The Expression of Connexin 37, 40, 43, 45 and Pannexin 1 in the Early Human Retina and Choroid Development and Tumorigenesis
Abstract
1. Introduction
2. Results
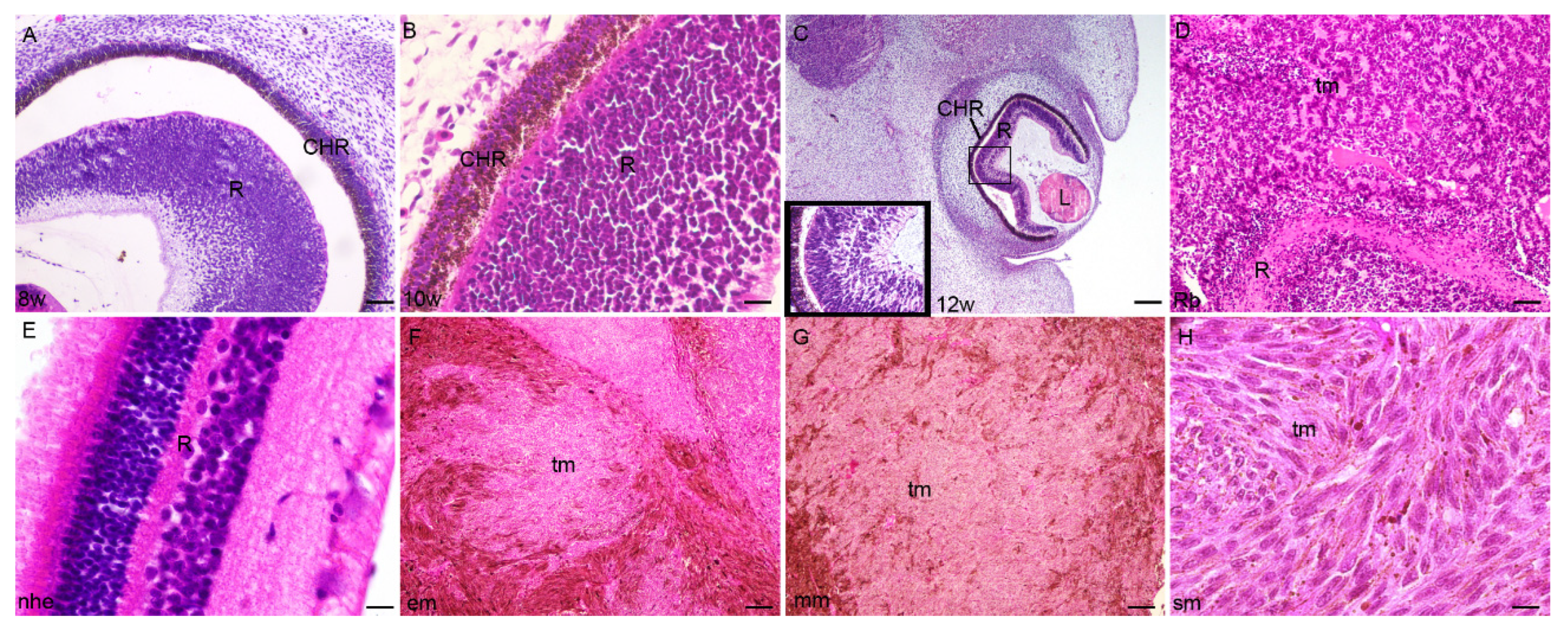
2.1. Developing Human Eye
2.2. Retinoblastoma
2.3. Epithelioid Melanoma
2.4. Myxoid Melanoma
2.5. Spindle Cell Melanoma
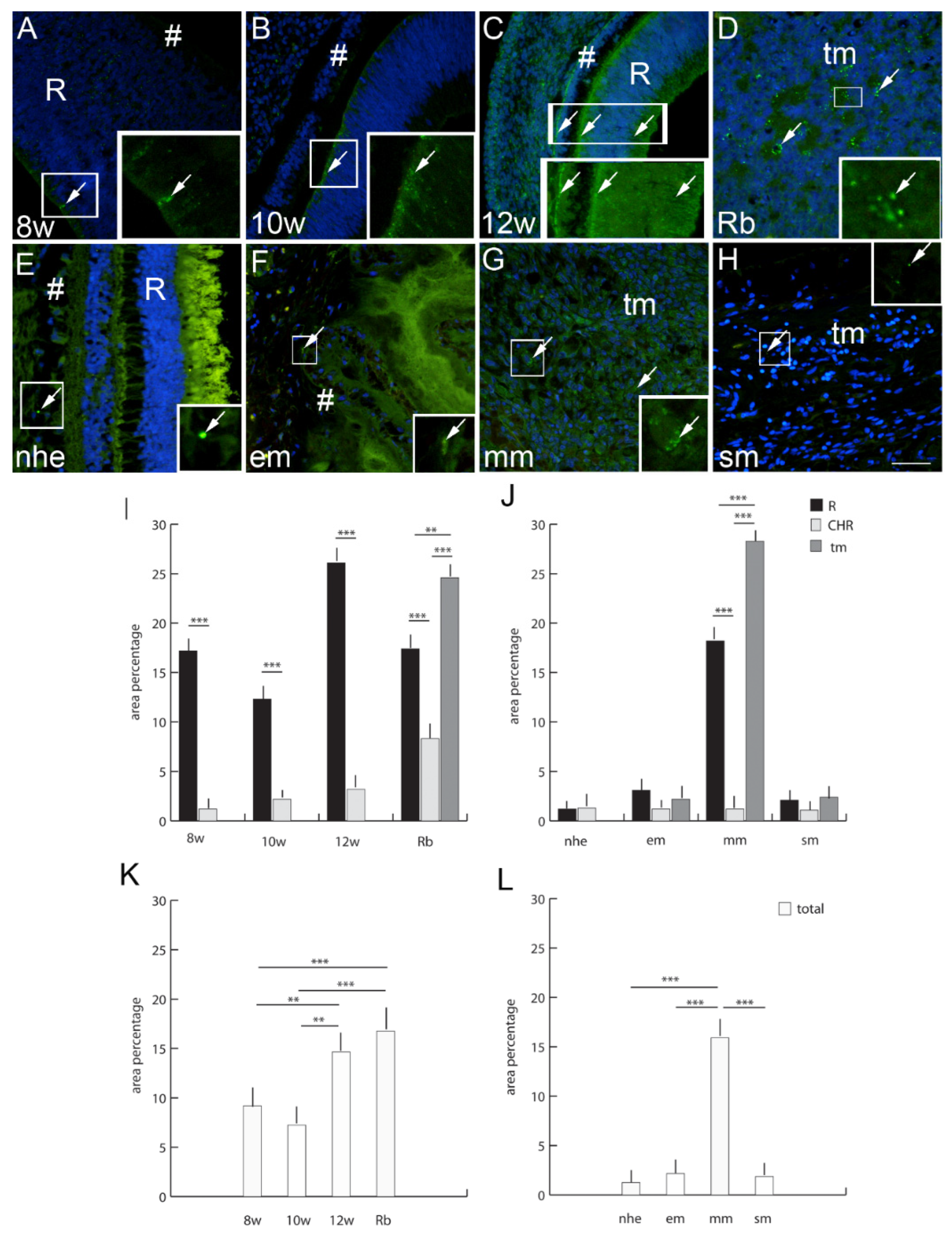
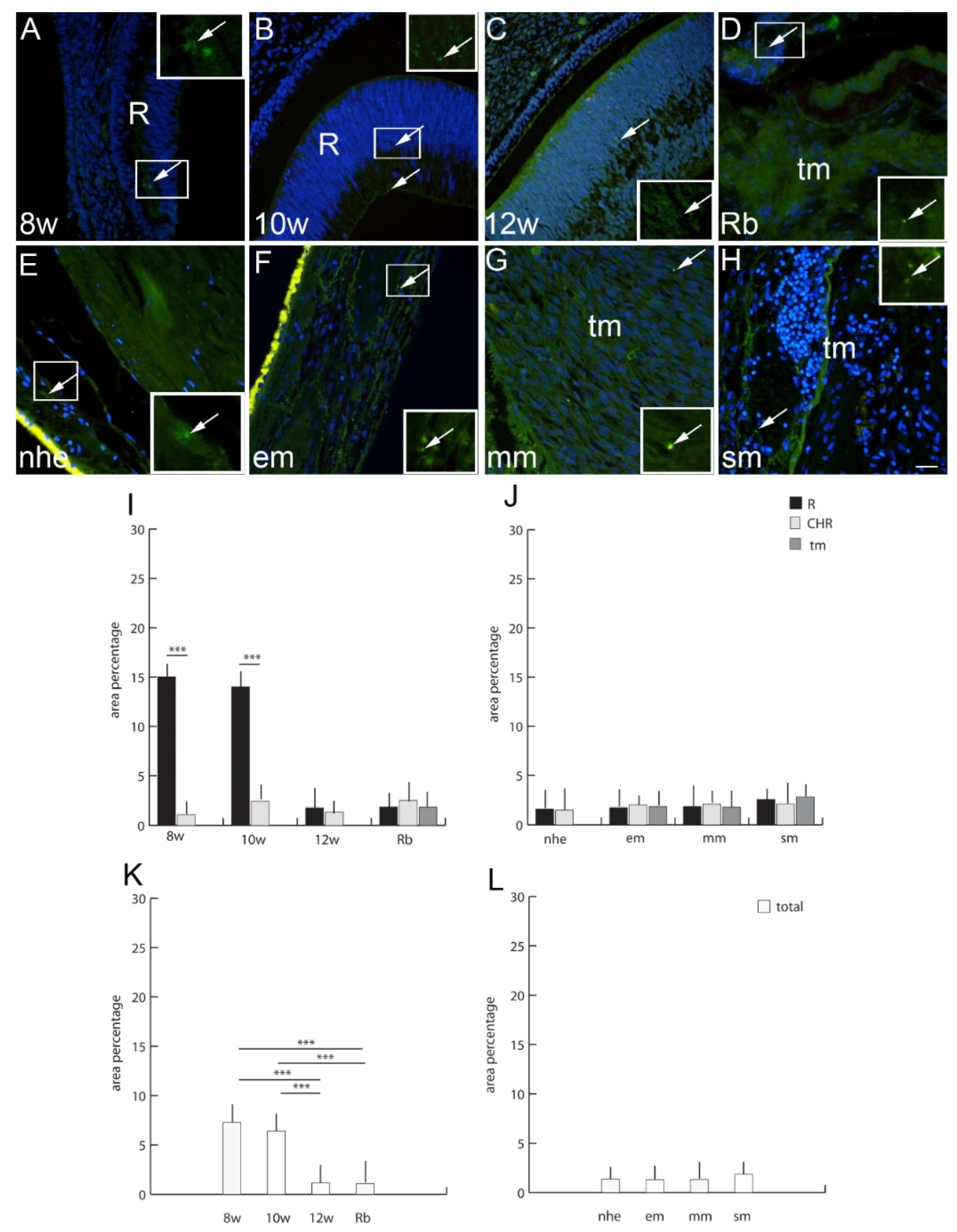
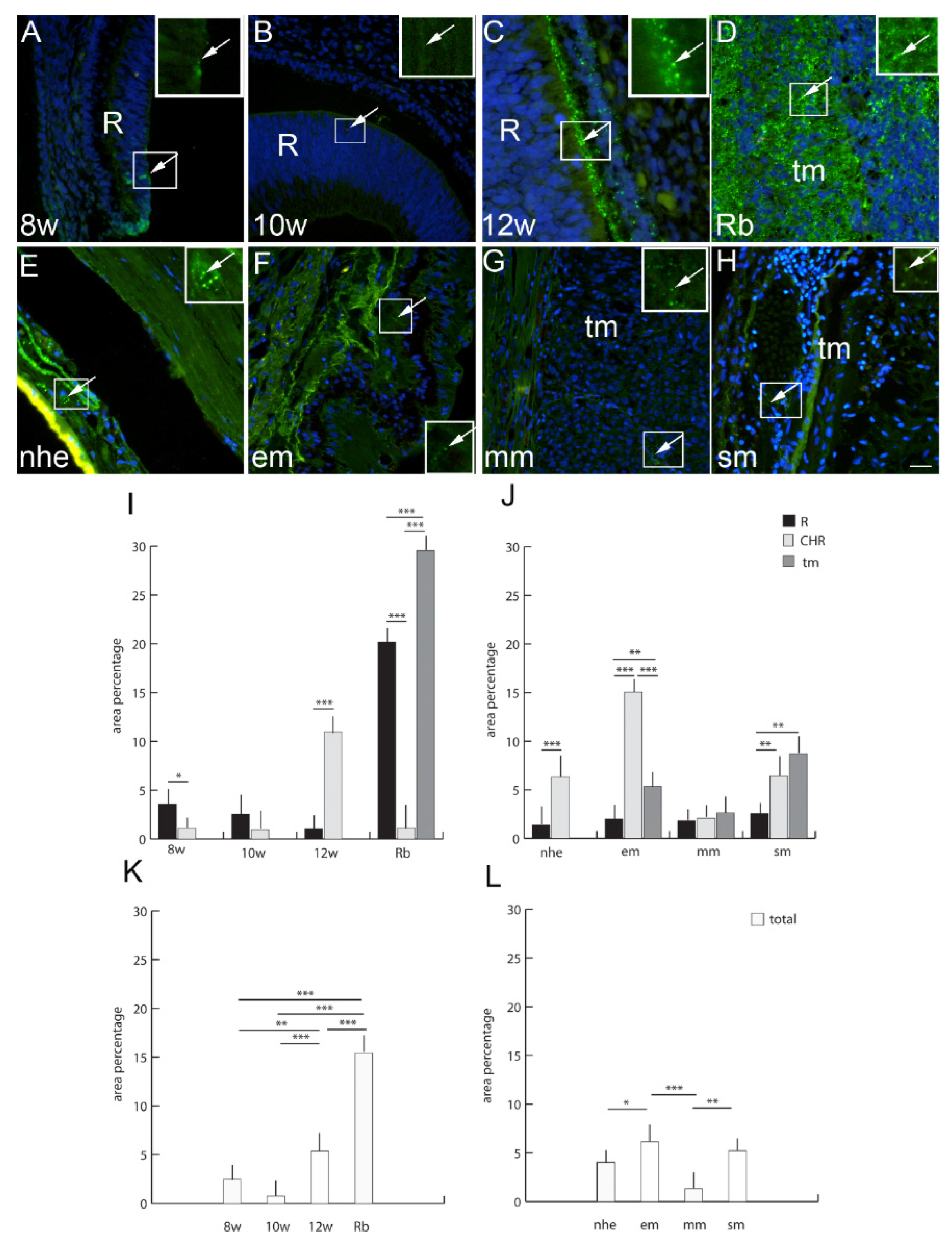
2.6. Connexin 37
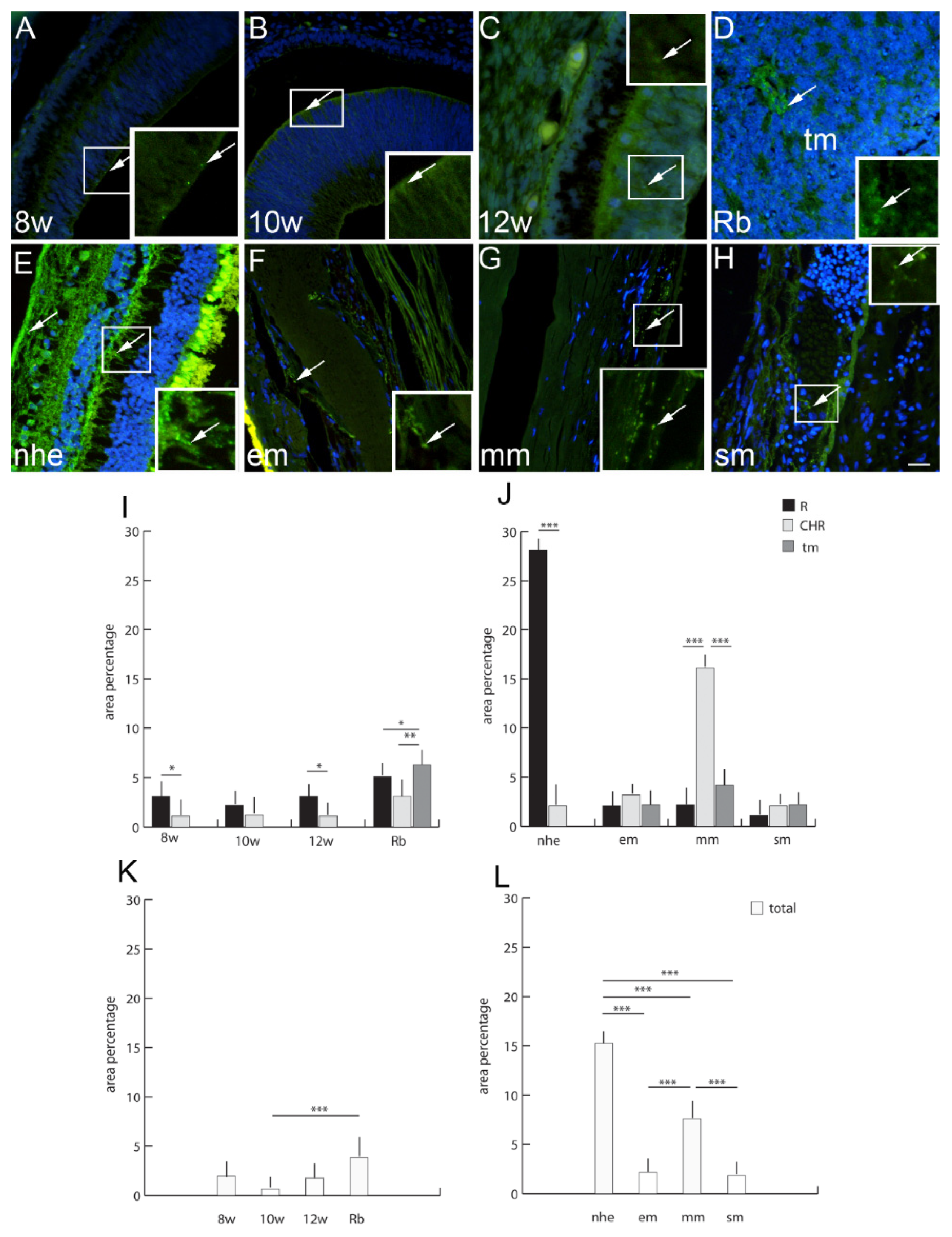
2.7. Connexin 40
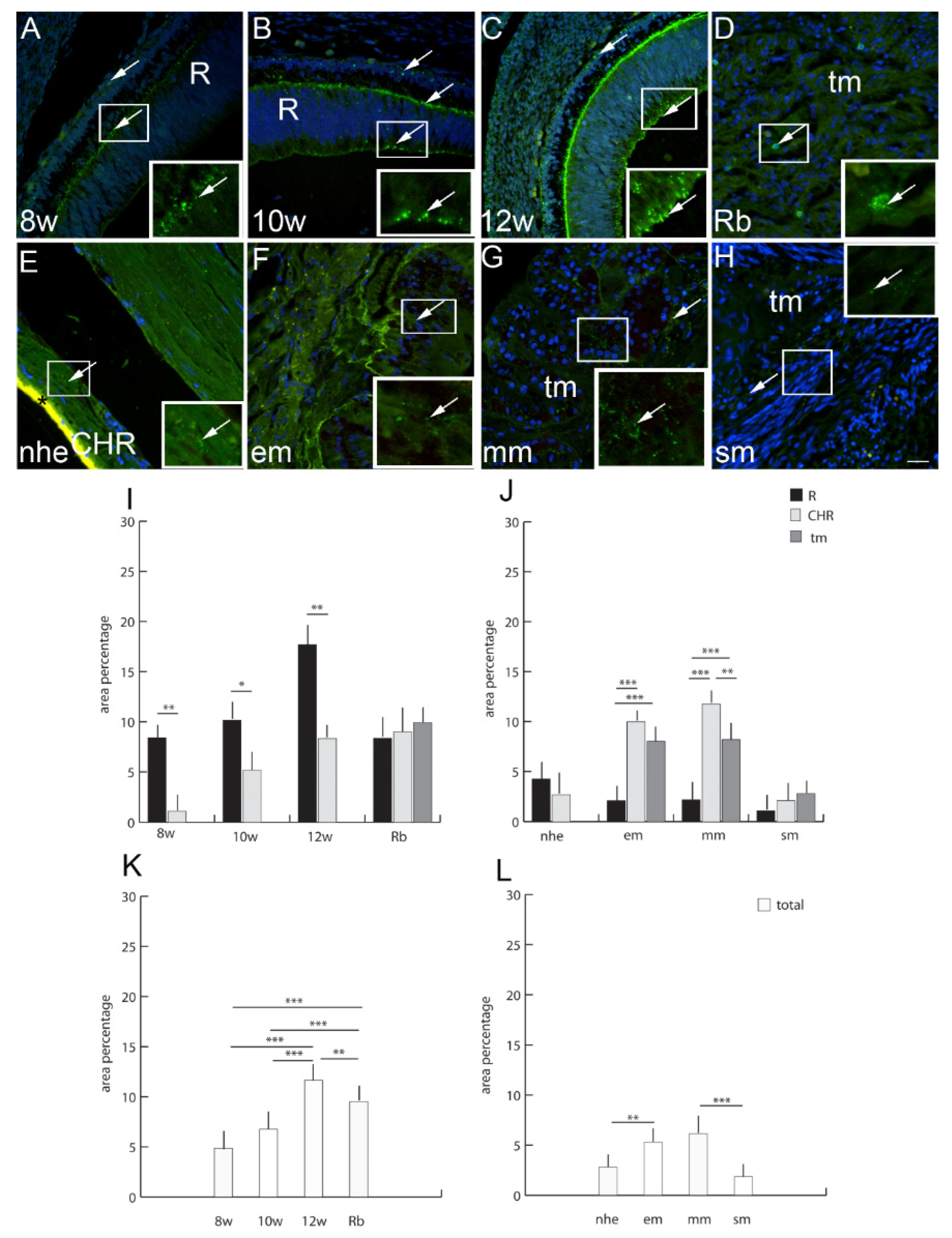
2.8. Connexin 43
2.9. Connexin 45
2.10. Panexin 1
3. Discussion
4. Materials and Methods
4.1. Tissue Processing and Procurement
4.2. Tissue Preparation for Indirect Immunofluorescence
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Prokosch, V. Energy Metabolism in the Inner Retina in Health and Glaucoma. Int. J. Mol. Sci. 2021, 22, 3689. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, Y.; Zhang, H. Ocular Autonomic Nervous System: An Update from Anatomy to Physiological Functions. Vision 2022, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Barishak, Y.R. Embryology of the eye and its adnexae. Dev. Ophthalmol. 1992, 24, 1–142. [Google Scholar] [PubMed]
- Lutty, G.A.; McLeod, D.S. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog. Retin. Eye Res. 2018, 62, 58–76. [Google Scholar] [CrossRef]
- Fruttiger, M. Development of the mouse retinal vasculature: Angiogenesis versus vasculogenesis. Investig. Ophthalmol. Vis. science 2002, 43, 522–527. [Google Scholar]
- Xiao, W.; Ye, H.; Zeng, H.; Tang, L.; Chen, R.; Gao, Y.; Mao, Y.; Yang, H. Associations among Socioeconomic Factors, Lag Time, and High-Risk Histopathologic Features in Eyes Primarily Enucleated for Retinoblastoma. Curr. Eye Res. 2019, 44, 1144–1149. [Google Scholar] [CrossRef]
- Lamas, N.J.; Martel, A.; Nahon-Esteve, S.; Goffinet, S.; Macocco, A.; Bertolotto, C.; Lassalle, S.; Hofman, P. Prognostic Biomarkers in Uveal Melanoma: The Status Quo, Recent Advances and Future Directions. Cancers 2021, 14, 96. [Google Scholar] [CrossRef]
- Matas, A.; Filipovic, N.; Znaor, L.; Mardesic, S.; Saraga-Babic, M.; Vukojevic, K. Interplay of proliferation and differentiation factors is revealed in the early human eye development. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2015, 253, 2187–2201. [Google Scholar] [CrossRef]
- Rancic, A.; Filipovic, N.; Marin Lovric, J.; Mardesic, S.; Saraga-Babic, M.; Vukojevic, K. Neuronal differentiation in the early human retinogenesis. Acta Histochem. 2017, 119, 264–272. [Google Scholar] [CrossRef]
- Zhang, M.H.; Niu, H.; Li, Z.; Huo, R.T.; Wang, J.M.; Liu, J. Activation of PI3K/AKT is involved in TINAG-mediated promotion of proliferation, invasion and migration of hepatocellular carcinoma. Cancer Biomark. Sect. A Dis. Markers 2018, 23, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lesko, J.; Rastović, P.; Mišković, J.; Šoljić, V.; Paštar, V.; Zovko, Z.; Filipović, N.; Katsuyama, Y.; Saraga-Babić, M.; Vukojević, K. The Interplay of Cx26, Cx32, Cx37, Cx40, Cx43, Cx45, and Panx1 in Inner-Ear Development of Yotari (dab1−/−) Mice and Humans. Biomedicines 2022, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int. J. Mol. Sci. 2020, 21, 8194. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Casanova, J.; Schmachtenberg, O.; Martinez, A.D.; Sanchez, H.A.; Harcha, P.A.; Rojas-Gomez, D. An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 3194. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Gao, J.; Minogue, P.J.; Jara, O.; Mathias, R.T.; Beyer, E.C. Connexin Mutants Compromise the Lens Circulation and Cause Cataracts through Biomineralization. Int. J. Mol. Sci. 2020, 21, 5822. [Google Scholar] [CrossRef]
- Pfenniger, A.; Wohlwend, A.; Kwak, B.R. Mutations in connexin genes and disease. Eur. J. Clin. Investig. 2011, 41, 103–116. [Google Scholar] [CrossRef]
- Yeung, A.K.; Patil, C.S.; Jackson, M.F. Pannexin-1 in the CNS: Emerging concepts in health and disease. J. Neurochem. 2020, 154, 468–485. [Google Scholar] [CrossRef]
- Kurtenbach, S.; Zoidl, G. Emerging functions of pannexin 1 in the eye. Front. Cell. Neurosci. 2014, 8, 263. [Google Scholar] [CrossRef][Green Version]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Leguillier, T.; Dargere, D.; Auzeil, N.; Laprevote, O.; Rat, P. P2X7-pannexin-1 and amyloid beta-induced oxysterol input in human retinal cell: Role in age-related macular degeneration? Biochimie 2016, 127, 70–78. [Google Scholar] [CrossRef]
- Su, L.; Jiang, X.; Yang, C.; Zhang, J.; Chen, B.; Li, Y.; Yao, S.; Xie, Q.; Gomez, H.; Murugan, R.; et al. Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J. Biol. Chem. 2019, 294, 19395–19404. [Google Scholar] [CrossRef]
- Aquilino, M.S.; Whyte-Fagundes, P.; Zoidl, G.; Carlen, P.L. Pannexin-1 channels in epilepsy. Neurosci. Lett. 2019, 695, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.H.; Mantovani de Castro, L.; Belmonte, M.A.; Yan, C.Y.; Moriscot, A.S.; Hamassaki, D.E. Expression of connexins 36, 43, and 45 during postnatal development of the mouse retina. J. Neurobiol. 2006, 66, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Boric Skaro, D.; Filipovic, N.; Mizdrak, M.; Glavina Durdov, M.; Solic, I.; Kosovic, I.; Lozic, M.; Racetin, A.; Juric, M.; Ljutic, D.; et al. SATB1 and PTEN expression patterns in biopsy proven kidney diseases. Acta Histochem. 2020, 122, 151631. [Google Scholar] [CrossRef] [PubMed]
- King, D.R.; Sedovy, M.W.; Leng, X.; Xue, J.; Lamouille, S.; Koval, M.; Isakson, B.E.; Johnstone, S.R. Mechanisms of Connexin Regulating Peptides. Int. J. Mol. Sci. 2021, 22, 10186. [Google Scholar] [CrossRef]
- Ivanova, E.; Kovacs-Oller, T.; Sagdullaev, B.T. Domain-specific distribution of gap junctions defines cellular coupling to establish a vascular relay in the retina. J. Comp. Neurol. 2019, 527, 2675–2693. [Google Scholar] [CrossRef]
- Pedri, D.; Karras, P.; Landeloos, E.; Marine, J.C.; Rambow, F. Epithelial-to-mesenchymal-like transition events in melanoma. FEBS J. 2022, 289, 1352–1368. [Google Scholar] [CrossRef]
- Hamard, L.; Santoro, T.; Allagnat, F.; Meda, P.; Nardelli-Haefliger, D.; Alonso, F.; Haefliger, J.A. Targeting connexin37 alters angiogenesis and arteriovenous differentiation in the developing mouse retina. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 8234–8249. [Google Scholar] [CrossRef]
- Alonso, F.; Domingos-Pereira, S.; Le Gal, L.; Derre, L.; Meda, P.; Jichlinski, P.; Nardelli-Haefliger, D.; Haefliger, J.A. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 2016, 7, 14015–14028. [Google Scholar] [CrossRef]
- Sohl, G.; Guldenagel, M.; Traub, O.; Willecke, K. Connexin expression in the retina. Brain Res. Brain Res. Rev. 2000, 32, 138–145. [Google Scholar] [CrossRef]
- Mou, Y.Y.; Zhao, G.Q.; Lin, J.Y.; Zhao, J.; Lin, H.; Hu, L.T.; Xu, Q.; Wang, Q.; Sun, W.R. Expression of connexin 43 and E-cadherin in choroidal melanoma. Int. J. Ophthalmol. 2011, 4, 156–161. [Google Scholar]
- Wong, P.; Tan, T.; Chan, C.; Laxton, V.; Chan, Y.W.; Liu, T.; Wong, W.T.; Tse, G. The Role of Connexins in Wound Healing and Repair: Novel Therapeutic Approaches. Front. Physiol. 2016, 7, 596. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Ji, L.; Wang, H.; Wang, W.; Zheng, H.; Zou, J.; Liu, L.; Qi, X.; Liu, Z.; Du, B.; et al. Connexin 43 upregulation by dioscin inhibits melanoma progression via suppressing malignancy and inducing M1 polarization. Int. J. Cancer 2017, 141, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Mat Nor, M.N.; Danesh-Meyer, H.V.; Vessey, K.A.; Fletcher, E.L.; O’Carroll, S.J.; Acosta, M.L.; Green, C.R. Connexin43 Mimetic Peptide Improves Retinal Function and Reduces Inflammation in a Light-Damaged Albino Rat Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3961–3973. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Tran, H.; Green, C.R.; Danesh-Meyer, H.V.; Acosta, M.L. Gap junction proteins in the light-damaged albino rat. Mol. Vis. 2014, 20, 670–682. [Google Scholar] [PubMed]
- Sayedyahossein, S.; Huang, K.; Li, Z.; Zhang, C.; Kozlov, A.M.; Johnston, D.; Nouri-Nejad, D.; Dagnino, L.; Betts, D.H.; Sacks, D.B.; et al. Pannexin 1 binds beta-catenin to modulate melanoma cell growth and metabolism. J. Biol. Chem. 2021, 296, 100478. [Google Scholar] [CrossRef]
- Nor, M.M.; Rupenthal, I.; Green, C.R.; Acosta, M.L. Differential Action of Connexin Hemichannel and Pannexin Channel Therapeutics for Potential Treatment of Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 1755. [Google Scholar]
- Vukojevic, K.; Kero, D.; Novakovic, J.; Kalibovic Govorko, D.; Saraga-Babic, M. Cell proliferation and apoptosis in the fusion of human primary and secondary palates. Eur. J. Oral Sci. 2012, 120, 283–291. [Google Scholar] [CrossRef]
- Vukojevic, K.; Petrovic, D.; Saraga-Babic, M. Nestin expression in glial and neuronal progenitors of the developing human spinal ganglia. Gene Expr. Patterns GEP 2010, 10, 144–151. [Google Scholar] [CrossRef]
| Antibodies | Host | Dilution | Source | |
|---|---|---|---|---|
| Primary | Anti-Cx37/GJA4 ab181701 | Rabbit | 1:500 | Abcam (Cambridge, UK) |
| Anti-Cx40/GJA5 ab213688 | Rabbit | 1:100 | Abcam (Cambridge, UK) | |
| Anti-Cx43&GJA1 ab87645 | Goat | 1:200 | Abcam (Cambridge, UK) | |
| Anti-Cx45/GJA7 ab135474 Anti-pannexin 1/PANX1 | Rabbit Rabbit | 1:100 1:300 | Abcam (Cambridge, UK) Merck KGaA (Darmstadt, Germany) | |
| Secondary | Anti-Goat IgG, Alexa Fluor® 488, ab150129 | Donkey | 1:400 | Abcam (Cambridge, UK) |
| Anti-Rabbit IgG, Alexa Fluor® 488, 711-545-152 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc. (Baltimore, PA, USA) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žužul, M.; Lozić, M.; Filipović, N.; Čanović, S.; Didović Pavičić, A.; Petričević, J.; Kunac, N.; Šoljić, V.; Saraga-Babić, M.; Konjevoda, S.; et al. The Expression of Connexin 37, 40, 43, 45 and Pannexin 1 in the Early Human Retina and Choroid Development and Tumorigenesis. Int. J. Mol. Sci. 2022, 23, 5918. https://doi.org/10.3390/ijms23115918
Žužul M, Lozić M, Filipović N, Čanović S, Didović Pavičić A, Petričević J, Kunac N, Šoljić V, Saraga-Babić M, Konjevoda S, et al. The Expression of Connexin 37, 40, 43, 45 and Pannexin 1 in the Early Human Retina and Choroid Development and Tumorigenesis. International Journal of Molecular Sciences. 2022; 23(11):5918. https://doi.org/10.3390/ijms23115918
Chicago/Turabian StyleŽužul, Matea, Mirela Lozić, Natalija Filipović, Samir Čanović, Ana Didović Pavičić, Joško Petričević, Nenad Kunac, Violeta Šoljić, Mirna Saraga-Babić, Suzana Konjevoda, and et al. 2022. "The Expression of Connexin 37, 40, 43, 45 and Pannexin 1 in the Early Human Retina and Choroid Development and Tumorigenesis" International Journal of Molecular Sciences 23, no. 11: 5918. https://doi.org/10.3390/ijms23115918
APA StyleŽužul, M., Lozić, M., Filipović, N., Čanović, S., Didović Pavičić, A., Petričević, J., Kunac, N., Šoljić, V., Saraga-Babić, M., Konjevoda, S., & Vukojevic, K. (2022). The Expression of Connexin 37, 40, 43, 45 and Pannexin 1 in the Early Human Retina and Choroid Development and Tumorigenesis. International Journal of Molecular Sciences, 23(11), 5918. https://doi.org/10.3390/ijms23115918







