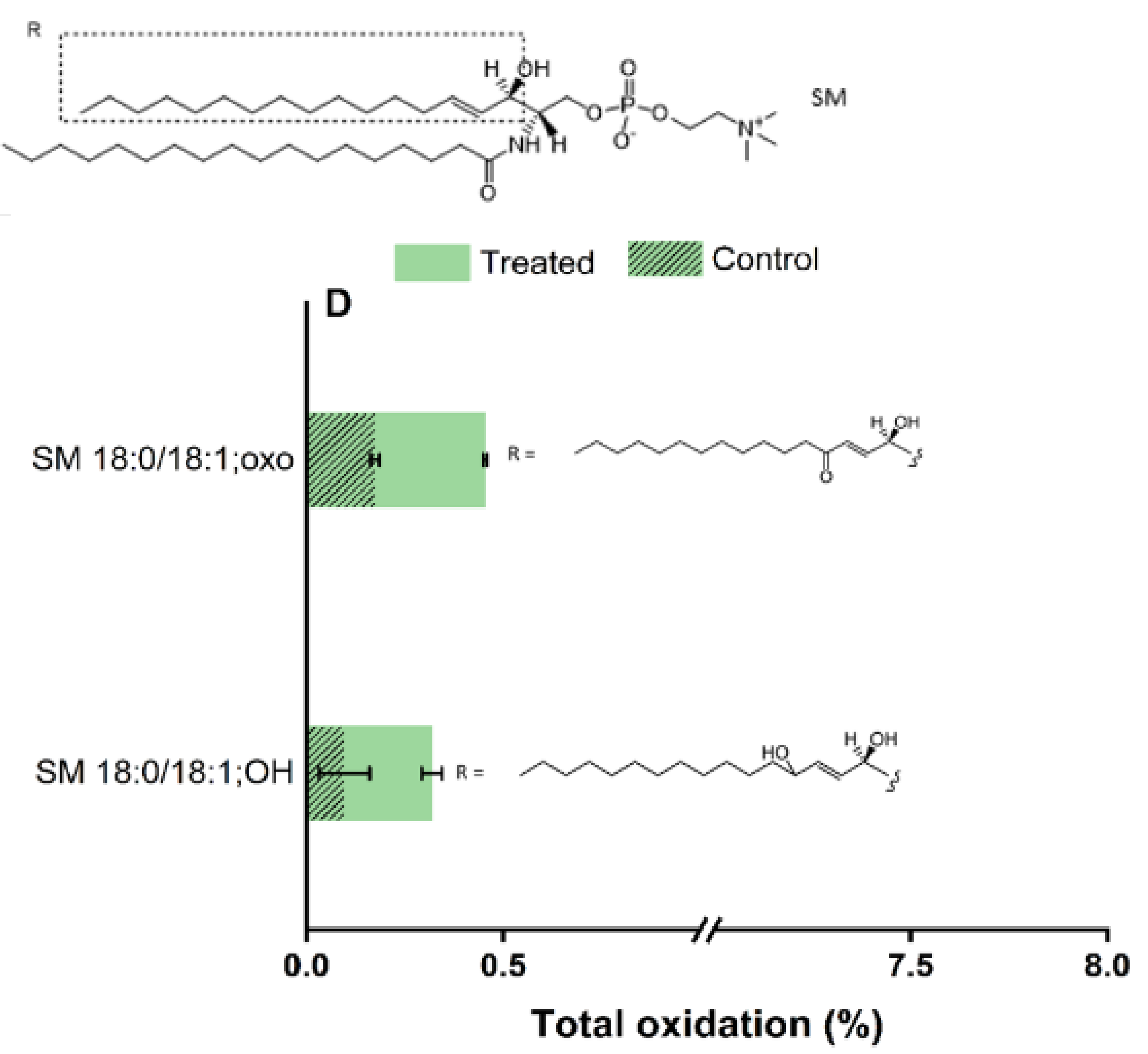
2.5. Plasma-Induced Chemical Modifications of the Model Lipids
3. Materials and Methods
3.1. Materials
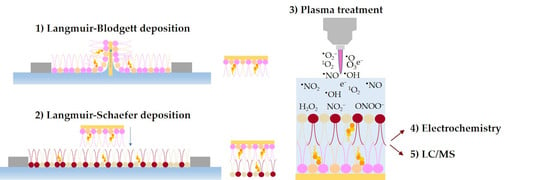
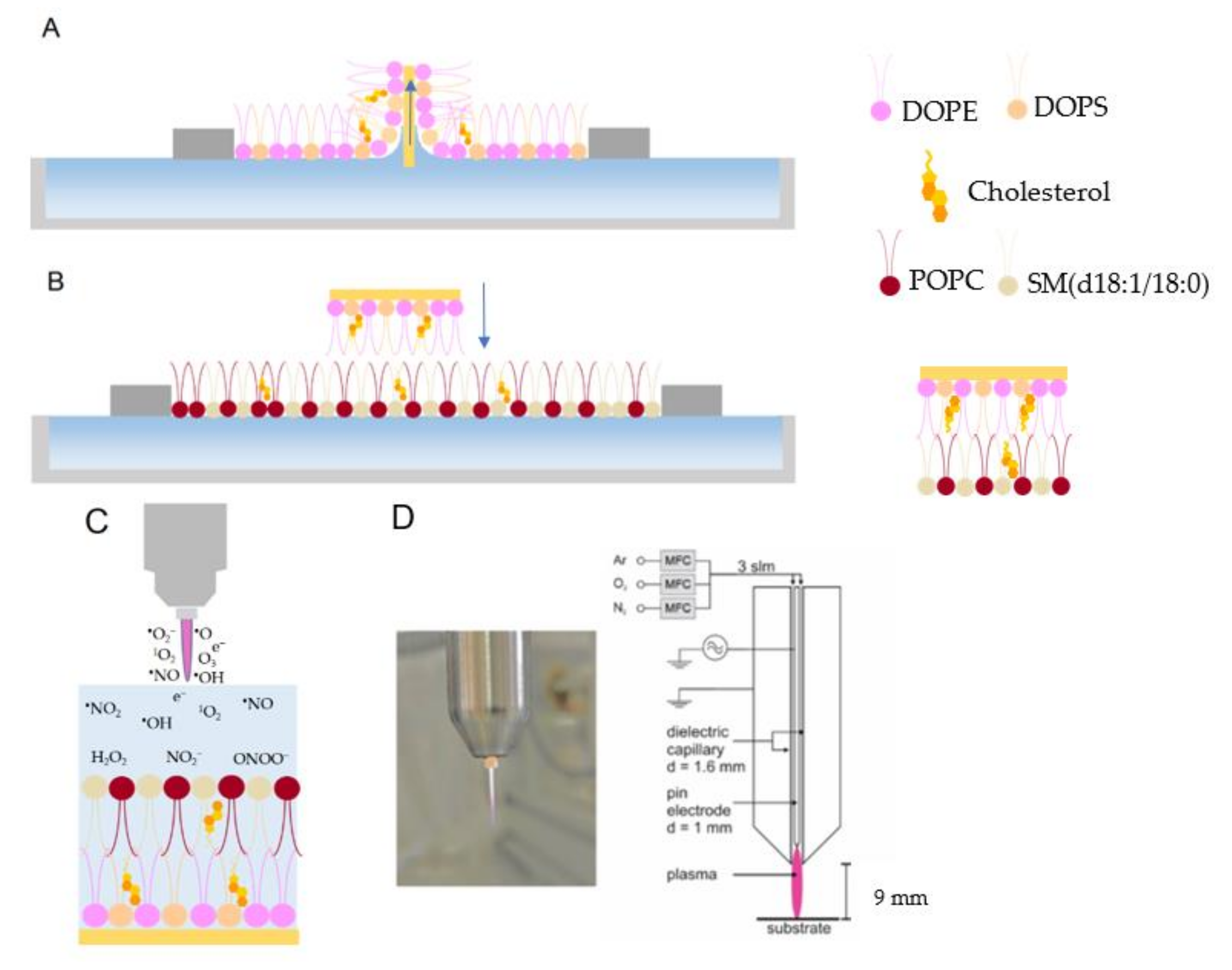
3.2. Langmuir-Blodgett (LB) and Langmuir-Schaefer (LS) Deposition
3.3. Electrochemical Measurements
3.4. Plasma Source
3.5. Reactive Species Quantification
3.6. Lipid Extraction
3.7. LC-MS2 Analysis
3.8. Identification of Lipid Peroxidation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Jiang, J.; Lei, Y.; Zhou, S.; Wei, Y.; Huang, C. Targeting Metabolic–Redox Circuits for Cancer Therapy. Trends Biochem. Sci. 2019, 44, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Isvoranu, G.; Comanescu, M.V.; Manea, A.; Butuner, B.D.; Korkmaz, K.S. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015, 5, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, L.; Chu, Y. Reactive oxygen species: The signal regulator of B cell. Free Radic. Biol. Med. 2019, 142, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031–014041. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Khalili, M.; Daniels, L.; Lin, A.; Krebs, F.C.; E Snook, A.; Bekeschus, S.; Bowne, W.B.; Miller, V. Non-thermal plasma-induced immunogenic cell death in cancer. J. Phys. D. Appl. Phys. 2019, 52, 423001. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, J.; Li, X.; Liu, D.; Lu, X. On the dose of plasma medicine: Equivalent total oxidation potential (ETOP). Phys. Plasmas 2020, 27, 063514. [Google Scholar] [CrossRef]
- Wende, K.; von Woedtke, T.; Weltmann, K.-D.; Bekeschus, S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biol. Chem. 2018, 400, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; von Woedtke, T.; Partecke, L.-I.; van der Linde, J. Platelets are key in cold physical plasma-facilitated blood coagulation in mice. Clin. Plasma Med. 2017, 7-8, 58–65. [Google Scholar] [CrossRef]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of Blood Coagulation by Nonthermal Atmospheric Pressure Dielectric Barrier Discharge Plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kINPen – A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical mechanisms of plasma assisted wound healing: Production and delivery of active species, Demanovska dolina, Slovakia, Demanovska dolina, Slovakia, 2011. Available online: http://enviro.fmph.uniba.sk/nato/pdfs/O/o16.pdf (accessed on 5 May 2022).
- Haertel, B.; von Woedtke, T.; Weltmann, K.-D.; Lindequist, U. Non-Thermal Atmospheric-Pressure Plasma Possible Application in Wound Healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, Y.; Xu, M.; Chen, H.; Lu, X.; Ostrikov, K. Cold atmospheric pressure plasmas in dermatology: Sources, reactive agents, and therapeutic effects. Plasma Process. Polym. 2020, 17, 1900218. [Google Scholar] [CrossRef]
- Heinlin, J.; Isbary, G.; Stolz, W.; Morfill, G.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 2010, 25, 1–11. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; von Woedtke, T.; Junger, M. Cold plasma antisepsis for skin and wounds: A new antimicrobial concept in dermatology. Exp. Dermatol. 2012, 21, e39. [Google Scholar]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2016, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirst, A.M.; Frame, F.M.; Arya, M.; Maitland, N.J.; O’Connell, D. Low temperature plasmas as emerging cancer therapeutics: The state of play and thoughts for the future. Tumor Biol. 2016, 37, 7021–7031. [Google Scholar] [CrossRef] [Green Version]
- Wenske, S.; Lackmann, J.-W.; Busch, L.M.; Bekeschus, S.; von Woedtke, T.; Wende, K. Reactive species driven oxidative modifications of peptides—Tracing physical plasma liquid chemistry. J. Appl. Phys. 2021, 129, 193305. [Google Scholar] [CrossRef]
- Wenske, S.; Lackmann, J.-W.; Bekeschus, S.; Weltmann, K.-D.; Von Woedtke, T.; Wende, K. Nonenzymatic post-translational modifications in peptides by cold plasma-derived reactive oxygen and nitrogen species. Biointerphases 2020, 15, 061008. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Wenske, S.; Lackmann, J.-W.; Lalk, M.; Von Woedtke, T.; Wende, K. On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas. Biomolecules 2020, 10, 1687. [Google Scholar] [CrossRef]
- Yan, D.; Xiao, H.; Zhu, W.; Nourmohammadi, N.; Zhang, L.G.; Bian, K.; Keidar, M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J. Phys. D Appl. Phys. 2017, 50, 055401. [Google Scholar] [CrossRef]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Cold Atmospheric Plasma and Plasma-Activated Medium Trigger RONS-Based Tumor Cell Apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef]
- Yusupov, M.; Wende, K.; Kupsch, S.; Neyts, E.C.; Reuter, S.; Bogaerts, A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci. Rep. 2017, 7, 5761. [Google Scholar] [CrossRef]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.-M.; Robert, E.; Pichon, C. New insights on molecular internalization and drug delivery following plasma jet exposures. Int. J. Pharm. 2020, 589, 119874. [Google Scholar] [CrossRef]
- LeDuc, M.; Guay, D.; Leask, R.; Coulombe, S. Cell permeabilization using a non-thermal plasma. New J. Phys. 2009, 11, 115021. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Hasan, S.W.; Yousuf, A.; Chakraborty, S.; Johnson, D.; Hilal, N. Biomimetic membranes: A critical review of recent progress. Desalination 2017, 420, 403–424. [Google Scholar] [CrossRef] [Green Version]
- Luchini, A.; Vitiello, G. Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Maheux, S.; Frache, G.; Thomann, J.S.; Clément, F.; Penny, C.; Belmonte, T.; Duday, D. Small unilamellar liposomes as a membrane model for cell inactivation by cold atmospheric plasma treatment. J. Phys. D Appl. Phys. 2016, 49, 344001. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-H.; Szili, E.J.; A Jenkins, A.T.; Short, R.D. Ionized gas (plasma) delivery of reactive oxygen species (ROS) into artificial cells. J. Phys. D Appl. Phys. 2014, 47, 362001. [Google Scholar] [CrossRef] [Green Version]
- Ki, S.H.; Park, J.K.; Sung, C.; Lee, C.B.; Uhm, H.; Choi, E.H.; Baik, K.Y. Artificial vesicles as an animal cell model for the study of biological application of non-thermal plasma. J. Phys. D Appl. Phys. 2016, 49, 085401. [Google Scholar] [CrossRef]
- Tero, R.; Suda, Y.; Kato, R.; Tanoue, H.; Takikawa, H. Plasma irradiation of artificial cell membrane system at solid–liquid interface. Appl. Phys. Express 2014, 7, 077001. [Google Scholar] [CrossRef]
- Van der Paal, J.; Hong, S.-H.; Yusupov, M.; Gaur, N.; Oh, J.-S.; Short, R.D.; Szili, E.J.; Bogaerts, A. How membrane lipids influence plasma delivery of reactive oxygen species into cells and subsequent DNA damage: An experimental and computational study. Phys. Chem. Chem. Phys. 2019, 21, 19327–19341. [Google Scholar] [CrossRef]
- Fadeel, B.; Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: Roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 264–277. [Google Scholar] [CrossRef]
- Bartlett, P.N. Bioelectrochemistry: Fundamentals, Experimental Techniques, and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kurniawan, J.; De Souza, J.F.V.; Dang, A.T.; Liu, G.-Y.; Kuhl, T.L. Preparation and Characterization of Solid-Supported Lipid Bilayers Formed by Langmuir–Blodgett Deposition: A Tutorial. Langmuir 2018, 34, 15622–15639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuypers, F.A. Red Cell Membrane Lipids in Hemoglobinopathies. Curr. Mol. Med. 2008, 8, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Striesow, J.; Lackmann, J.-W.; Ni, Z.; Wenske, S.; Weltmann, K.-D.; Fedorova, M.; von Woedtke, T.; Wende, K. Oxidative modification of skin lipids by cold atmospheric plasma (CAP): A standardizable approach using RP-LC/MS2 and DI-ESI/MS2. Chem. Phys. Lipids 2019, 226, 104786. [Google Scholar] [CrossRef] [PubMed]
- Bassereau, P.; Pincet, F. Quantitative Analysis of Holes in Supported Bilayers Providing the Adsorption Energy of Surfactants on Solid Substrate. Langmuir 1997, 13, 7003–7007. [Google Scholar] [CrossRef]
- Benz, M.; Gutsmann, T.; Chen, N.; Tadmor, R.; Israelachvili, J. Correlation of AFM and SFA Measurements Concerning the Stability of Supported Lipid Bilayers. Biophys. J. 2004, 86, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Marsh, D. Liquid-ordered phases induced by cholesterol: A compendium of binary phase diagrams. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 688–699. [Google Scholar] [CrossRef] [Green Version]
- Ravandeh, M.; Coliva, G.; Kahlert, H.; Azinfar, A.; Helm, C.A.; Fedorova, M.; Wende, K. Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress. Biomolecules 2021, 11, 276. [Google Scholar] [CrossRef]
- Ravandeh, M.; Kahlert, H.; Jablonowski, H.; Lackmann, J.-W.; Striesow, J.; Hernández, V.A.; Wende, K. A combination of electrochemistry and mass spectrometry to monitor the interaction of reactive species with supported lipid bilayers. Sci. Rep. 2020, 10, 18683. [Google Scholar] [CrossRef]
- Graves, D.B. Reactive Species from Cold Atmospheric Plasma: Implications for Cancer Therapy. Plasma Process. Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- de Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef] [Green Version]
- Van Der Paal, J.; Verheyen, C.; Neyts, E.C.; Bogaerts, A. Hampering Effect of Cholesterol on the Permeation of Reactive Oxygen Species through Phospholipids Bilayer: Possible Explanation for Plasma Cancer Selectivity. Sci. Rep. 2017, 7, 39526. [Google Scholar] [CrossRef]
- Bogaerts, A.; Yusupov, M.; Razzokov, J.; Van der Paal, J. Plasma for cancer treatment: How can RONS penetrate through the cell membrane? Answers from computer modeling. Front. Chem. Sci. Eng. 2019, 13, 253–263. [Google Scholar] [CrossRef]
- Valentine, M.L.; Waterland, M.K.; Fathizadeh, A.; Elber, R.; Baiz, C.R. Interfacial Dynamics in Lipid Membranes: The Effects of Headgroup Structures. J. Phys. Chem. B 2021, 125, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. The importance of hydrogen bonding in sphingomyelin’s membrane interactions with co-lipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Madrid, E.; Horswell, S.L. Effect of Headgroup on the Physicochemical Properties of Phospholipid Bilayers in Electric Fields: Size Matters. Langmuir 2013, 29, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Pasenkiewicz-Gierula, M.; Takaoka, Y.; Miyagawa, H.; Kitamura, A.K.; Kusumi, A. Hydrogen Bonding of Water to Phosphatidylcholine in the Membrane As Studied by a Molecular Dynamics Simulation: Location, Geometry, and Lipid−Lipid Bridging via Hydrogen-Bonded Water. J. Phys. Chem. A 1997, 101, 3677–3691. [Google Scholar] [CrossRef]
- Ripa, I.; Andreu, S.; López-Guerrero, J.A.; Bello-Morales, R. Membrane Rafts: Portals for Viral Entry. Front. Microbiol. 2021, 12, 631274. [Google Scholar] [CrossRef] [PubMed]
- Verlackt, C.C.W.; Neyts, E.; Bogaerts, A. Atomic scale behavior of oxygen-based radicals in water. J. Phys. D. Appl. Phys. 2017, 50, 11LT01. [Google Scholar] [CrossRef]
- Wong-Ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.-M.; Tieleman, D.P.; Monticelli, L. Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [Green Version]
- Riske, K.A.; Sudbrack, T.P.; Archilha, N.L.; Uchoa, A.F.; Schroder, A.P.; Marques, C.M.; Baptista, M.S.; Itri, R. Giant Vesicles under Oxidative Stress Induced by a Membrane-Anchored Photosensitizer. Biophys. J. 2009, 97, 1362–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellert, F.; Ahrens, H.; Helm, C.A. Oxidation of Unsaturated Phospholipids: A Monolayer Study. Langmuir 2020, 36, 12213–12220. [Google Scholar] [CrossRef] [PubMed]
- Bahja, J.; Dymond, M.K. Does membrane curvature elastic energy play a role in mediating oxidative stress in lipid membranes? Free. Radic. Biol. Med. 2021, 171, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Tero, R.; Yamashita, R.; Hashizume, H.; Suda, Y.; Takikawa, H.; Hori, M.; Ito, M. Nanopore formation process in artificial cell membrane induced by plasma-generated reactive oxygen species. Arch. Biochem. Biophys. 2016, 605, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Tsubone, T.M.; Junqueira, H.C.; Baptista, M.S.; Itri, R. Contrasting roles of oxidized lipids in modulating membrane microdomains. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1861, 660–669. [Google Scholar] [CrossRef]
- O’Donnell, V.B. Mass spectrometry analysis of oxidized phosphatidylcholine and phosphatidylethanolamine. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2011, 1811, 818–826. [Google Scholar] [CrossRef]
- Madrid, E.; Horswell, S.L. The electrochemical phase behaviour of chemically asymmetric lipid bilayers supported at Au (111) electrodes. J. Electroanal. Chem. 2018, 819, 338–346. [Google Scholar] [CrossRef]
- Mann, M.S.; Tiede, R.; Gavenis, K.; Daeschlein, G.; Bussiahn, R.; Weltmann, K.-D.; Emmert, S.; Von Woedtke, T.; Ahmed, R. Introduction to DIN-specification 91315 based on the characterization of the plasma jet kINPen® MED. Clin. Plasma Med. 2016, 4, 35–45. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Linxiang, L.; Abe, Y.; Nagasawa, Y.; Kudo, R.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. An HPLC assay of hydroxyl radicals by the hydroxylation reaction of terephthalic acid. Biomed. Chromatogr. 2004, 18, 470–474. [Google Scholar] [CrossRef]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rödder, K.; Jablonowski, H.; Schmidt, A.; von Woedtke, T.; Weltmann, K.-D.; Benedikt, J. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.-D.; Wende, K. Basic Research in Plasma Medicine—A Throughput Approach from Liquids to Cells. J. Vis. Exp. 2017, e56331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, Z.; Bruno, G.; Bekeschus, S.; Weltmann, K.-D.; von Woedtke, T.; Wende, K. Development of an electrochemical sensor for in-situ monitoring of reactive species produced by cold physical plasma. Sens. Actuators B Chem. 2020, 326, 129007. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Koefeler, H.; et al. Update on LIPID MAPS Classification, Nomenclature and Shorthand Notation for MS-derived Lipid Structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Nasri, Z.; Memari, S.; Wenske, S.; Clemen, R.; Martens, U.; Delcea, M.; Bekeschus, S.; Weltmann, K.; von Woedtke, T.; Wende, K. Singlet-Oxygen-Induced Phospholipase A 2 Inhibition: A Major Role for Interfacial Tryptophan Dioxidation. Chem. A Eur. J. 2021, 27, 14702–14710. [Google Scholar] [CrossRef]
- Clemen, R.; Freund, E.; Mrochen, D.; Miebach, L.; Schmidt, A.; Rauch, B.H.; Lackmann, J.; Martens, U.; Wende, K.; Lalk, M.; et al. Gas Plasma Technology Augments Ovalbumin Immunogenicity and OT-II T Cell Activation Conferring Tumor Protection in Mice. Adv. Sci. 2021, 8, 2003395. [Google Scholar] [CrossRef]
- Jackman, J.A.; Knoll, W.; Cho, N.-J. Biotechnology Applications of Tethered Lipid Bilayer Membranes. Materials 2012, 5, 2637–2657. [Google Scholar] [CrossRef] [Green Version]
- Denisov, I.G.; Sligar, S.G. Nanodiscs in Membrane Biochemistry and Biophysics. Chem. Rev. 2017, 117, 4669–4713. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, Z.; Ahmadi, M.; Striesow, J.; Ravandeh, M.; von Woedtke, T.; Wende, K. Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models. Int. J. Mol. Sci. 2022, 23, 5932. https://doi.org/10.3390/ijms23115932
Nasri Z, Ahmadi M, Striesow J, Ravandeh M, von Woedtke T, Wende K. Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models. International Journal of Molecular Sciences. 2022; 23(11):5932. https://doi.org/10.3390/ijms23115932
Chicago/Turabian StyleNasri, Zahra, Mohsen Ahmadi, Johanna Striesow, Mehdi Ravandeh, Thomas von Woedtke, and Kristian Wende. 2022. "Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models" International Journal of Molecular Sciences 23, no. 11: 5932. https://doi.org/10.3390/ijms23115932
APA StyleNasri, Z., Ahmadi, M., Striesow, J., Ravandeh, M., von Woedtke, T., & Wende, K. (2022). Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models. International Journal of Molecular Sciences, 23(11), 5932. https://doi.org/10.3390/ijms23115932