Osteosarcopenia: A Narrative Review on Clinical Studies
Abstract
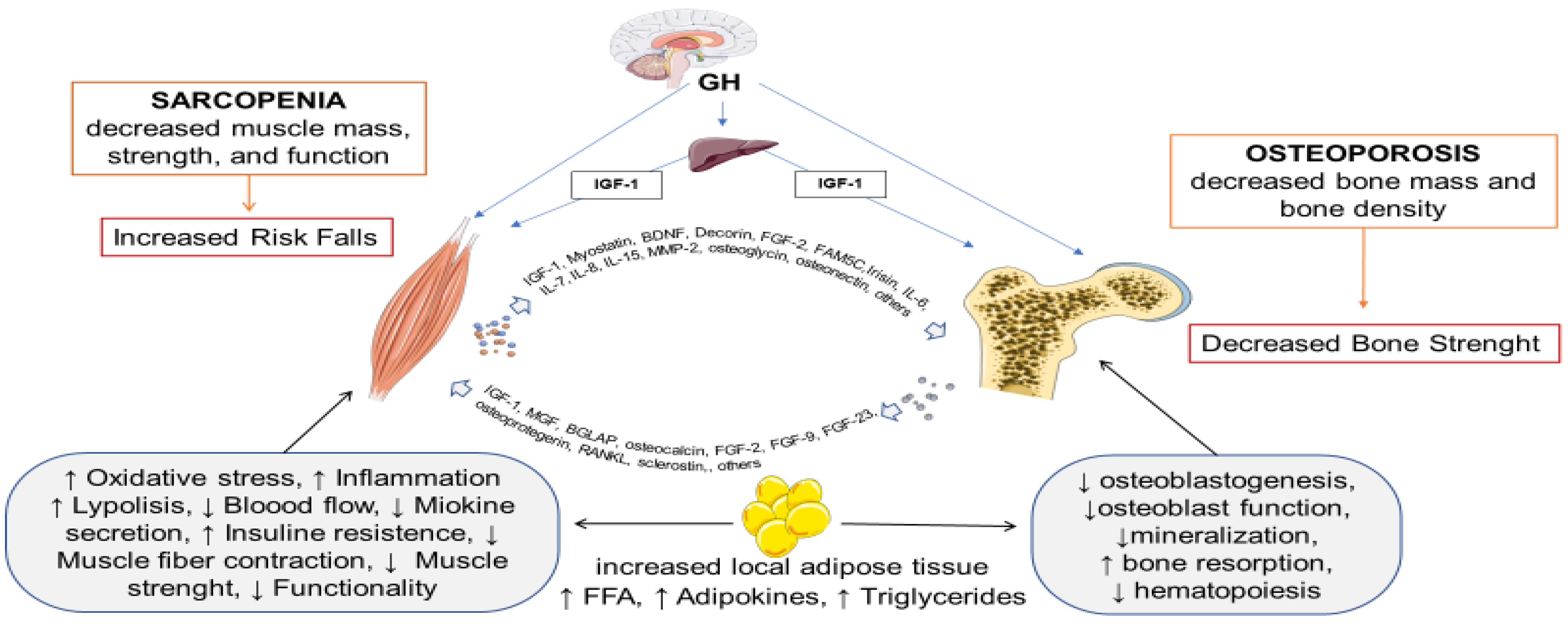
1. Introduction
2. Diagnostic Criteria
3. Clinical Studies on Osteosarcopenia and Associated Risk Factors
3.1. Osteosarcopenia and the Association with Falls, Fractures, and Frailty
3.2. Osteosarcopenia and Type 2 Diabetes Mellitus
3.3. Osteosarcopenia and Obesity
3.4. Osteosarcopenia and Polycystic Ovary Syndrome
3.5. Osteosarcopenia and Cardiovascular Diseases
3.6. Osteosarcopenia and Anemia
| Author, Year [Ref] | Sample Size Age and Sex | % of OS | Relevant Results |
|---|---|---|---|
| Huo, 2015 [39] | 680 (65% W) mean age 79 yrs | 38% | OS subjects were older, mostly women, with a body mass index (BMI) below 25 and at higher risk of depression and malnutrition |
| Reiss, 2019 [40] | 141 (60% W) 80.6 ± 5.5 yrs | 14.2% | BMI and Mini Nutritional Assessment-Short form were lower in OS compared to sarcopenia or osteoporosis alone (p < 0.05) |
| Okayama, 2022 [41] | 61 W 77.6 ± 8.1 yrs | 39.3% | Patients with OS had lower quality of life scores, greater postural instability, and a higher incidence of falls. |
| Park, 2021 [42] | 885 (67.1% W) 70.3 ± 6.2 yrs | 19.2% | Disability (17.5, 95% CI: 14.8–20.1), frailty (3.0, 95% CI: 2.6–3.4), and depression mean score (4.6, 95% CI: 3.9–5.4) were statistically significantly higher in the OS group compared the other groups. |
| Pourhassan, 2021 [43] | 572 (78% W) 75.1 ± 10.8 yrs | 8% | OS patients were older and frailer and had lower BMI, fat, muscle mass, handgrip strength, and T-score compared to non-OS patients. |
| Salech, 2020 [44] | 1119 (68.6% W) 72.0± 6.7 yrs | 16.4% | OS increases with age from 8.9% at 60–69.9 years), to 33.7% (>80 years) (p < 0.0001; mortality was significantly higher for the group with OS (15.9%) compared with those without the condition (6.1%). The risk of falls, fractures and functional impairment increases in OS (falls: HR 1.60; CI 1.07–2.38; p < 0.05; fractures HR 1.54; CI 1.13–2.08; p < 0.01; functional impairment: HR 1.83; CI 1.41–2.38; p < 0.001). |
| Fahimfar, 2022 [45] | 341 M 73.3 ± 7.4 yrs | 100% | Risk of falls: positively associated with age (OR = 1.09, 95% CI: 1.04–1.14), fasting blood glucose, an increase of 10 mg/dL increased the chance of falling by 14% (OR = 1.14, 95% CI = 1.06–1.23); negatively associated with triglyceridemia (OR = 0.33, CI 95% = 0.12 to 0.89). |
| Di Monaco, 2020 [46] | 350 W2 79.7 ±7.2 yrs | 65.7% | Significant difference in Spine Deformity Index (SDI) scores across the 3 groups (no osteoporosis and sarcopenia; osteoporosis or sarcopenia and osteosarcopenia (p < 0.001). |
| Drey, 2016 [48] | 68 pre-frail older (47 W, 21 M) 65–94 yrs | 41% | OS participants showed significantly reduced hand-grip increased chair rising time, and STS power time as well as significantly increased bone turnover markers. |
| Saeki, 2020 [49] | 291 (137 M 154 W) 59–76 yrs | 16.8% | OS and vertebral fracture were often seen in patients with frailty than in those without frailty (48.1% vs. 4.8% and 49.4% vs. 18.1%, respectively; p < 0.001). Frailty was an independent factor associated with OS (OR= 9.837; p < 0.001), and vice versa (OR = 10.069; p < 0.001). |
| Inoue, 2021 [50] | 495 (68.7% W) 76.5 ± 7.2 yrs | 11.1% | Logistic regression analysis revealed that OS was significantly associated with social frailty (pooled OR: 2.117; 95%CI: 1.104–4.213) |
| Inoue, 2022 [51] | 432 patients (298 W) 75.9 ± 7.3 yrs | 10.2% | Logistic regression analysis revealed that OS was independently associated with cognitive frailty with a higher odds ratio (OR: 8.246, 95% CI 3.319−20.487) than osteoporosis or sarcopenia alone. |
| Liu, 2021 [54] | 150 (80 M and 70 W) patients with T2DM aged ≥50 yrs. | 29% | Patients with OS had lower body mass index, waist circumference, body fat percentage (p < 0.001), AUC-Ins/Glu (p = 0.01), and AUC-CP/Glu (p = 0.013). Both AUC-Ins/Glu (OR = 0.634, p = 0.008) and AUC-CP/Glu (OR = 0.491, p = 0.009) were negatively associated with the presence of OS. |
| Pechmann, 2021 [55] | T2DM group n = 177, (64.4% W) 65.1 8.2 yrs; Control group n = 146, (54.7% W) 68.8 ± 11.0 yrs | 11.9% (T2DM group); 2.14% (control group) | T2DM group versus the control group had higher rates fractures (29.9% vs. 18.5%, respectively, p= 0.019), lower handgrip strength values (24.4 ± 10.3 kg vs. 30.9 ± 9.15 kg, respectively, p < 0.001), but comparable BMD values. OS was associated with diabetes complications (p = 0.03), calcium and vitamin D supplementation (p = 0.01), and all components of OS diagnosis (p < 0.05). |
| Park, 2018 [56] | 1344 Post-menopausal W >50 yrs | 24.1% | Pro-inflammatory diet was associate with increased odds for osteopenic obesity (OR = 2.757, 95% CI: 1.398–5.438, p < 0.01) and OS obesity (OR = 2.186, 95% CI: 1.182–4.044, p < 0.05) respectivelyA deficiency of antioxidant vitamins (A and E) was found in OS subjects compared to control (p < 0.001). |
| Bazdyrev, 2021 [63] | 387 stable coronary artery disease (26.9% W) 50–82 yrs | 6.5% | Patients with OS had a higher score on the SARC-F questionnaire, low handgrip strength, small area of muscle tissue, low musculoskeletal index, as well as low values of bone mineral density. |
| Fahimfar, 2020 [64] | 2353 (51.2% W) >60 yrs | 34% | OS increases with age (from 14.3% in aged 60–64 years to 59.4% in aged ≥75 years in men and from 20.3% in aged 60–64 years to 48.3% in aged ≥75 years in women- p = 0.019). BMI was inversely associated with OS. High-fat mass was positively associated with OS [PR 1.46 (95% CI 1.11–1.92) in men, and 2.25 (95% CI 1.71–2.95) in women]. OS was more likely in diabetic men (adjusted PR: 1.33, 95% CI 1.04–1.69), but not in women. No association between OS and smoking and lipid profiles has been found. |
| Hassan, 2020 [69] | 558 community-dwelling participants older (79 ± 7.5 yrs) | 36% | OS patients on average had 6.3 g/L lower Hb levels compared to controls (p = 0.001), and 3.7 g/L lower Hb than patients with osteoporosis/penia (p < 0.026). Sarcopenia and OS (but not osteoporosis alone) are associated with anemia |
4. Management of Osteosarcopenia
4.1. Non-Pharmacological Interventions
4.2. Pharmacological Interventions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzymes |
| ALM | appendicular lean mass |
| BALP | alkaline phosphatase bone |
| BIA | bioimpedance analysis |
| BMD | bone mass density |
| BMI | body mass index |
| CAD | coronary artery disease |
| COP | Circulating osteoprogenitor |
| CTX | C-terminal cross-linked telopeptide |
| DEXA | Dual-energy X-ray absorptiometry |
| EWGSOP2 | European Working Group on Sarcopenia in the Elderly |
| FDA | Food and Drug Administration |
| FFC | Falls and Fractures Clinic |
| FrOST | Franconian Osteopenia and Sarcopenia Trial |
| GH | the growth hormone |
| GMV | gluteus maximus muscle volume |
| HbA1c | glycated hemoglobin |
| Hb | hemoglobin |
| HIT-DRT | High-intensity dynamic resistance exercise |
| hpCRP | high-sensitivity C-reactive protein |
| HR | hazard ratio |
| GH | growth hormone |
| IGF-1; | insulin-like growth factor 1 |
| GH-IGF-1 axis | growth hormone-insulin-like growth factor 1 axis |
| IR | insulin resistance |
| MRI | Magnetic resonance imaging |
| MSCs | Mesenchymal stem cells |
| NCDs | Noncommunicable diseases |
| NOF | Nepean Osteoporosis and Frailty Study |
| OC | osteocalcin |
| OS | Osteosarcopenia; |
| PCOS | Polycystic ovary syndrome |
| PTH | parathyroid hormone |
| RANK-L | receptor activator of nuclear factor-kappa-β ligand |
| RCT | randomized controlled trials |
| RE | resistance exercise |
| SDI | Spine Deformity Index |
| SHBG | sex hormone-binding globulin |
| SMI | skeletal muscle index |
| SMD | standardized mean difference |
| SPPB | Short Physical Performance Battery |
| STS | sit-to-stand test |
| TBS | trabecular bone score |
| TD2M | Type 2 diabetes mellitus |
| TRAP | tartrate-resistant acid phosphatase |
| WPS | whey protein supplementation |
References
- Binkley, N.; Buehring, B. Beyond FRAX®: It’s Time to Consider “Sarco-Osteopenia”. J. Clin. Densitom. 2009, 12, 413–416. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Kaplan, S.J.; Pham, T.N.; Arbabi, S.; Gross, J.A.; Damodarasamy, M.; Bentov, I.; Taitsman, L.A.; Mitchell, S.H.; Reed, M.J. Associationof radiologic indicators of frailty with 1-year mortality in older trauma patients: Opportunistic screening for sarcopenia and os-teopenia. JAMA Surg. 2017, 152, e164604. [Google Scholar] [CrossRef]
- Nielsen, B.R.; Abdulla, J.; Andersen, H.E.; Schwarz, P.; Suetta, C. Sarcopenia and osteoporosis in older people: A systematic review and meta-analysis. Eur. Geriatr. Med. 2018, 9, 419–434. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Report of the European working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Fatima, M.; Brennan-Olsen, S.L.; Duque, G. Therapeutic approaches to osteosarcopenia: Insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19867009. [Google Scholar] [CrossRef]
- Paintin, J.; Cooper, C.; Dennison, E. Osteosarcopenia. Br. J. Hosp. Med. 2018, 79, 253–258. [Google Scholar] [CrossRef]
- Azzolino, D.; Spolidoro, G.C.I.; Saporiti, E.; Luchetti, C.; Agostoni, C.; Cesari, M. Musculoskeletal Changes Across the Lifespan: Nutrition and the Life-Course Approach to Prevention. Front. Med. 2021, 8, 697954. [Google Scholar] [CrossRef]
- Schoenau, E. From mechanistic theory to development of the “Functional Muscle-Bone-Unit”. J. Musculoskelet. Neuronal. Interact. 2005, 5, 232–238. [Google Scholar]
- Lara-Castillo, N.; Johnson, M.L. Bone-Muscle Mutual Interactions. Curr. Osteoporos. Rep. 2020, 18, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Solute Transport in the Bone Lacunar-Canalicular System (LCS). Curr. Osteoporos. Rep. 2018, 16, 32–41. [Google Scholar] [CrossRef]
- Deb, A. How Stem Cells Turn into Bone and Fat. N. Engl. J. Med. 2019, 380, 2268–2270. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Singh, L.; Tyagi, S.; Myers, D.; Duque, G. Good, Bad, or Ugly: The Biological Roles of Bone Marrow Fat. Curr. Osteoporos. Rep. 2018, 16, 130–137. [Google Scholar] [CrossRef]
- Carter, C.S.; Justice, J.N.; Thompson, L. Lipotoxicity, aging, and muscle contractility: Does fiber type matter? GeroScience 2019, 41, 297–308. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef]
- Rivas, D.A.; McDonald, D.J.; Rice, N.P.; Haran, P.H.; Dolnikowski, G.G.; Fielding, R.A. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R561–R569. [Google Scholar] [CrossRef]
- Lindsey, R.C.; Mohan, S. Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol. Cell. Endocrinol. 2015, 432, 44–55. [Google Scholar] [CrossRef]
- Ferrucci, L.; Baroni, M.; Ranchelli, A.; Lauretani, F.; Maggio, M.; Mecocci, P.; Ruggiero, C. Interaction between Bone and Muscle in Older Persons with Mobility Limitations. Curr. Pharm. Des. 2014, 20, 3178–3197. [Google Scholar] [CrossRef]
- Colón, C.J.P.; Molina-Vicenty, I.L.; Frontera-Rodríguez, M.; García-Ferré, A.; Rivera, B.P.; Cintrón-Vélez, G.; Frontera-Rodríguez, S. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J. Biomed. 2018, 3, 40–49. [Google Scholar] [CrossRef]
- Russo, C.R.; Ricca, M.; Ferrucci, L. Sarcopenia, the physiological decline in muscle mass and function occurring in the elderly, may influence age-related osteoporosis. J. Am. Geriatr. Soc. 2000, 48, 1738–1739. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, M.; Castiglioni, C.; Milano, E.; Massazza, G. Is there a definition of low lean mass that captures the associated low bone mineral density? A cross-sectional study of 80 men with hip fracture. Aging 2018, 30, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.R.; Andersen, H.E.; Haddock, B.; Hovind, P.; Schwarz, P.; Suetta. Prevalence of muscle dysfunction concomitant with osteoporosis in a home-dwelling Danish population aged 65–93 years—The Copenhagen Sarcopenia Study. Exp. Gerontol. 2020, 138, 110974. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Phu, S.; Hassan, E.B.; Brennan-Olsen, S.L.; Zanker, J.; Vogrin, S.; Conzade, R.; Kirk, B.; Al Saedi, A.; Probst, V.; et al. The joint occur-rence of osteoporosis and sarcopenia (osteosarcopenia): Definitions and characteristics. J. Am. Med. Dir. Assoc. 2020, 21, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention and Management of Osteoporosis; WHO Technical Report Series n. 921; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Peppa, M.; Stefanaki, C.; Papaefstathiou, A.; Boschiero, D.; Dimitriadis, G.; Chrousos, G.P. Bioimpedance analysis vs. DEXA as a screening tool for osteosarcopenia in lean, overweight and obese Caucasian postmenopausal females. Hormones 2017, 16, 181–193. [Google Scholar]
- Hamad, B.; Basaran, S.; Benlidayi, I.C. Osteosarcopenia among postmenopausal women and handgrip strength as a practical method for predicting the risk. Aging 2019, 32, 1923–1930. [Google Scholar] [CrossRef]
- Fathi, M.; Heshmat, R.; Ebrahimi, M.; Salimzadeh, A.; Ostovar, A.; Fathi, A.; Razi, F.; Nabipour, I.; Moghaddassi, M.; Shafiee, G. Association between biomarkers of bone health and osteosarcopenia among Iranian older people: The Bushehr Elderly Health (BEH) program. BMC Geriatr. 2021, 21, 654. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Cherry, K.E.; Su, L.J.; Kim, S.; Myers, L.; Welsh, D.A.; Jazwinski, S.M.; Ravussin, E. Body Composition, IGF1 Status, and Physical Functionality in Nonagenarians: Implications for Osteosarcopenia. J. Am. Med. Dir. Assoc. 2018, 20, 70–75.e2. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Kassem, M. Circulating osteogenic cells: Implications for injury, repair, and regeneration. J. Bone Miner. Res. 2011, 26, 1685–1693. [Google Scholar] [CrossRef]
- Gunawardene, P.; Bermeo, S.; Vidal, C.; Al-Saedi, A.; Chung, P.; Boersma, D.; Phu, S.; Pokorski, I.; Suriyaarachchi, P.; Demontiero, O.; et al. Association between circulating osteogenic progenitor cells and disability and frailty in older persons: The Nepean osteoporosis and frailty study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Leli, C.; Fabbriciani, G.; Manfredelli, M.R.; Callarelli, L.; Bagaglia, F.; Scarponi, A.M.; Mannarino, E. Association between circulating osteoprogenitor cell numbers and bone mineral density in postmenopausal osteoporosis. Osteoporos. Int. 2010, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, P.; Al Saedi, A.; Singh, L.; Bermeo, S.; Vogrin, S.; Phu, S.; Suriyaarachchi, P.; Pignolo, R.J.; Duque, G. Age, gender, and percentage of circulating osteoprogenitor (COP) cells: The COP Study. Exp. Gerontol. 2017, 96, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Al Saedi, A.; Gunawardene, P.; Bermeo, S.; Vogrin, S.; Boersma, D.; Phu, S.; Duque, G. Lamin A expression in circulat-ing osteoprogenitors as a potential biomarker for frailty: The Nepean Osteoporosis and Frailty (NOF) Study. Exp. Gerontol. 2018, 102, 69–75. [Google Scholar] [CrossRef]
- Cedeno-Veloz, B.; Bonnardeauxa, P.L.D.; Duque, G. Osteosarcopenia: Una revisión narrativa. Rev. Españ. Geriatr. Gerontol. 2019, 54, 103–108. [Google Scholar] [CrossRef]
- Clynes, M.A.; Gregson, C.L.; Bruyère, O.; Cooper, C.; Dennison, E.M. Osteosarcopenia: Where osteoporosis and sarcopenia collide. Rheumatology 2020, 60, 529–537. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, Y.; Teng, Y.; Long, Q.; Hao, Q.; Yu, X.; Yang, L.; Lv, Y.; Liu, J.; Zeng, Y.; et al. The analysis of osteosarcopenia as a risk factor for fractures, mortality, and falls. Osteoporos. Int. 2021, 32, 2173–2183. [Google Scholar] [CrossRef]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Muir, S.W.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of osteosarco-penia in older individuals with a history of falling. J. Am. Med. Dir. Assoc. 2015, 16, 290–295. [Google Scholar] [CrossRef]
- Reiss, J.; Iglseder, B.; Alzner, R.; Mayr-Pirker, B.; Pirich, C.; Kässmann, H.; Kreutzer, M.; Dovjak, P.; Reiter, R. Sarcopenia and osteoporosis are interrelated in geriatric inpatients. Z. Für Gerontol. Und Geriatr. 2019, 52, 688–693. [Google Scholar] [CrossRef]
- Okayama, A.; Nakayama, N.; Kashiwa, K.; Horinouchi, Y.; Fukusaki, H.; Nakamura, H.; Katayama, S. Prevalence of Sarcopenia and Its Association with Quality of Life, Postural Stability, and Past Incidence of Falls in Postmenopausal Women with Osteoporosis: A Cross-Sectional Study. Healthcare 2022, 10, 192. [Google Scholar] [CrossRef]
- Park, K.-S.; Lee, G.-Y.; Seo, Y.-M.; Seo, S.-H.; Yoo, J.-I. Disability, Frailty and Depression in the community-dwelling older adults with Osteosarcopenia. BMC Geriatr. 2021, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Pourhassan, M.; Buehring, B.; Stervbo, U.; Rahmann, S.; Mölder, F.; Rütten, S.; Trampisch, U.; Babel, N.; Westhoff, T.H.; Wirth, R. Osteosarcopenia, an Asymmetrical Overlap of Two Connected Syndromes: Data from the OsteoSys Study. Nutrients 2021, 13, 3786. [Google Scholar] [CrossRef] [PubMed]
- Salech, F.; Marquez, C.; Lera, L.; Angel, B.; Saguez, R.; Albala, C. Osteosarcopenia Predicts Falls, Fractures, and Mortality in Chilean Community-Dwelling Older Adults. J. Am. Med Dir. Assoc. 2021, 22, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Fahimfar, N.; Yousefi, S.; Noorali, S.; Gharibzadeh, S.; Sanjari, M.; Khalagi, K.; Mehri, A.; Shafiee, G.; Heshmat, R.; Nabipour, I.; et al. The association of cardio-metabolic risk factors and history of falling in men with osteosarcopenia: A cross-sectional analysis of Bushehr Elderly Health (BEH) program. BMC Geriatr. 2022, 22, 46. [Google Scholar] [CrossRef]
- Di Monaco, M.; Castiglioni, C.; Bardesono, F.; Milano, E.; Massazza, G. Sarcopenia, osteoporosis and the burden of prevalent vertebral fractures: A cross-sectional study of 350 women with hip fracture. Eur. J. Phys. Rehabil. Med. 2020, 56, 184–190. [Google Scholar] [CrossRef]
- Turkmen, I.; Ozcan, C. Osteosarcopenia increases hip fracture risk: A case-controlled study in the elderly. J. Back Musculoskelet. Rehabil. 2019, 32, 613–618. [Google Scholar] [CrossRef]
- Drey, M.; Sieber, C.C.; Bertsch, T.; Bauer, J.M.; Schmidmaier, R.; The FiAT intervention group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin. Exp. Res. 2016, 28, 895–899. [Google Scholar] [CrossRef]
- Saeki, C.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Abo, M.; Saruta, M.; Tsubota, A. Relationship between osteosarco-penia and frailty in patients with chronic liver disease. J. Clin. Med. 2020, 9, 2381. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Satake, S.; Matsui, Y.; Arai, H. Osteosarcopenia, the co-existence of osteoporosis and sarcopenia, is associated with social frailty in older adults. Aging Clin. Exp. Res. 2021, 34, 291. [Google Scholar] [CrossRef]
- Inoue, T.; Shimizu, A.; Satake, S.; Matsui, Y.; Ueshima, J.; Murotani, K.; Arai, H.; Maeda, K. Association between osteosarcopenia and cognitive frailty in older outpatients visiting a frailty clinic. Arch. Gerontol. Geriatr. 2021, 98, 104530. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: International consensus group. J. Nutr. Health Aging 2013, 17, 2002. [Google Scholar]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment—Facts and numbers. J. Cachex-Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- Liu, J.; Yu, D.; Xu, M.; Feng, R.; Sun, Y.; Yin, X.; Lai, H.; Wang, C.; Liu, J. β-Cell function is associated with osteosarcopenia in middle-aged and older nonobese patients with type 2 diabetes: A cross-sectional study. Open Med. 2021, 16, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, L.M.; Petterle, R.R.; Moreira, C.A.; Borba, V.Z.C. Osteosarcopenia and trabecular bone score in patients with type 2 diabetes mellitus. Arch. Endocrinol. Metab. 2021, 65, 801–810. [Google Scholar] [CrossRef]
- Park, S.; Na, W.; Sohn, C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal Korean women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J. Clin. Biochem. Nutr. 2018, 63, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of sarcopenic obesity in older individuals with a history of falling. Arch. Gerontol. Geriatr. 2016, 65, 255–259. [Google Scholar] [CrossRef]
- Stefanaki, C.; Peppa, M.; Boschiero, D.; Chrousos, G.P. Healthy overweight/obese youth: Early osteosarcopenic obesity features. Eur. J. Clin. Investig. 2016, 46, 767–778. [Google Scholar] [CrossRef]
- Piovezan, J.M.; Premaor, M.O.; Comim, F.V. Negative impact of polycystic ovary syndrome on bone health: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 634–646. [Google Scholar] [CrossRef]
- Mario, F.M.; do Amarante, F.; Toscani, M.K.; Spritzer, P.M. Lean muscle mass in classic or ovulatory PCOS: Association with central obesity and insulin resistance. Exp. Clin. Endocrinol. Diabetes 2012, 120, 511–516. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, challenges, and guiding treatment. J. Clin. Endocrinol. Metab. 2021, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Kazemi, M.; Jarrett, B.Y.; Parry, S.A.; Thalacker-Mercer, A.E.; Hoeger, K.M.; Spandorfer, S.D.; Lujan, M.E. Osteosarcopenia in reproductive-aged women with polycystic ovary syndrome: A multicenter case-control study. J. Clin. Endocrinol. Metab. 2020, 105, e3400–e3414. [Google Scholar] [CrossRef] [PubMed]
- Bazdyrev, E.D.; Terentyeva, N.A.; Krivoshapova, K.E.; Masenko, V.L.; Wegner, E.A.; Kokov, N.; Pomeshkina, S.A.; Barbarash, O.L. Prevalence of Musculoskeletal Disorders in Patients with Coronary Artery Disease. Ration. Pharmacother. Cardiol. 2021, 17, 369–375. [Google Scholar] [CrossRef]
- Fahimfar, N.; Zahedi Tajrishi, F.; Gharibzadeh, S.; Shafiee, G.; Tanha, K.; Heshmat, R.; Nabipour, I.; Raeisi, A.; Jalili, A.; Larijani, B.; et al. Prevalence of osteosarcopenia and its association with cardiovascular risk factors in Iranian older people: Bushehr Elderly Health (BEH) Program. Calcif. Tissue Int. 2020, 106, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Espallargues, M.; Sampietro-Colom, L.; Estrada, M.D.; Solà, M.; Del Río, L.; Setoain, J.; Granados, A. Identifying Bone-Mass-Related Risk Factors for Fracture to Guide Bone Densitometry Measurements: A Systematic Review of the Literature. Osteoporos. Int. 2001, 12, 811–822. [Google Scholar] [CrossRef]
- Hirani, V.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Hsu, B.; Cumming, R.G. Low hemoglobin concentrations are associated with sarcopenia, physical performance, and disability in older Australian men in cross sectional and longitudinal analysis: The concord health and ageing in men project. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1667–1675. [Google Scholar] [CrossRef]
- Duh, M.S.; Mody, S.H.; Lefebvre, P.; Woodman, R.C.; Buteau, S.; Piech, C.T. Anaemia and the Risk of Injurious Falls in a Community-Dwelling Elderly Population. Drugs Aging 2008, 25, 325–334. [Google Scholar] [CrossRef]
- Valderrábano, R.J.; Lee, J.; Lui, L.-Y.; Hoffman, A.R.; Cummings, S.R.; Orwoll, E.S.; Wu, J.Y. Older men with anemia have increased fracture risk independent of bone mineral density. J. Clin. Endocrinol. Metab. 2017, 102, 2199–2206. [Google Scholar] [CrossRef]
- Hassan, E.B.; Vogrin, S.; Viña, I.H.; Boersma, D.; Suriyaarachchi, P.; Duque, G. Hemoglobin Levels are Low in Sarcopenic and Osteosarcopenic Older Persons. Calcif. Tissue Res. 2020, 107, 135–142. [Google Scholar] [CrossRef]
- Choi, M.-K.; Bae, Y.-J. Dietary calcium, phosphorus, and osteosarcopenic adiposity in Korean adults aged 50 years and older. Arch. Osteoporos. 2021, 16, 89. [Google Scholar] [CrossRef]
- Gomez, F.; Curcio, C.L.; Brennan-Olsen, S.L.; Boersma, D.; Phu, S.; Vogrin, S.; Suriyaarachchi, P.; Duque, G. Effects of the falls and frac-tures clinic as an integrated multidisciplinary model of care in Australia: A pre–post study. BMJ Open 2019, 9, e027013. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Daly, R.M.; Singh, M.A.; Taaffe, D.R. Exercise and Sports Science Australia (ESSA) position statement on exercise pre-scription for the prevention and management of osteoporosis. J. Sci. Med. Sport. 2017, 20, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Fleg, J.L. Aerobic exercise in the elderly: A key to successful aging. Discov. Med. 2012, 13, 223–228. [Google Scholar] [PubMed]
- Palombaro, K.M.; Black, J.D.; Buchbinder, R.; Jette, D.U. Effectiveness of exercise for managing osteoporosis in women postmeno-pause. Phys. Ther. 2013, 93, 1021–1025. [Google Scholar] [CrossRef][Green Version]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Galea, G.L.; Lanyon, L.E.; Price, J.S. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone 2016, 96, 38–44. [Google Scholar] [CrossRef]
- Lichtenberg, T.; von Stengel, S.; Sieber, C.C.; Kemmler, W. The Favorable Effects of a High-Intensity Resistance Training on Sarcopenia in Older Community-Dwelling Men with Osteosarcopenia: The Randomized Controlled FrOST Study. Clin. Interv. Aging 2019, 14, 2173–2186. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Fröhlich, M.; Jakob, F.; Engelke, K.; Von Stengel, S.; Schoene, D. Effects of High—Intensity Resistance Training on Osteopenia and Sarcopenia Parameters in Older Men with Osteosarcopenia—One-Year Results of the Randomized Controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). J. Bone Miner. Res. 2020, 35, 1634–1644. [Google Scholar] [CrossRef]
- Kemmler, W.; Schoene, D.; Kohl, M.; von Stengel, S. Changes in body composition and cardiometabolic health after de-training in older men with osteosarcopenia: 6-month follow-up of the randomized controlled franconian osteopenia and sar-copenia trial (FrOST) study. Clin. Interv. Aging 2021, 16, 571. [Google Scholar] [CrossRef]
- Kirk, B.; Miller, S.; Zanker, J.; Duque, G. A clinical guide to the pathophysiology, diagnosis and treatment of osteosarcopenia. Maturitas 2020, 140, 27–33. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef] [PubMed]
- Phu, S.; Hassan, E.B.; Vogrin, S.; Kirk, B.; Duque, G. Effect of Denosumab on Falls, Muscle Strength, and Function in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2019, 67, 2660–2661. [Google Scholar] [CrossRef]
- Klein, G.L. Pharmacologic Treatments to Preserve Bone and Muscle Mass in Osteosarcopenia. Curr. Osteoporos. Rep. 2020, 18, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.C.; Maddocks, M.; Cella, D.; Muscaritoli, M. Efficacy of Anamorelin, a Novel Non-Peptide Ghrelin Analogue, in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC) and Cachexia—Review and Expert Opinion. Int. J. Mol. Sci. 2018, 19, 3471. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R. Denosumab for the treatment of osteoporosis. Osteoporos Sarcopenia 2017, 8–17, 74. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.; Lin, C.; Brown, J.; Wang, A.; Yin, X.; Ebeling, P.; Fahrleitner-Pammer, A.; Franek, E.; Gilchrist, N.; Miller, P.; et al. Long-term denosumab treatment restores cortical bone loss and reduces fracture risk at the forearm and humerus: Analyses from the FREEDOM Extension cross-over group. Osteoporos. Int. 2019, 30, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Belchior, G.F.; Kirk, B.; Da Silva, E.A.P.; Duque, G. Osteosarcopenia: Beyond age-related muscle and bone loss. Eur. Geriatr. Med. 2020, 11, 715–724. [Google Scholar] [CrossRef]
| Author, Year [Ref] | Sample Size Age and Sex | Type of Intervention | % of OS | Relevant Results |
|---|---|---|---|---|
| Gomez, 2018 [71] | 106 (68% W) 78 ± 8 yrs | Multifactorial interventions: e.g., vitamin D/calcium supplement, osteoporosis medications, supervised group exercise programs; protein supplement, etc | 53% | At 6-month follow-up, the multidisciplinary interventions reduce falls by more than 80% and 50% fracture risk. In addition, 65% of patients had a reduced risk for falling and a 57% reduction in 10-year fracture probability. |
| Lichtenberg, 2019 [78] | 43 M (21 EG group; 22 Inactive Control group CG 73 to 91 yrs. | FROST Study 18 months trial High-intensity dynamic resistance exercise (HIT-DRT), whey protein supplement (up to 1.5 g/kg/day in HIT-DRT and 1.2 g/kg/day in CG); vitamin D supplements (up to 800 IE/day). | 100% | The results show a significant effect of the exercise intervention on the sarcopenia Z-score (p < 0.001), a significant increase in the skeletal muscle mass index (SMI) (p < 0.001), and in handgrip strength (p < 0.001) in the HI-RT group and a significant worsening on the sarcopenia Z score in the CG group (p = 0.012). |
| Kemmler, 2020 [79] | 43 M (21 EG group; 22 Inactive Control group CG 73 to 91 yrs. | FROST Study 18 months high-intensity dynamic resistance exercise (HIT-DRT), whey protein supplement (up to 1.5 g/kg/day in HIT-DRT and 1.2 g/kg/day in CG); vitamin D supplements (up to 800 IE/day). | 100% | After 12 months the lumbar spine (LS) BMD was maintained in the EG and decreased significantly in the CG (p < 0.001; standardized mean difference (SMD) = 0.90); SMI increased significantly in the EG and decreased significantly in the CG (p < 0.001; SMD = 1.95). Changes in maximum hip−/leg extensor strength were much more prominent (p < 0.001; SMD = 1.92) in the EG. |
| Kemmler, 2021 [80] | 43 M (21 EG group; 22 Inactive Control group CG 73 to 91 yrs. | FROST Study 6 months of detraining after 18 months of intervention. | 100% | During detraining, the EG group lost approximately one-third of the HIT-DRT-induced gain and showed a significantly (p = 0.001) higher reduction in muscle quality than the CG. The negative effect was only significant for skeletal muscle mass index and hip/leg extensor strength (p = 0.002 and p = 0.013), but not for lumbar spine BMD (p = 0.068), total hip BMD (p = 0.069), handgrip strength (p = 0.066) and gait velocity (p = 0.067). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polito, A.; Barnaba, L.; Ciarapica, D.; Azzini, E. Osteosarcopenia: A Narrative Review on Clinical Studies. Int. J. Mol. Sci. 2022, 23, 5591. https://doi.org/10.3390/ijms23105591
Polito A, Barnaba L, Ciarapica D, Azzini E. Osteosarcopenia: A Narrative Review on Clinical Studies. International Journal of Molecular Sciences. 2022; 23(10):5591. https://doi.org/10.3390/ijms23105591
Chicago/Turabian StylePolito, Angela, Lorenzo Barnaba, Donatella Ciarapica, and Elena Azzini. 2022. "Osteosarcopenia: A Narrative Review on Clinical Studies" International Journal of Molecular Sciences 23, no. 10: 5591. https://doi.org/10.3390/ijms23105591
APA StylePolito, A., Barnaba, L., Ciarapica, D., & Azzini, E. (2022). Osteosarcopenia: A Narrative Review on Clinical Studies. International Journal of Molecular Sciences, 23(10), 5591. https://doi.org/10.3390/ijms23105591








