Allelic Diversification of the Wx and ALK Loci in Indica Restorer Lines and Their Utilisation in Hybrid Rice Breeding in China over the Last 50 Years
Abstract
:1. Introduction
2. Results
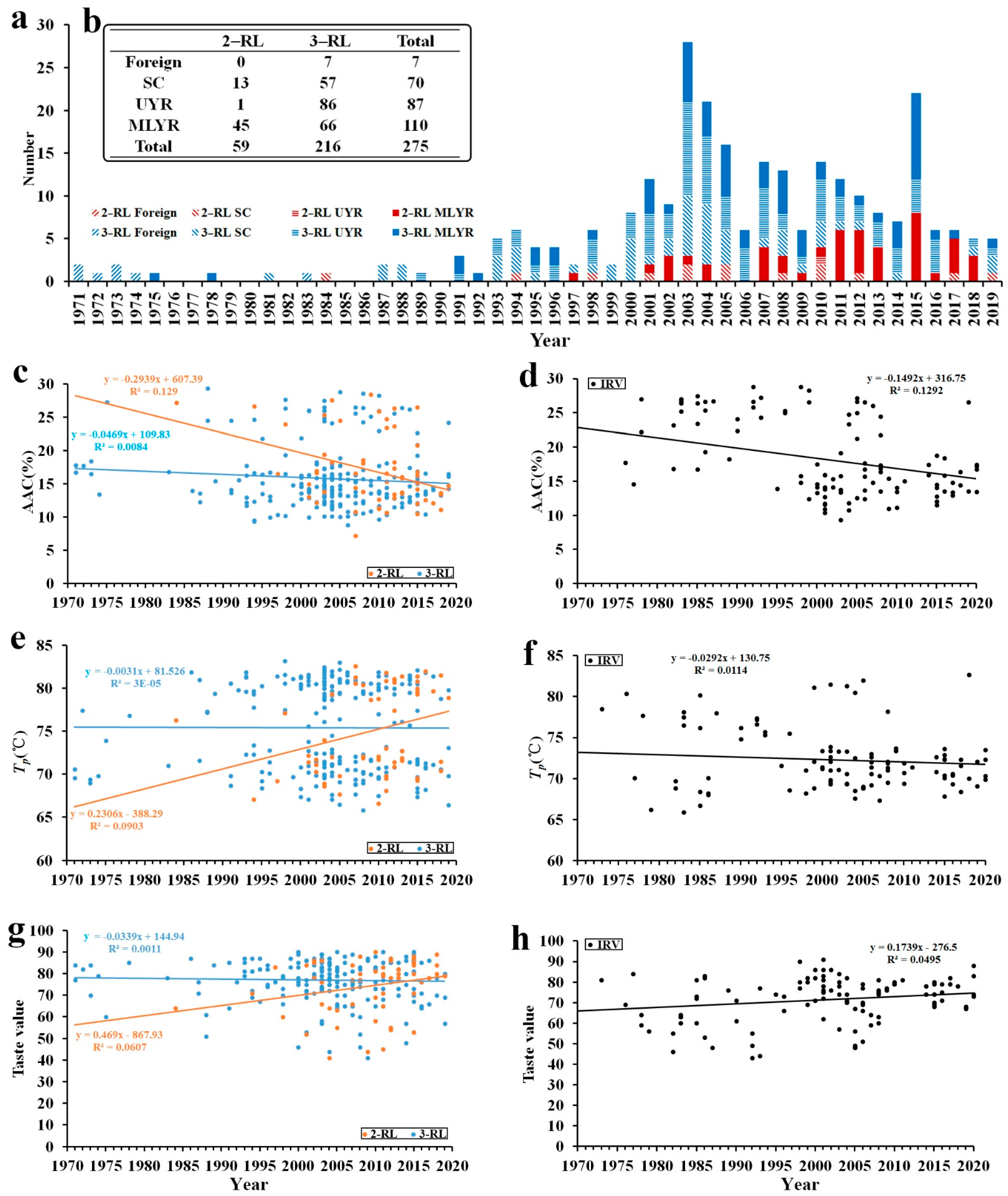
2.1. Characteristics of ECQ among Indica RLs
2.2. ECQ Differentiation with Years and Places
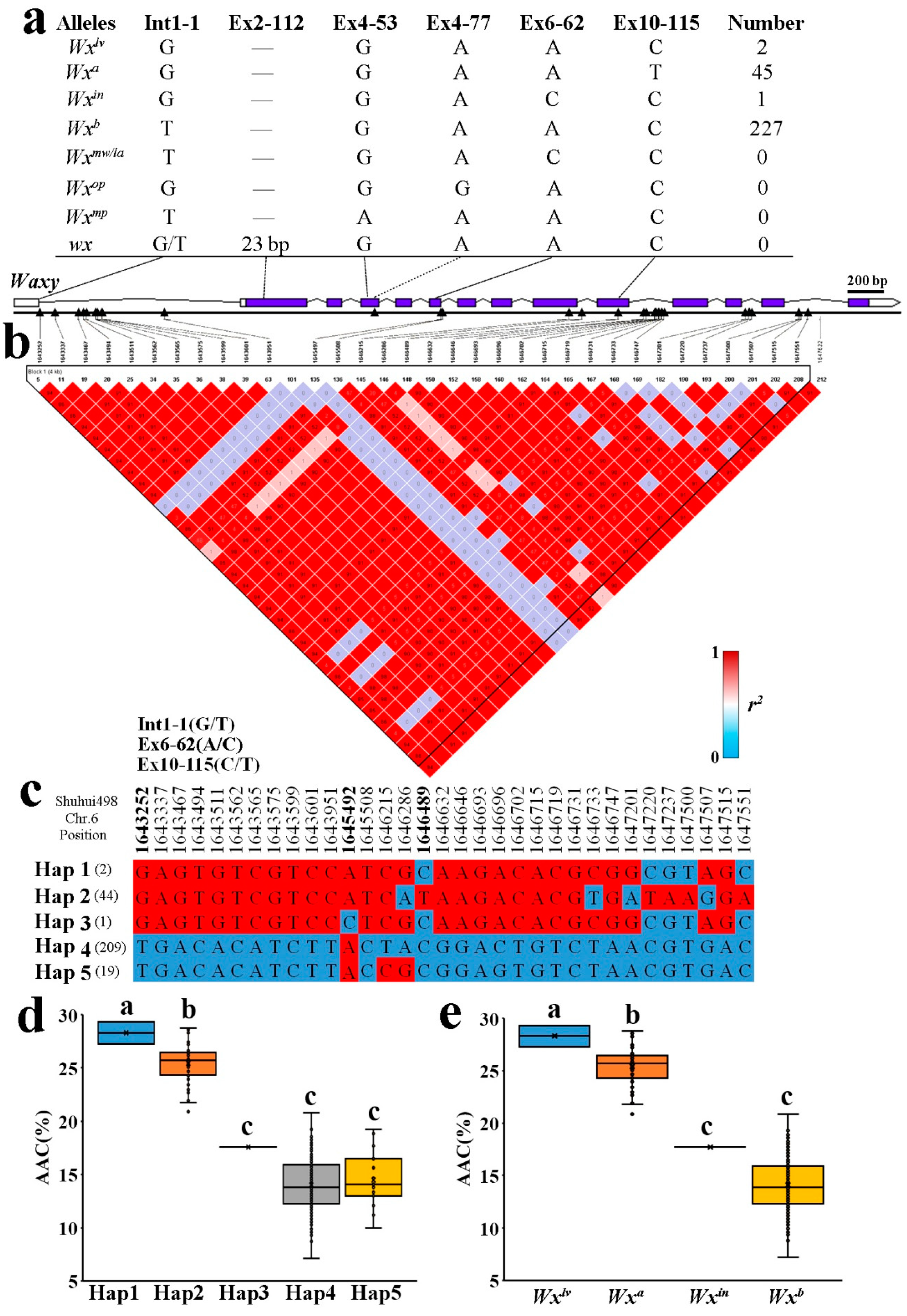
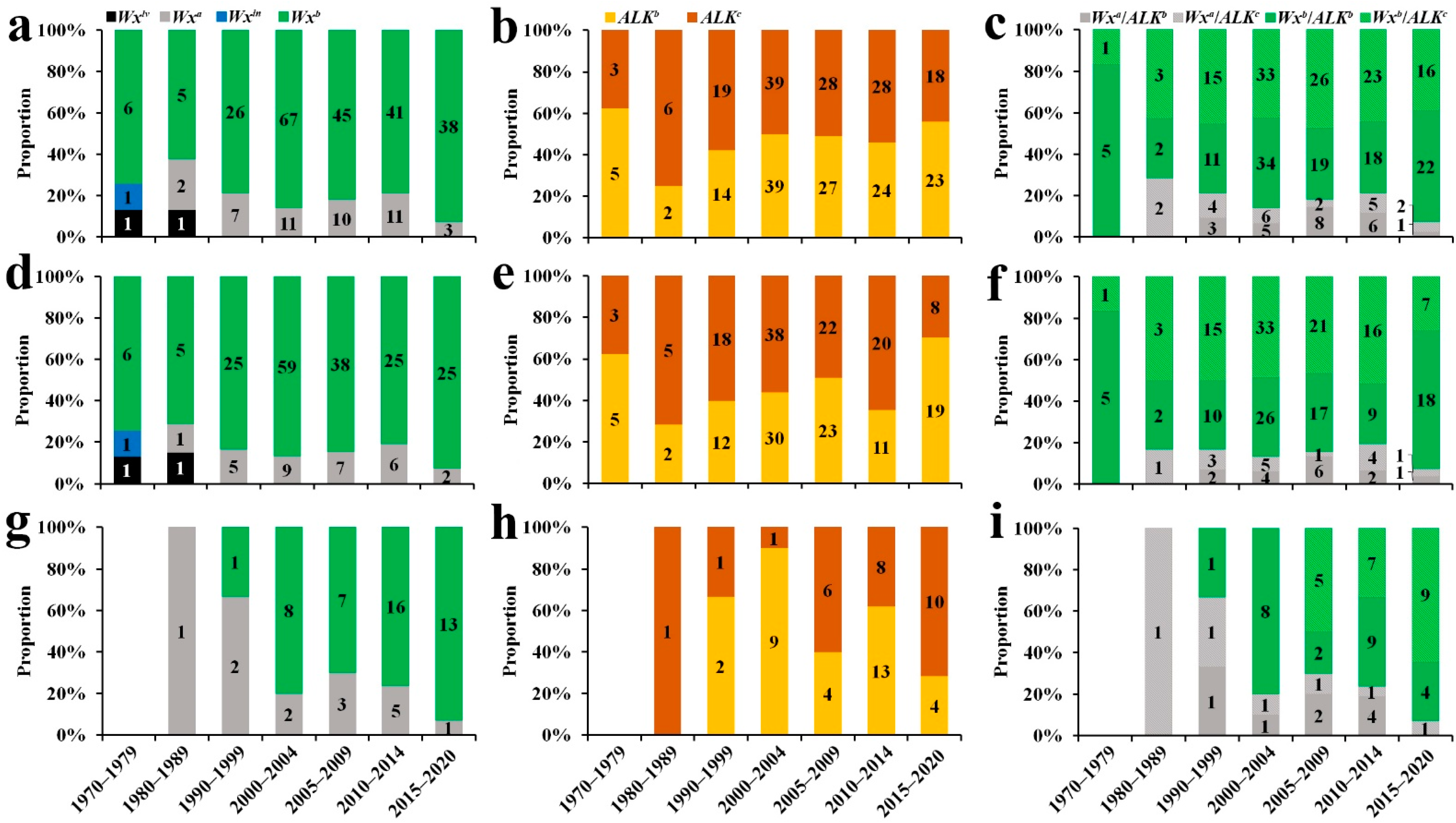
2.3. Allelic Diversification of the Wx Locus in Indica RLs
2.4. Allelic Diversification of the ALK Locus in Indica RLs
2.5. Combining Wx and ALK Alleles and Their Utilisation in Indica RLs
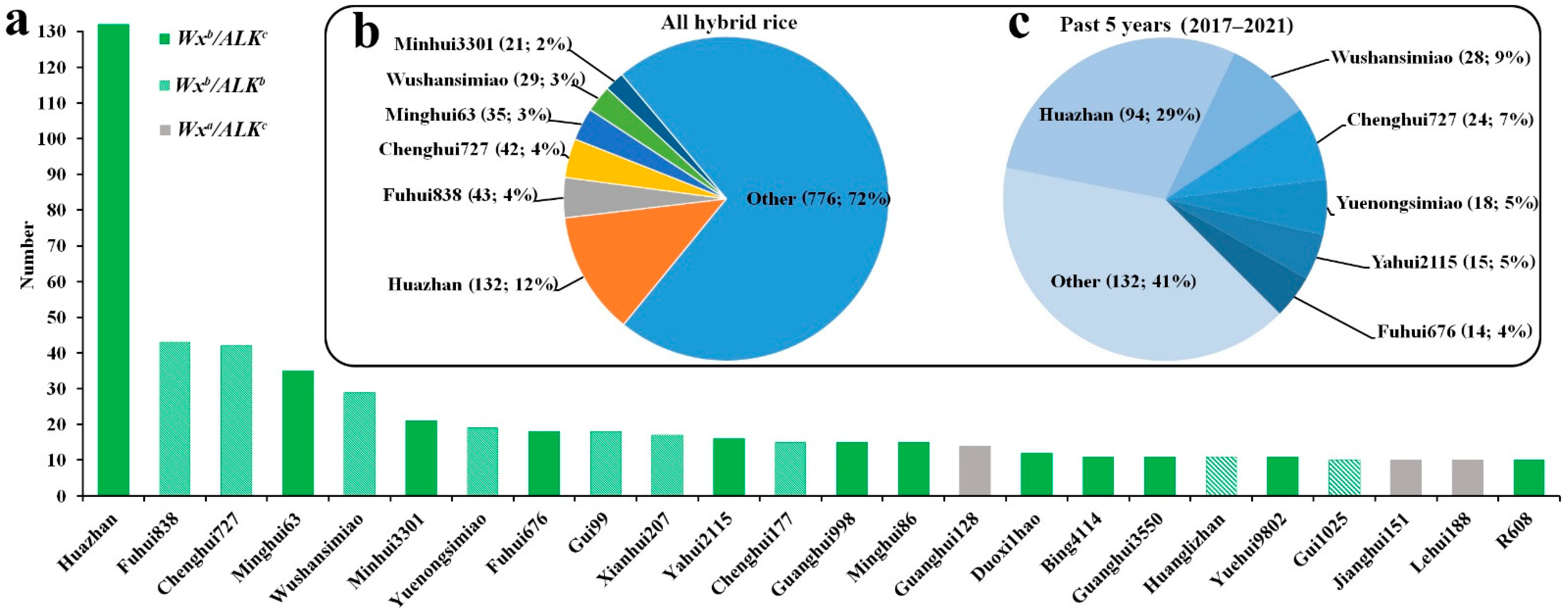
2.6. Contributions of Elite Ls in the Development of Hybrid Rice
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Polished Rice and Flour Preparation
4.3. Determination of Grain Physicochemical Properties
4.4. Measurement of Rice Taste Value
4.5. Sequencing and Genotype Annotation
4.6. Detection of Wx and ALK Alleles
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECQ | Eating and cooking quality |
| RL | Restorer line |
| IRV | Inbred rice variety |
| AC | Amylose content |
| GT | Gelatinisation temperature |
| DSC | Differential scanning calorimetry |
| RVA | Rapid visco analyzer |
| Tp | Peak temperature of gelatinisation |
| SNP | Single-nucleotide polymorphism |
| KASP | Kompetitive allele-specific PCR |
| BDV | Breakdown viscosity |
| SBV | Setback viscosity |
| SC | South China |
| MLYR | Middle and lower reaches of the Yangtze River |
| UYR | Upper reaches of Yangtze River |
References
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G.; et al. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- Angira, B.; Addison, C.K.; Cerioli, T.; Rebong, D.B.; Wang, D.R.; Pumplin, N.; Ham, J.H.; Oard, J.H.; Linscombe, S.D.; Famoso, A.N. Haplotype characterization of the sd1 semidwarf gene in United States rice. Plant Genome 2019, 12, 190010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.-P. Strategic hypotheses of breeding for hybrid rice. Hybrid Rice 1987, 1, 1–3. [Google Scholar]
- Cheng, H.; Zhuang, Y.; Fan, Y.; Du, H.; Cao, Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef]
- Peng, S.; Khush, G.; Virk, P.; Tang, Q.; Zou, Y. Progress in ideotype breeding to increase rice yield potential. Field Crop. Res. 2008, 108, 32–38. [Google Scholar] [CrossRef]
- Chen, L.-T.; Liu, Y.-G. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J.; et al. Genomic architecture of heterosis for yield traits in rice. Nature 2016, 537, 629–633. [Google Scholar] [CrossRef]
- Chen, E.; Huang, X.; Tian, Z.; Wing, R.-A.; Han, B. The genomics of Oryza species provides insights into rice domestication and heterosis. Annu. Rev. Plant Biol. 2019, 70, 639–665. [Google Scholar] [CrossRef]
- Xie, Y.; Shen, R.; Chen, L.; Liu, Y.-G. Molecular mechanisms of hybrid sterility in rice. Sci. China Life Sci. 2019, 62, 737–743. [Google Scholar] [CrossRef]
- Lv, Q.; Li, W.; Sun, Z.; Ouyang, N.; Jing, X.; He, Q.; Wu, J.; Zheng, J.; Zheng, J.; Tang, S.; et al. Resequencing of 1,143 indica rice accessions reveals important genetic variations and different heterosis patterns. Nat. Commun. 2020, 11, 4778. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y. Corrigendum: Correlations between parental lines and indica hybrid rice in terms of eating quality traits. Front. Nutr. 2021, 8, 663504. [Google Scholar]
- Peng, Y.; Mao, B.; Zhang, C.; Shao, Y.; Wu, T.; Hu, L.; Hu, Y.; Tang, L.; Li, Y.; Tang, W.; et al. Influence of physicochemical properties and starch fine structure on the eating quality of hybrid rice with similar apparent amylose content. Food Chem. 2021, 353, 129461. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Qian, Q.; Liu, Q.; Yan, M.; Liu, X.; Yan, C.; Liu, G.; Gao, Z.; Tang, S.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Yang, Y.; Chen, Z.; Chen, F.; Pan, L.; Lu, Y.; Li, Q.; Fan, X.; Sun, Z.; Liu, Q. Characteristics of grain physicochemical properties and the starch structure in rice carrying a mutated ALK/SSIIa gene. J. Agric. Food Chem. 2020, 68, 13950–13959. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Ren, X.; Lu, Y.; Liu, D.; Cai, X.; Li, Q.; Gao, J.; Liu, Q. Molecular structure and physicochemical properties of starches from rice with different amylose contents resulting from modification of OsGBSSI activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef]
- Suwannaporn, P.; Linnemann, A.R. Rice-eating quality among consumers in different rice grain preference countries. J. Sens. Stud. 2008, 23, 1–13. [Google Scholar] [CrossRef]
- Zhao, J. Comparison of Grain Yield and Quality between Hybrid Rice and Inbred Rice in China. Hybrid Rice 2008, 23, 1–4. [Google Scholar]
- Ball, S.G.; Morell, M.K. From bacterial glycogen to starch: Understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 2003, 54, 207–233. [Google Scholar] [CrossRef]
- Nakamura, Y. Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: Rice endosperm as a model tissue. Plant Cell Physiol. 2002, 43, 718–725. [Google Scholar] [CrossRef]
- Smith, A.M.; Denyer, K.; Martin, C. The synthesis of the starch granule. Annu. Rev. Plant Phys. 1997, 48, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.; Kossmann, J.; Smith, A. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakata, M.; Miyashita, T.; Kimura, R.; Nakata, Y.; Takagi, H.; Kuroda, M.; Yamaguchi, T.; Umemoto, T.; Yamakawa, H. MutMapPlus identified novel mutant alleles of a rice starch branching enzyme IIb gene for fine-tuning of cooked rice texture. Plant Biotechnol. J. 2018, 16, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhu, J.; Chen, S.; Fan, X.; Li, Q.; Lu, Y.; Wang, M.; Yu, H.; Yi, C.; Tang, S.; et al. Wx(lv), the ancestral allele of rice Waxy gene. Mol. Plant 2019, 12, 1157–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Ma, X.; Liu, S.; Zhu, C.; Jiang, L.; Wang, Y.; Shen, Y.; Ren, Y.; Dong, H.; Chen, L.; et al. Identification and characterization of a novel Waxy allele from a Yunnan rice landrace. Plant Mol. Biol. 2009, 71, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Mikami, I.; Aikawa, M.; Hirano, H.; Sano, Y. Altered tissue-specific expression at the Wx gene of the opaque mutants in rice. Euphytica 1999, 105, 91–97. [Google Scholar] [CrossRef]
- Mikami, I.; Dung, L.; Hirano, H.; Sano, Y. Effects of the two most common Wx alleles on different genetic backgrounds in rice. Plant Breed. 2000, 119, 505–508. [Google Scholar] [CrossRef]
- Mikami, I.; Uwatoko, N.; Ikeda, Y.; Yamaguchi, J.; Hirano, H.Y.; Suzuki, Y.; Sano, Y. Allelic diversification at the wx locus in landraces of Asian rice. Theor. Appl. Genet. 2008, 116, 979–989. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Chen, S.; Liu, X.; Zhu, J.; Zhou, L.; Lu, Y.; Li, Q.; Fan, X.; Tang, S.; et al. A rare Waxy allele coordinately improves rice eating and cooking quality and grain transparency. J. Integr. Plant Biol. 2021, 63, 889–901. [Google Scholar] [CrossRef]
- Huang, L.; Sreenivasulu, N.; Liu, Q. Waxy editing: Old meets new. Trends Plant Sci. 2020, 25, 963–966. [Google Scholar] [CrossRef]
- Gao, Z.; Zeng, D.; Cheng, F.; Tian, Z.; Guo, L.; Su, Y.; Yan, M.; Jiang, H.; Dong, G.; Huang, Y.; et al. ALK, the key gene for gelatinization temperature, is a modifier gene for gel consistency in rice. J. Integr. Plant Biol. 2011, 53, 756–765. [Google Scholar] [PubMed]
- Bao, J.-S.; Corke, H.; Sun, M. Nucleotide diversity in starch synthase IIa and validation of single nucleotide polymorphisms in relation to starch gelatinization temperature and other physicochemical properties in rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 113, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.; Henry, R.; Reinke, R.; Fitzgerald, M. Gelatinization temperature of rice explained by polymorphisms in starch synthase. Plant Biotechnol. J. 2006, 4, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, Y.; Feng, L.; Hao, W.; Li, C.; Yang, Y.; Fan, X.; Li, Q.; Zhang, C.; Liu, Q. Genetic dissection and functional differentiation of ALKa and ALKb, two natural alleles of the ALK/SSIIa gene, responding to low gelatinization temperature in rice. Rice 2020, 13, 39. [Google Scholar] [CrossRef]
- You, L.; Yu, L.; Liang, R.; Sun, R.; Hu, F.; Lu, X.; Zhao, J. Identification and analysis of genes involved in double fertilization in rice. Int. J. Mol. Sci. 2021, 22, 12850. [Google Scholar] [CrossRef]
- Friedman, W.E. Comparative embryology of basal angiosperms. Curr. Opin. Plant Biol. 2001, 4, 14–20. [Google Scholar] [CrossRef]
- Bleckmann, A.; Alter, S.; Dresselhaus, T. The beginning of a seed: Regulatory mechanisms of double fertilization. Front. Plant Sci. 2014, 5, 452. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Peng, Y.; Mao, B.; Lv, Q.; Yuan, D.; Liu, X.; Zhao, B. Allelic variations of the Wx locus in cultivated rice and their use in the development of hybrid rice in China. PLoS ONE 2020, 15, e0232279. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Yan, Y.; Hu, Z.; Wang, K.; Zhou, J.; Zhou, Y.; Cao, L.; Wu, S. Influence of starch fine structure and storage proteins on the eating quality of rice varieties with similar amylose contents. J Sci Food Agric. 2021, 101, 811–3818. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; He, L.-H.; Wang, T.; Lu, H.; Yang, F.; Deng, F.; Chen, Y.; Tao, Y.-F.; Li, M.; et al. Correlation of taste values with chemical compositions and Rapid Visco Analyser profiles of 36 indica rice (Oryza sativa L.) varieties. Food Chem. 2021, 349, 129176. [Google Scholar] [CrossRef]
- Cai, X.-L.; Wang, Z.-Y.; Xing, Y.-Y.; Zhang, J.-L.; Hong, M.-M. Aberrant splicing of intron 1 leads to the heterogeneous 5′ UTR and decreased expression of Waxy gene in rice cultivars of intermediate amylose content. Plant J. 1998, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Guan, H.P.; James, M.; Myers, A.; Keeling, P.; Mouille, G.; Buléon, A.; Colonna, P.; Preiss, J. From glycogen to amylopectin: A model for the biogenesis of the plant starch granule. Cell 1996, 86, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Dian, W.; Liu, F.; Wu, P. Molecular cloning and expression analysis of three genes encoding starch synthase II in rice. Planta 2004, 218, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Francisco, P.B.; Hosaka, Y.; Sato, A.; Sawada, T.; Kubo, A.; Fujita, N. Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties. Plant Mol. Biol. 2005, 58, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Horibata, T.; Aoki, N.; Hiratsuka, M.; Yano, M.; Inouchi, N. Effects of variations in starch synthase on starch properties and eating quality of rice. Plant Prod. Sci. 2008, 11, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cao, P.; Wu, P.; Yu, W.; Gilbert, R.G.; Li, E. Effects of endogenous proteins on rice digestion during small intestine (in vitro) digestion. Food Chem. 2020, 344, 128687. [Google Scholar] [CrossRef]
- Butardo, V.M., Jr.; Luo, J.; Li, Z.; Gidley, M.J.; Bird, A.R.; Tetlow, I.J.; Fitzgerald, M.; Jobling, S.A.; Rahman, S. Functional genomic validation of the roles of soluble starch synthase IIa in Japonica rice endosperm. Front. Genet. 2020, 11, 289. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Zhang, Q.; Wei, X.; Huang, X. Exploring the molecular basis of heterosis for plant breeding. J. Integr. Plant Biol. 2020, 62, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-J.; Zhang, D. Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci. 2018, 23, 53–65. [Google Scholar] [CrossRef]
- Wu, B.; Hu, W.; Xing, Y.Z. The history and prospect of rice genetic breeding in China. Hereditas 2018, 40, 841–857. [Google Scholar]
- Zhang, C.; Feng, L.; Gu, M.; Liu, Q. Progress on inheritance and gene cloning for rice grain quality in Jiangsu province. Hereditas 2021, 43, 425–441. [Google Scholar]
- Yao, S.; Zhang, Y.; Liu, Y.; Zhao, C.; Zhou, L.; Chen, T.; Zhao, Q.Y.; Pillay, B.; Wang, C. Effects of soluble starch synthase genes on eating and cooking quality in semi Waxy japonica rice with Wxmp. Food Prod. Process. Nutr. 2020, 2, 22. [Google Scholar] [CrossRef]
- Juliano, B.O.; Perez, C.M.; Blakeney, A.B.; Castillo, T.; Kongseree, N.; Laignelet, B.; Lapis, E.T.; Murty, V.V.S.; Paule, C.M.; Webb, B.D. International cooperative testing on the amylose content of milled rice. Starch—Stärke 1981, 33, 157–162. [Google Scholar] [CrossRef]
- Tao, K.; Li, C.; Yu, W.; Gilbert, R.G.; Li, E. How amylose molecular fine structure of rice starch affects functional properties. Carbohydr. Polym. 2019, 204, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the Human genome. Science 2002, 296, 2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.-X.; Sun, Z.-Z.; Zhang, C.-Q.; Li, B.; Yang, Q.-Q.; Chen, F.; Fan, X.-L.; Zhao, D.-S.; Lv, Q.-M.; Yuan, D.-Y.; et al. Allelic Diversification of the Wx and ALK Loci in Indica Restorer Lines and Their Utilisation in Hybrid Rice Breeding in China over the Last 50 Years. Int. J. Mol. Sci. 2022, 23, 5941. https://doi.org/10.3390/ijms23115941
Pan L-X, Sun Z-Z, Zhang C-Q, Li B, Yang Q-Q, Chen F, Fan X-L, Zhao D-S, Lv Q-M, Yuan D-Y, et al. Allelic Diversification of the Wx and ALK Loci in Indica Restorer Lines and Their Utilisation in Hybrid Rice Breeding in China over the Last 50 Years. International Journal of Molecular Sciences. 2022; 23(11):5941. https://doi.org/10.3390/ijms23115941
Chicago/Turabian StylePan, Li-Xu, Zhi-Zhong Sun, Chang-Quan Zhang, Bu Li, Qing-Qing Yang, Fei Chen, Xiao-Lei Fan, Dong-Sheng Zhao, Qi-Ming Lv, Ding-Yang Yuan, and et al. 2022. "Allelic Diversification of the Wx and ALK Loci in Indica Restorer Lines and Their Utilisation in Hybrid Rice Breeding in China over the Last 50 Years" International Journal of Molecular Sciences 23, no. 11: 5941. https://doi.org/10.3390/ijms23115941
APA StylePan, L.-X., Sun, Z.-Z., Zhang, C.-Q., Li, B., Yang, Q.-Q., Chen, F., Fan, X.-L., Zhao, D.-S., Lv, Q.-M., Yuan, D.-Y., & Liu, Q.-Q. (2022). Allelic Diversification of the Wx and ALK Loci in Indica Restorer Lines and Their Utilisation in Hybrid Rice Breeding in China over the Last 50 Years. International Journal of Molecular Sciences, 23(11), 5941. https://doi.org/10.3390/ijms23115941





