Mitochondrial Inhibition by Sodium Azide Induces Assembly of eIF2α Phosphorylation-Independent Stress Granules in Mammalian Cells
Abstract
:1. Introduction
2. Results
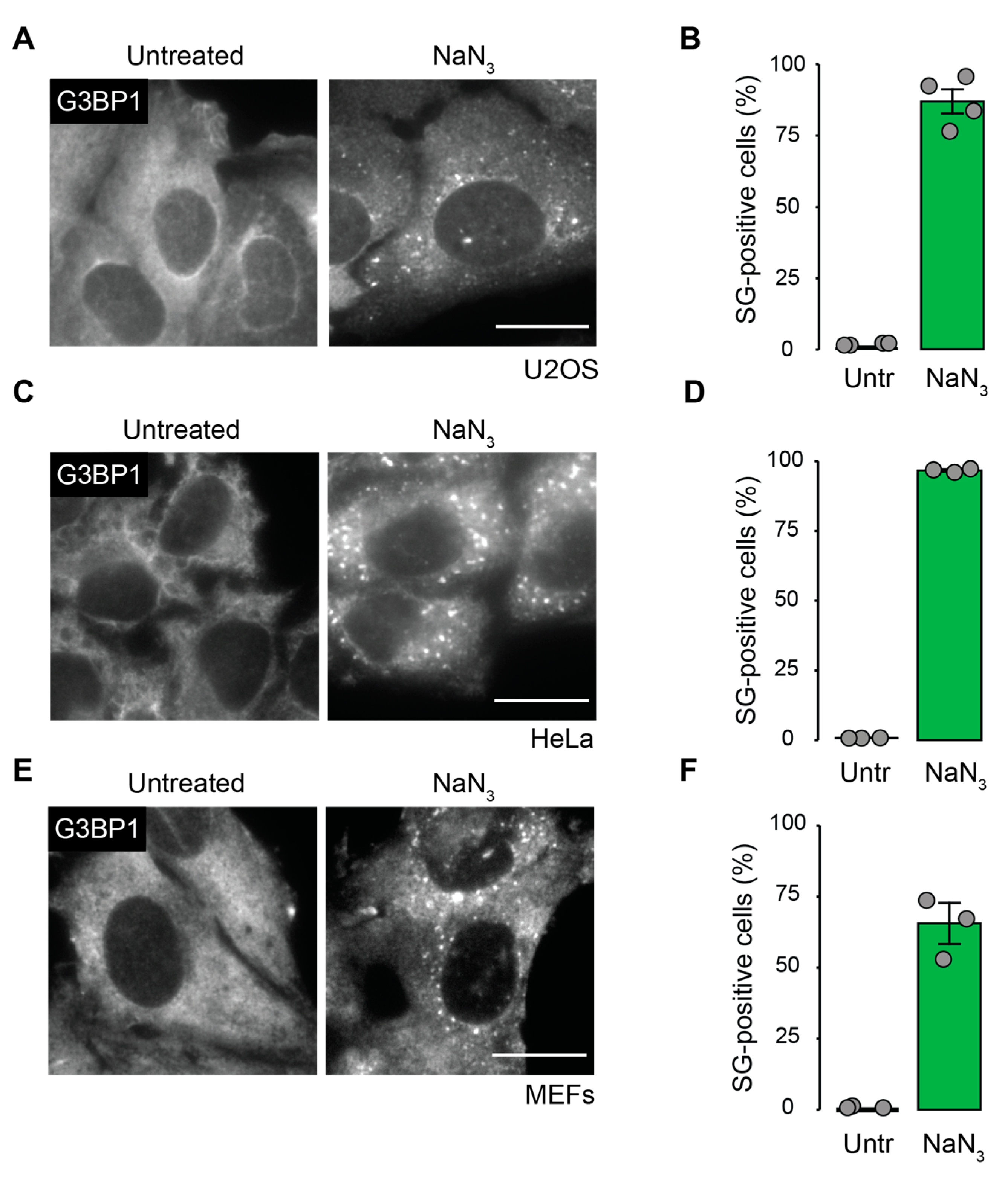
2.1. Mitochondrial Inhibition by NaN3 Is a Potent Trigger for SG Formation in Mammalian Cells
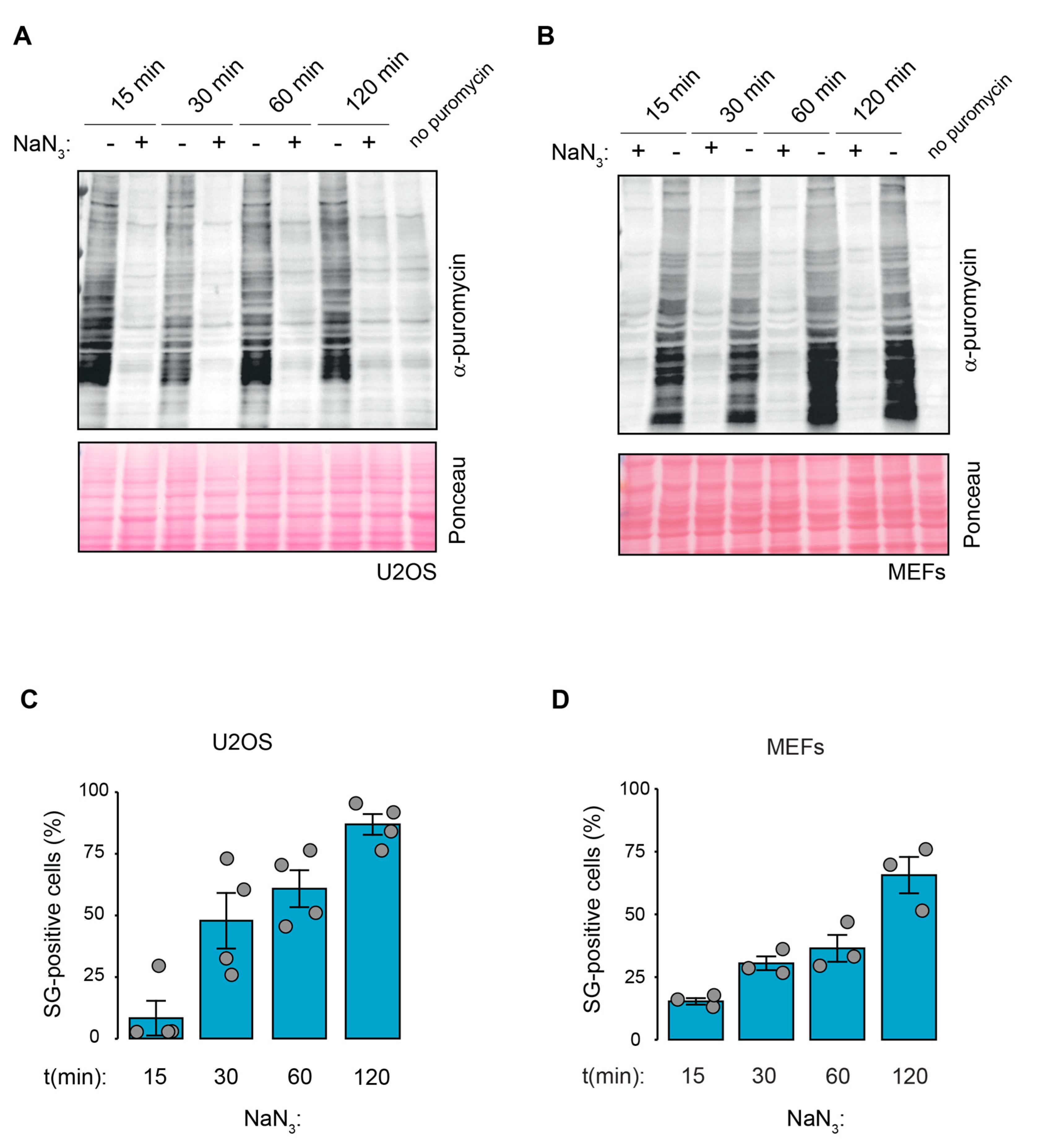
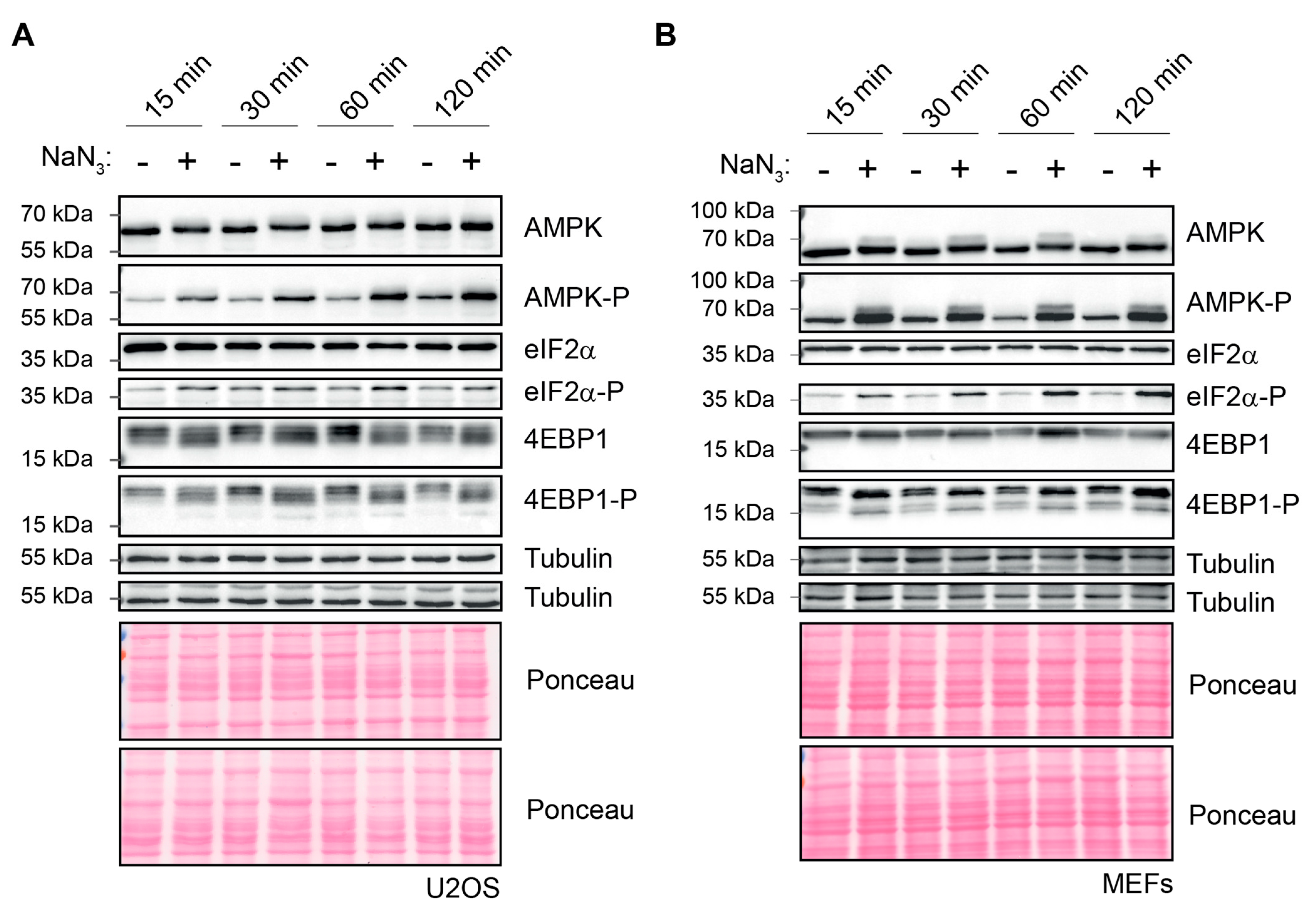
2.2. Kinetics of NaN3-Induced SG Formation Coincide with Translation Inhibition and Stress Signaling
2.3. NaN3-Induced SGs Are Assembled Independently of eIF2α Phosphorylation
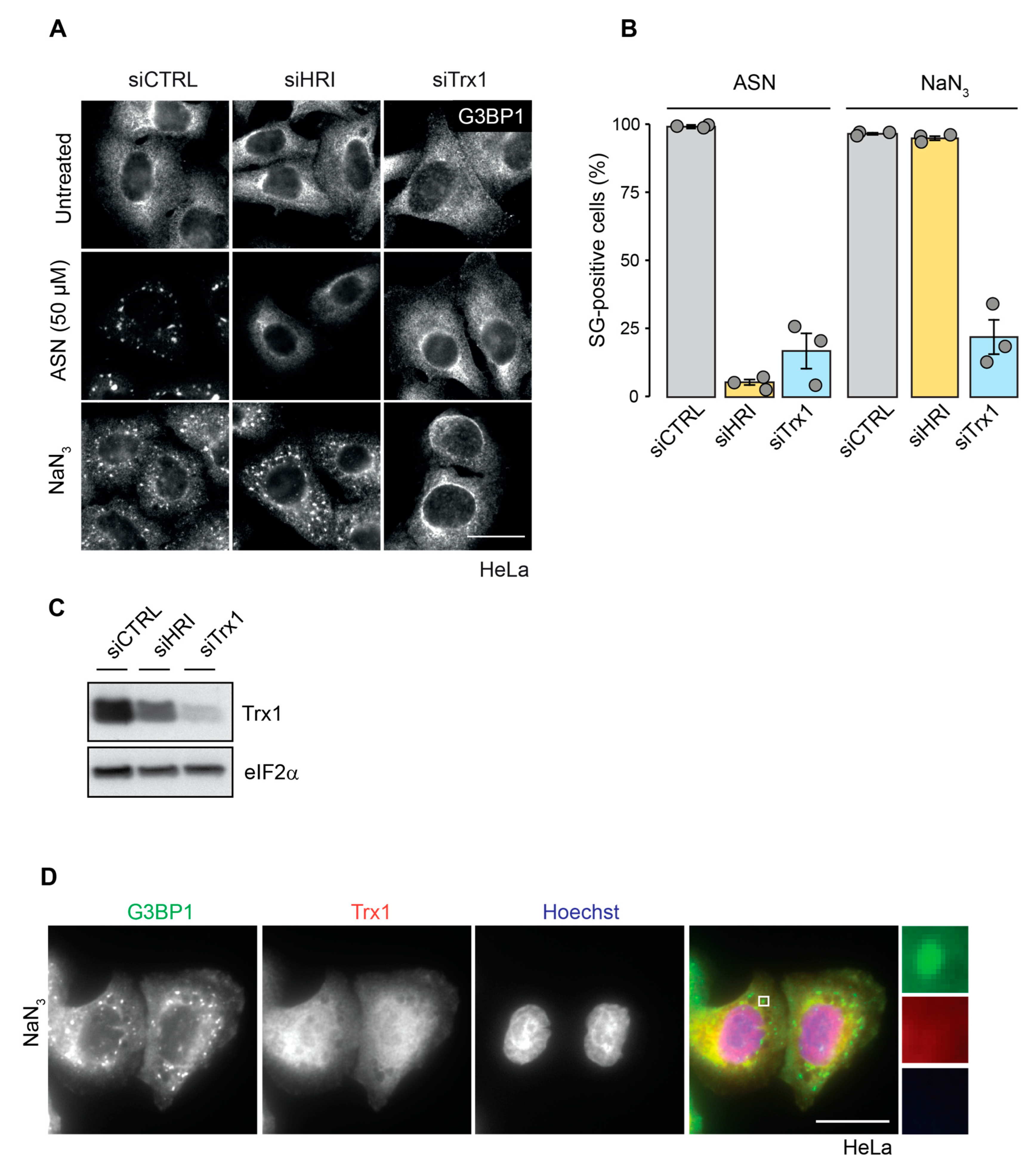
2.4. Trx1 Depletion Prevents Formation of NaN3-Induced SGs
3. Discussion
Conlusion
4. Materials and Methods
4.1. Cell Culture
4.2. Transfection of Cells
4.3. Immunofluorescence Analysis
4.4. Fluorescent In Situ Hybridization
4.5. Puromycin Incorporation Assay
4.6. Western Blot Analysis
4.7. MitoTrackerTM Staining
4.8. Antibodies
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkar, A.; Chattopadhyay, S.; Kaul, R.; Pal, J.K. Lead Exposure and Heat Shock Inhibit Cell Proliferation in Human HeLa and K562 Cells by Inducing Expression and Activity of the Heme-Regulated EIF-2alpha Kinase. J. Biochem. Mol. Biol. Biophys. 2002, 6, 391–396. [Google Scholar] [CrossRef]
- Holcik, M.; Sonenberg, N. Translational Control in Stress and Apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 318–327. [Google Scholar] [CrossRef]
- De Nadal, E.; Posas, F. Multilayered Control of Gene Expression by Stress-Activated Protein Kinases. EMBO J. 2010, 29, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Costa-Mattioli, M.; Walter, P. The Integrated Stress Response: From Mechanism to Disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The Integrated Stress Response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The EIF2α Kinases: Their Structures and Functions. Cell. Mol. Life Sci. 2013, 70, 3493–3511. [Google Scholar] [CrossRef]
- Eiermann, N.; Haneke, K.; Sun, Z.; Stoecklin, G.; Ruggieri, A. Dance with the Devil: Stress Granules and Signaling in Antiviral Responses. Viruses 2020, 12, 984. [Google Scholar] [CrossRef]
- Stoecklin, G.; Kedersha, N. Relationship of GW/P-Bodies with Stress Granules. In Ten Years of Progress in GW/P Body Research; Chan, E.K.L., Fritzler, M.J., Eds.; Springer: New York, NY, USA, 2013; Volume 768, pp. 197–211. ISBN 978-1-4614-5106-8. [Google Scholar]
- Mahboubi, H.; Stochaj, U. Cytoplasmic Stress Granules: Dynamic Modulators of Cell Signaling and Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 884–895. [Google Scholar] [CrossRef]
- Kedersha, N.; Anderson, P. Stress Granules: Sites of MRNA Triage That Regulate MRNA Stability and Translatability. Biochem. Soc. Trans. 2002, 30, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Kimball, S.R.; Horetsky, R.L.; Ron, D.; Jefferson, L.S.; Harding, H.P. Mammalian Stress Granules Represent Sites of Accumulation of Stalled Translation Initiation Complexes. AJP Cell Physiol. 2003, 284, C273–C284. [Google Scholar] [CrossRef] [Green Version]
- Cherkasov, V.; Hofmann, S.; Druffel-Augustin, S.; Mogk, A.; Tyedmers, J.; Stoecklin, G.; Bukau, B. Coordination of Translational Control and Protein Homeostasis during Severe Heat Stress. Curr. Biol. 2013, 23, 2452–2462. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Buchan, J.R.; Yoon, J.-H.; Parker, R. Stress-Specific Composition, Assembly and Kinetics of Stress Granules in Saccharomyces Cerevisiae. J. Cell Sci. 2011, 124, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Kedersha, N.; Ivanov, P.; Anderson, P. Stress Granules and Cell Signaling: More than Just a Passing Phase? Trends Biochem. Sci. 2013, 38, 494–506. [Google Scholar] [CrossRef] [Green Version]
- Emara, M.M.; Fujimura, K.; Sciaranghella, D.; Ivanova, V.; Ivanov, P.; Anderson, P. Hydrogen Peroxide Induces Stress Granule Formation Independent of EIF2α Phosphorylation. Biochem. Biophys. Res. Commun. 2012, 423, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Sama, R.R.K.; Ward, C.L.; Kaushansky, L.J.; Lemay, N.; Ishigaki, S.; Urano, F.; Bosco, D.A. FUS/TLS Assembles into Stress Granules and Is a Prosurvival Factor during Hyperosmolar Stress. J. Cell Physiol. 2013, 228, 2222–2231. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.J.; Back, S.H.; Kim, V.; Ryu, I.; Jang, S.K. Sequestration of TRAF2 into Stress Granules Interrupts Tumor Necrosis Factor Signaling under Stress Conditions. Mol. Cell. Biol. 2005, 25, 2450–2462. [Google Scholar] [CrossRef] [Green Version]
- Reineke, L.C.; Cheema, S.A.; Dubrulle, J.; Neilson, J.R. Chronic Starvation Induces Non-Canonical pro-Death Stress Granules. J. Cell Sci. 2018, 131, jcs220244. [Google Scholar] [CrossRef] [Green Version]
- Fessler, E.; Eckl, E.-M.; Schmitt, S.; Mancilla, I.A.; Meyer-Bender, M.F.; Hanf, M.; Philippou-Massier, J.; Krebs, S.; Zischka, H.; Jae, L.T. A Pathway Coordinated by DELE1 Relays Mitochondrial Stress to the Cytosol. Nature 2020, 579, 433–437. [Google Scholar] [CrossRef]
- Guo, X.; Aviles, G.; Liu, Y.; Tian, R.; Unger, B.A.; Lin, Y.-H.T.; Wiita, A.P.; Xu, K.; Correia, M.A.; Kampmann, M. Mitochondrial Stress Is Relayed to the Cytosol by an OMA1-DELE1-HRI Pathway. Nature 2020, 579, 427–432. [Google Scholar] [CrossRef]
- Kedersha, N.; Chen, S.; Gilks, N.; Li, W.; Miller, I.J.; Stahl, J.; Anderson, P. Evidence That Ternary Complex (EIF2-GTP-TRNA i Met)–Deficient Preinitiation Complexes Are Core Constituents of Mammalian Stress Granules. Mol. Biol. Cell 2002, 13, 195–210. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, B.; Eiermann, N.; Talwar, D.; Boulougouri, M.; Dick, T.P.; Stoecklin, G. Thioredoxin 1 Is Required for Stress Granule Assembly upon Arsenite-Induced Oxidative Stress. Food Chem. Toxicol. 2021, 156, 112508. [Google Scholar] [CrossRef]
- Fu, X.; Gao, X.; Ge, L.; Cui, X.; Su, C.; Yang, W.; Sun, X.; Zhang, W.; Yao, Z.; Yang, X.; et al. Malonate Induces the Assembly of Cytoplasmic Stress Granules. FEBS Lett. 2016, 590, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Samluk, L.; Urbanska, M.; Kisielewska, K.; Mohanraj, K.; Kim, M.-J.; Machnicka, K.; Liszewska, E.; Jaworski, J.; Chacinska, A. Cytosolic Translational Responses Differ under Conditions of Severe Short-Term and Long-Term Mitochondrial Stress. Mol. Biol. Cell 2019, 30, 1864–1877. [Google Scholar] [CrossRef]
- Igarashi, J.; Sato, A.; Kitagawa, T.; Sagami, I.; Shimizu, T. CO Binding Study of Mouse Heme-Regulated EIF-2alpha Kinase: Kinetics and Resonance Raman Spectra. Biochim. Biophys. Acta 2003, 1650, 99–104. [Google Scholar] [CrossRef]
- Chen, Y.; Joe, Y.; Park, J.; Song, H.-C.; Kim, U.-H.; Chung, H.T. Carbon Monoxide Induces the Assembly of Stress Granule through the Integrated Stress Response. Biochem. Biophys. Res. Commun. 2019, 512, 289–294. [Google Scholar] [CrossRef]
- Ishii, H.; Shirai, T.; Makino, C.; Nishikata, T. Mitochondrial Inhibitor Sodium Azide Inhibits the Reorganization of Mitochondria-Rich Cytoplasm and the Establishment of the Anteroposterior Axis in Ascidian Embryo. Develop. Growth Differ. 2014, 56, 175–188. [Google Scholar] [CrossRef]
- Bowler, M.W.; Montgomery, M.G.; Leslie, A.G.W.; Walker, J.E. How Azide Inhibits ATP Hydrolysis by the F-ATPases. Proc. Natl. Acad. Sci. USA 2006, 103, 8646–8649. [Google Scholar] [CrossRef] [Green Version]
- Grousl, T.; Vojtova, J.; Hasek, J.; Vomastek, T. Yeast Stress Granules at a Glance. Yeast 2021, 39, 247–261. [Google Scholar] [CrossRef]
- Khong, A.; Matheny, T.; Jain, S.; Mitchell, S.F.; Wheeler, J.R.; Parker, R. The Stress Granule Transcriptome Reveals Principles of MRNA Accumulation in Stress Granules. Mol. Cell 2017, 68, 808–820. [Google Scholar] [CrossRef]
- Wang, S.; Kwon, S.-H.; Su, Y.; Dong, Z. Stress Granules Are Formed in Renal Proximal Tubular Cells during Metabolic Stress and Ischemic Injury for Cell Survival. Am. J. Physiol. Ren. Physiol. 2019, 317, F116–F123. [Google Scholar] [CrossRef]
- Matsuki, H.; Takahashi, M.; Higuchi, M.; Makokha, G.N.; Oie, M.; Fujii, M. Both G3BP1 and G3BP2 Contribute to Stress Granule Formation. Genes Cells 2013, 18, 135–146. [Google Scholar] [CrossRef]
- Aulas, A.; Caron, G.; Gkogkas, C.G.; Mohamed, N.-V.; Destroismaisons, L.; Sonenberg, N.; Leclerc, N.; Parker, J.A.; Velde, C.V. G3BP1 Promotes Stress-Induced RNA Granule Interactions to Preserve Polyadenylated MRNA. J. Cell Biol. 2015, 209, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Advani, V.M.; Ivanov, P. Stress Granule Subtypes: An Emerging Link to Neurodegeneration. Cell. Mol. Life Sci. 2020, 77, 4827–4845. [Google Scholar] [CrossRef]
- Wheeler, J.R.; Matheny, T.; Jain, S.; Abrisch, R.; Parker, R. Distinct Stages in Stress Granule Assembly and Disassembly. eLife 2016, 5, e18413. [Google Scholar] [CrossRef]
- Kedersha, N.; Panas, M.D.; Achorn, C.A.; Lyons, S.; Tisdale, S.; Hickman, T.; Thomas, M.; Lieberman, J.; McInerney, G.M.; Ivanov, P.; et al. G3BP–Caprin1–USP10 Complexes Mediate Stress Granule Condensation and Associate with 40S Subunits. J. Cell Biol. 2016, 212, e201508028. [Google Scholar] [CrossRef] [Green Version]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of Translation Initiation in Eukaryotes: Mechanisms and Biological Targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [Green Version]
- Taniuchi, S.; Miyake, M.; Tsugawa, K.; Oyadomari, M.; Oyadomari, S. Integrated Stress Response of Vertebrates Is Regulated by Four EIF2α Kinases. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Scheuner, D.; Song, B.; McEwen, E.; Liu, C.; Laybutt, R.; Gillespie, P.; Saunders, T.; Bonner-Weir, S.; Kaufman, R.J. Translational Control Is Required for the Unfolded Protein Response and In Vivo Glucose Homeostasis. Molecular Cell 2001, 7, 1165–1176. [Google Scholar] [CrossRef]
- Mokas, S.; Mills, J.R.; Garreau, C.; Fournier, M.-J.; Robert, F.; Arya, P.; Kaufman, R.J.; Pelletier, J.; Mazroui, R. Uncoupling Stress Granule Assembly and Translation Initiation Inhibition. Mol. Biol. Cell 2009, 20, 2673–2683. [Google Scholar] [CrossRef] [Green Version]
- Aulas, A.; Fay, M.M.; Lyons, S.M.; Achorn, C.A.; Kedersha, N.; Anderson, P.; Ivanov, P. Stress-Specific Differences in Assembly and Composition of Stress Granules and Related Foci. J. Cell Sci. 2017, 130, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Nadezhdina, E.S.; Lomakin, A.J.; Shpilman, A.A.; Chudinova, E.M.; Ivanov, P.A. Microtubules Govern Stress Granule Mobility and Dynamics. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Horn, A.; Van der Meulen, J.H.; Defour, A.; Hogarth, M.; Sreetama, S.C.; Reed, A.; Scheffer, L.; Chandel, N.S.; Jaiswal, J.K. Mitochondrial Redox Signaling Enables Repair of Injured Skeletal Muscle Cells. Sci. Signal. 2017, 10, eaaj1978. [Google Scholar] [CrossRef] [Green Version]
- Harvey, J.; Hardy, S.C.; Ashford, M.L.J. Dual Actions of the Metabolic Inhibitor, Sodium Azide on Katp Channel Currents in the Rat CRI-G1 Insulinoma Cell Line: Azide Activates K ATP Channels. Br. J. Pharmacol. 1999, 126, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Muneyuki, E.; Makino, M.; Kamata, H.; Kagawa, Y.; Yoshida, M.; Hirata, H. Inhibitory Effect of NaN3 on the F0F1 ATPase of Submitochondrial Particles as Related to Nucleotide Binding. Biochim. Biophys. Acta 1993, 1144, 62–68. [Google Scholar] [CrossRef]
- Hofmann, S.; Cherkasova, V.; Bankhead, P.; Bukau, B.; Stoecklin, G. Translation Suppression Promotes Stress Granule Formation and Cell Survival in Response to Cold Shock. Mol. Biol. Cell 2012, 23, 3786–3800. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, H.J.; Zhang, H.; Huang, Y.; Hinojosa-Kirschenbaum, F.; Fan, P.; Yao, L.; Belardinelli, L.; Tellides, G.; Giordano, F.J.; et al. Thioredoxin-2 Inhibits Mitochondrial Reactive Oxygen Species Generation and Apoptosis Stress Kinase-1 Activity to Maintain Cardiac Function. Circulation 2015, 131, 1082–1097. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.-I.; Chin, A.; Park, J.Y.; Ikeda, S.; Mizushima, W.; Ralda, G.; Zhai, P.; Tong, M.; Byun, J.; Tang, F.; et al. Thioredoxin-1 Maintains Mitochondrial Function via Mechanistic Target of Rapamycin Signalling in the Heart. Cardiovasc. Res. 2020, 116, 1742–1755. [Google Scholar] [CrossRef]
- Zaobornyj, T.; Mazo, T.; Perez, V.; Gomez, A.; Contin, M.; Tripodi, V.; D’Annunzio, V.; Gelpi, R.J. Thioredoxin-1 Is Required for the Cardioprotecive Effect of Sildenafil against Ischaemia/Reperfusion Injury and Mitochondrial Dysfunction in Mice. Free. Radic. Res. 2019, 53, 993–1004. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Ravussin, E. The Role of Mitochondria in Health and Disease. Curr. Opin. Pharmacol. 2009, 9, 780–786. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiermann, N.; Stoecklin, G.; Jovanovic, B. Mitochondrial Inhibition by Sodium Azide Induces Assembly of eIF2α Phosphorylation-Independent Stress Granules in Mammalian Cells. Int. J. Mol. Sci. 2022, 23, 5600. https://doi.org/10.3390/ijms23105600
Eiermann N, Stoecklin G, Jovanovic B. Mitochondrial Inhibition by Sodium Azide Induces Assembly of eIF2α Phosphorylation-Independent Stress Granules in Mammalian Cells. International Journal of Molecular Sciences. 2022; 23(10):5600. https://doi.org/10.3390/ijms23105600
Chicago/Turabian StyleEiermann, Nina, Georg Stoecklin, and Bogdan Jovanovic. 2022. "Mitochondrial Inhibition by Sodium Azide Induces Assembly of eIF2α Phosphorylation-Independent Stress Granules in Mammalian Cells" International Journal of Molecular Sciences 23, no. 10: 5600. https://doi.org/10.3390/ijms23105600
APA StyleEiermann, N., Stoecklin, G., & Jovanovic, B. (2022). Mitochondrial Inhibition by Sodium Azide Induces Assembly of eIF2α Phosphorylation-Independent Stress Granules in Mammalian Cells. International Journal of Molecular Sciences, 23(10), 5600. https://doi.org/10.3390/ijms23105600





