Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF
Abstract
:1. Introduction
2. Results
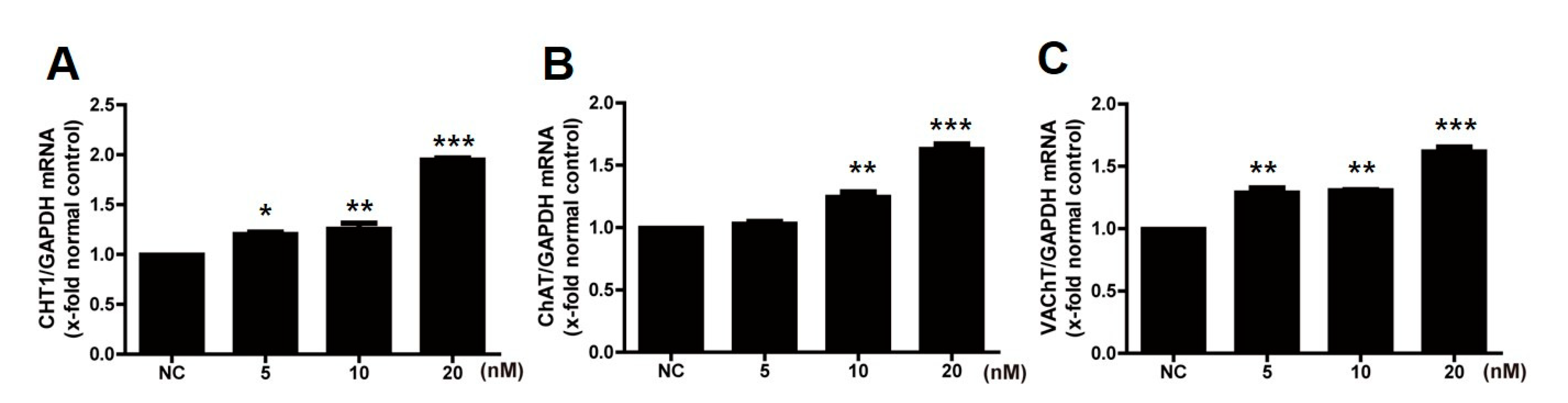
2.1. Treatment with Estradiol Upregulates the Cholinergic System
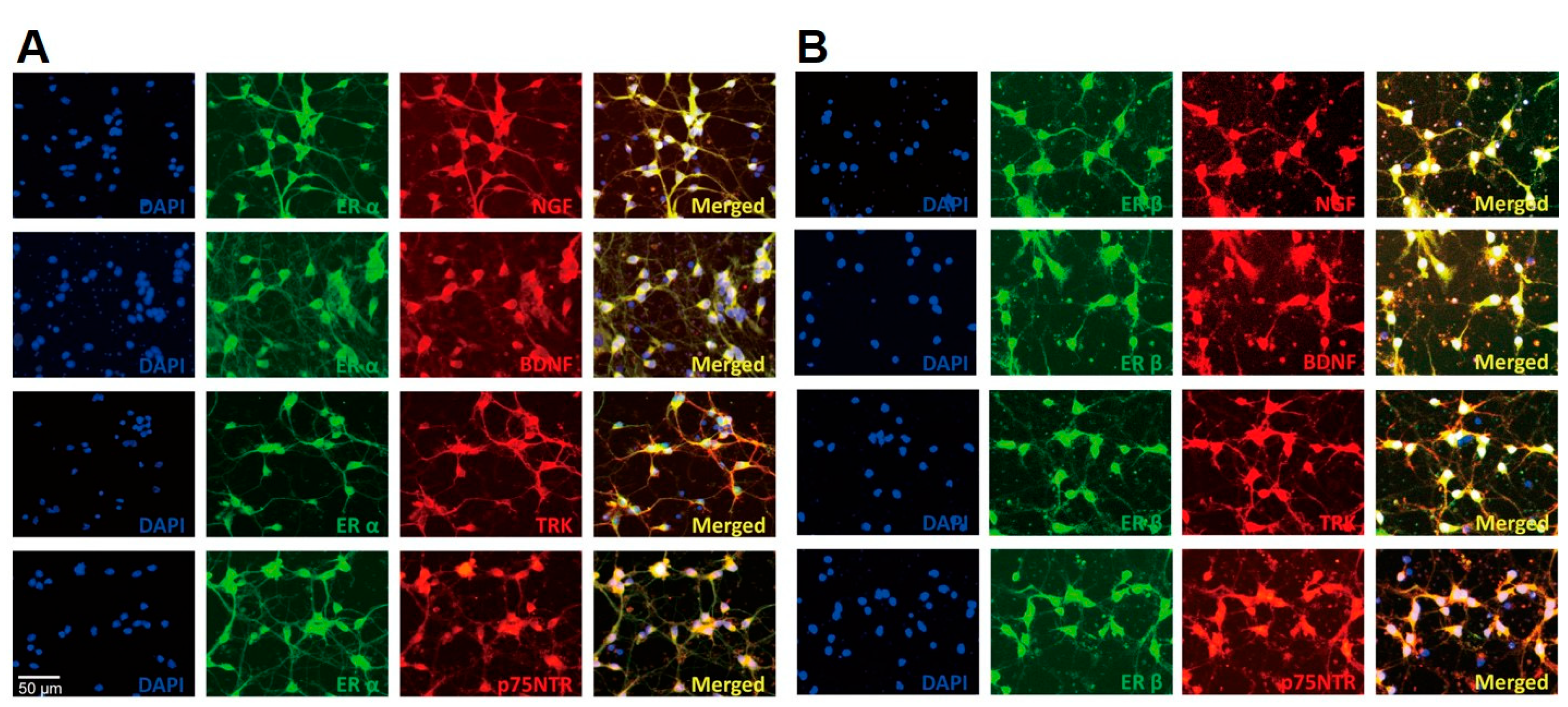
2.2. Estrogen Receptors Co-Localize with Neurotrophin Family
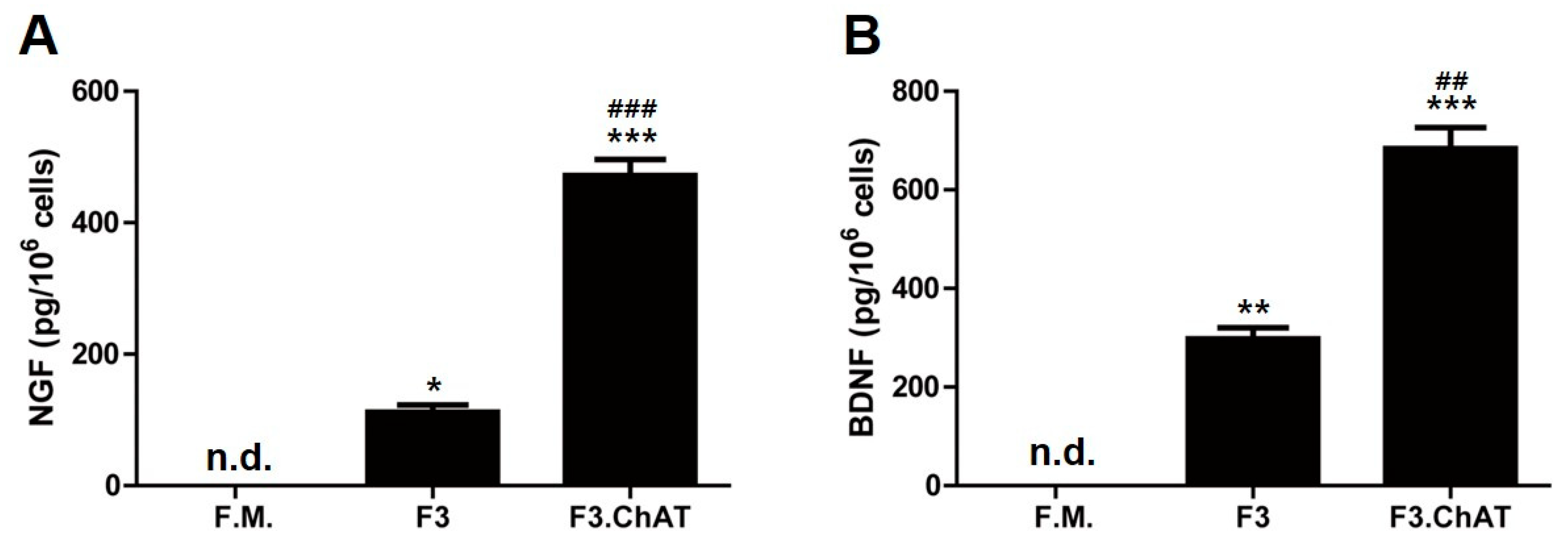
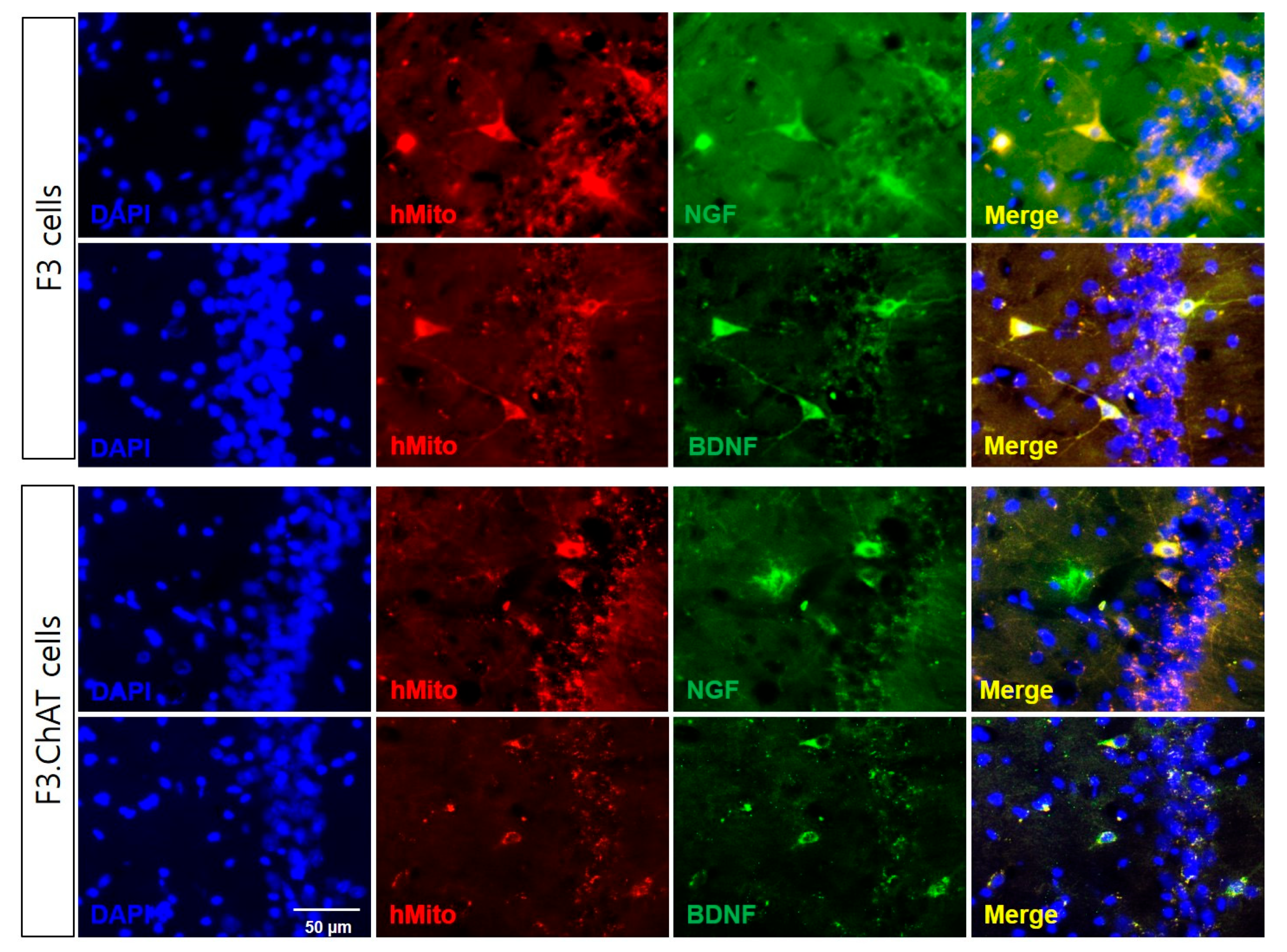
2.3. F3.ChAT Cells Release NGF and BDNF
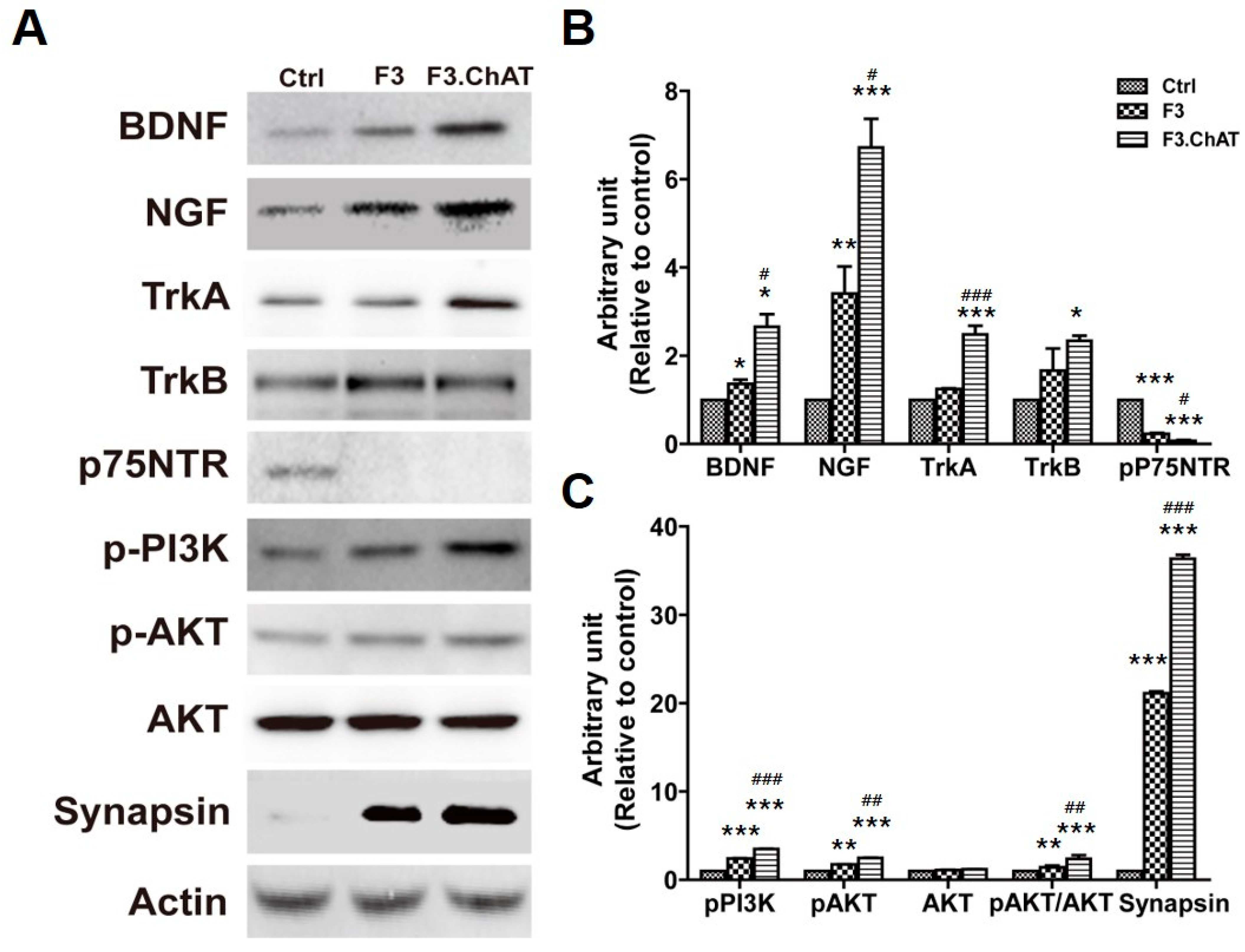
2.4. Co-Culture with F3.ChAT Cells Enhances Neurotrophin Signaling Pathway
2.5. Co-Culture with F3.ChAT Cells Upregulates the Cholinergic System
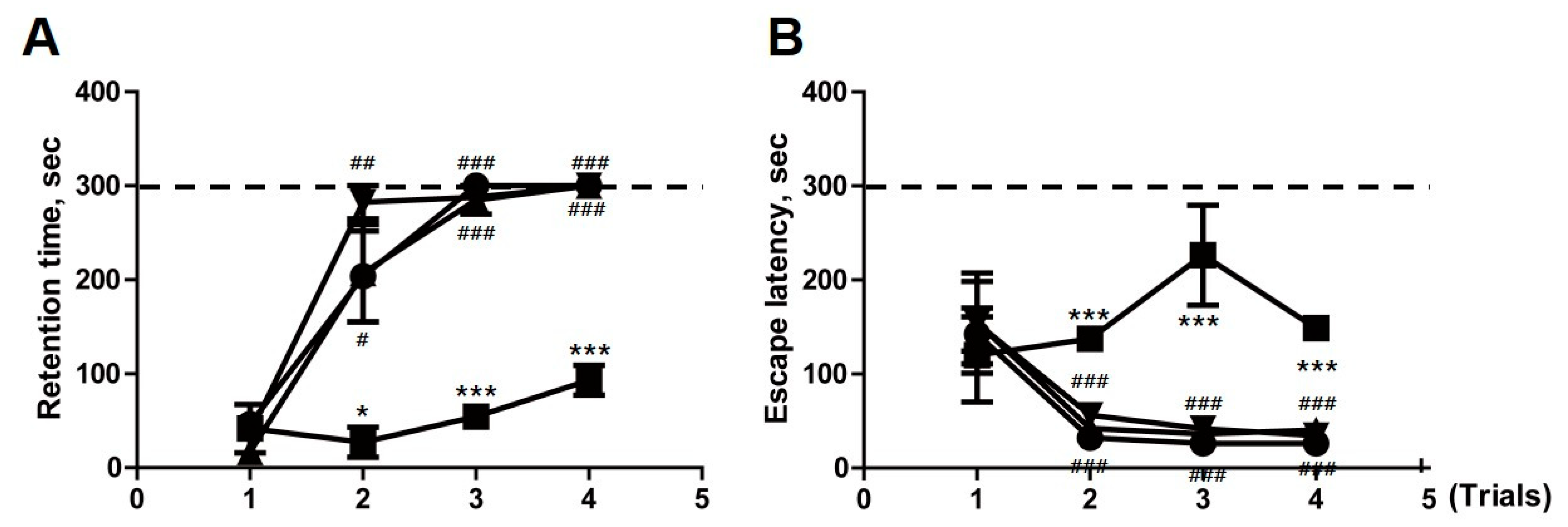
2.6. Transplantation of F3.ChAT Cells Improves Memory Deficit in Ovariectomized Rat
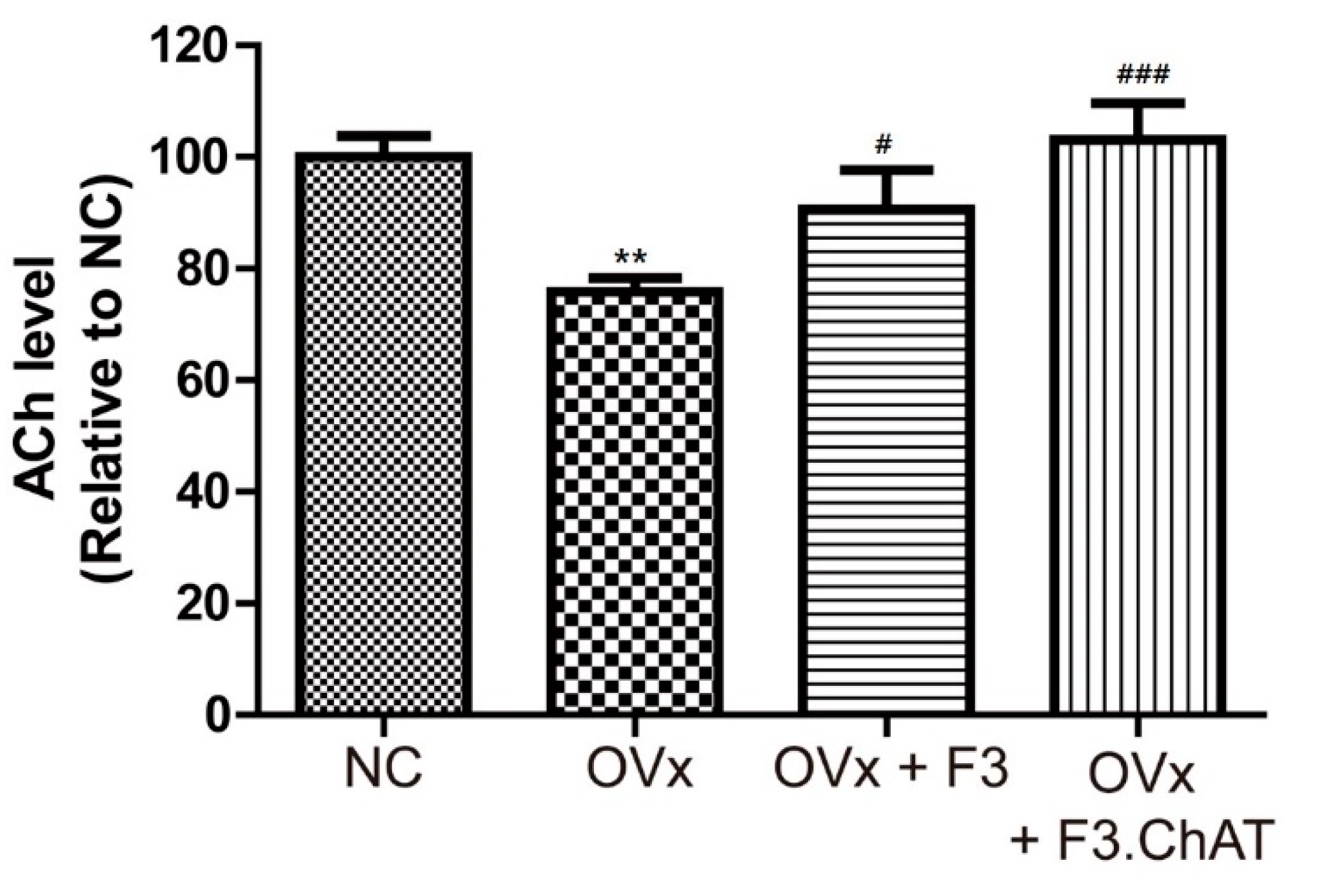
2.7. Transplantation of F3.ChAT Cells Restores ACh Concentration in CSF
2.8. Transplantation of F3.ChAT Cells Enhances Expression of the Cholinergic System via Upregulation of NGF and BDNF
3. Discussion
4. Materials and Methods
4.1. Primary Culture Cholinergic Neuron in Basal Forebrain
4.2. F3.ChAT Cell Culture
4.3. Assessing BDNF and NGF Levels
4.4. Immunocytochemistry of Estrogen Receptors and Neurophin Family in Primary Cells
4.5. Quantitative Real Time PCR Analysis
4.6. Animals
4.7. Ovariectomy and Stem Cell Transplantation
4.8. Passive Avoidance Performance
4.9. Morris Water-Maze Performance
4.10. ACh Analysis in CSF
4.11. Western Blot Analysis in Primary Cells and Brain Tissues
4.12. Immunohistochemistry in Brain Sections
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genazzani, A.R.; Pluchino, N.; Luisi, S.; Luisi, M. Estrogen, cognition and female ageing. Hum. Reprod. Update 2007, 13, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwin, B.B. Estrogen and cognitive functioning in women. Endocr. Rev. 2003, 24, 133–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [Green Version]
- Newhouse, P.; Dumas, J. Estrogen–cholinergic interactions: Implications for cognitive aging. Horm. Behav. 2015, 74, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Kim, K.-L.; Park, D. Effects of Resistance Exercise on mRNA Expression of Brain Neuroplasticity Related Factors in Hippocampus and Cognitive Function in Ovariectomized Rats. Brain Digit. Learn. 2021, 11, 577–587. [Google Scholar]
- Harte-Hargrove, L.C.; Maclusky, N.J.; Scharfman, H.E. Brain-derived neurotrophic factor–estrogen interactions in the hippocampal mossy fiber pathway: Implications for normal brain function and disease. Neuroscience 2013, 239, 46–66. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.L.; Waters, E.M.; Romeo, R.D.; Wood, G.E.; Milner, T.A.; McEwen, B.S. Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocr. 2008, 29, 219–237. [Google Scholar] [CrossRef] [Green Version]
- Cowansage, K.K.; LeDoux, J.E.; Monfils, M.-H. Brain-derived neurotrophic factor: A dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. 2010, 3, 12–29. [Google Scholar] [CrossRef]
- Korsching, S. The role of nerve growth factor in the CNS. Trends Neurosci. 1986, 9, 570–573. [Google Scholar] [CrossRef]
- Tapia-Arancibia, L.; Aliaga, E.; Silhol, M.; Arancibia, S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res. Rev. 2008, 59, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Yang, J.H.; Barba, D.; U, H.-S.; Bakay, R.A.E.; Pay, M.M.; Masliah, E.; Conner, J.M.; Kobalka, P.; Roy, S.; et al. Nerve Growth Factor Gene Therapy. JAMA Neurol. 2015, 72, 1139. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yang, G.; Bae, D.K.; Lee, S.H.; Yang, Y.-H.; Kyung, J.; Kim, D.; Choi, E.-K.; Choi, K.-C.; Kim, S.U.; et al. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J. Neurosci. Res. 2013, 91, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yang, Y.H.; Bae, D.K.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.K.; Lee, S.W.; Kim, G.H.; et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol. Aging 2013, 34, 2639–2646. [Google Scholar] [CrossRef]
- Pramanik, S.; Sulistio, Y.A.; Heese, K. Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy. Mol. Neurobiol. 2017, 54, 7401–7459. [Google Scholar] [CrossRef]
- Blokland, A. Acetylcholine: A neurotransmitter for learning and memory? Brain Res. Rev. 1995, 21, 285–300. [Google Scholar] [CrossRef]
- Da Penha Berzaghi, M.; Cooper, J.; Castren, E.; Zafra, F.; Sofroniew, M.; Thoenen, H.; Lindholm, D. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J. Neurosci. 1993, 13, 3818–3826. [Google Scholar] [CrossRef] [Green Version]
- Tyler, W.J.; Pozzo-Miller, L.D. BDNF Enhances Quantal Neurotransmitter Release and Increases the Number of Docked Vesicles at the Active Zones of Hippocampal Excitatory Synapses. J. Neurosci. 2001, 21, 4249–4258. [Google Scholar] [CrossRef]
- Li, Y.-X.; Zhang, Y.; Lester, H.A.; Schuman, E.M.; Davidson, N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J. Neurosci. 1998, 18, 10231–10240. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, R.B. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: Effects of estrogen and progesterone. J. Neurosci. 1996, 16, 1049–1055. [Google Scholar] [CrossRef]
- Kaur, G.; Janik, J.; Isaacson, L.G.; Callahan, P. Estrogen regulation of neurotrophin expression in sympathetic neurons and vascular targets. Brain Res. 2007, 1139, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Choi, E.K.; Cho, T.H.; Joo, S.S.; Kim, Y.B. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Abeta Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2020, 21, 3958. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Kesslak, J.P.; Pike, C.J.; Adlard, P.A.; Cotman, C.W. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur. J. Neurosci. 2001, 14, 1992–2002. [Google Scholar] [CrossRef]
- Russo-Neustadt, A.; Ha, T.; Ramirez, R.; Kesslak, J.P. Physical activity–antidepressant treatment combination: Impact on brain-derived neurotrophic factor and behavior in an animal model. Behav. Brain Res. 2001, 120, 87–95. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Hu, W.; Gao, Y.; Ni, X.; Sheng, H.; Liu, Y. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neurosci. Lett. 2014, 573, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Gu, X.; Dong, Y.; Tan, F.; Liu, Z.; Thiele, C.J.; Li, Z. PI3K and MAPK pathways mediate the BDNF/TrkB-increased metastasis in neuroblastoma. Tumour Biol. 2016, 37, 16227–16236. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Alegria, K.; Flores-Leon, M.; Avila-Munoz, E.; Rodriguez-Corona, N.; Arias, C. PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions. Int. J. Mol. Sci. 2018, 19, 3725. [Google Scholar] [CrossRef] [Green Version]
- Roman-Blas, J.A.; Castañeda, S.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Amp. Ther. 2009, 11, 241. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Shukla, P.; Anjum, B.; Gupta, H.P.; Pal, S.; Arjaria, N.; Gupta, K.; Chattopadhyay, N.; Sinha, R.A.; Bandyopadhyay, S. Estrogen deficiency induces memory loss via altered hippocampal HB-EGF and autophagy. J. Endocrinol. 2020, 244, 53–70. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Sarrel, P.M.; Nelson, L.M. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil. Steril. 2016, 106, 1588–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adu-Nti, F.; Gao, X.; Wu, J.-M.; Li, J.; Iqbal, J.; Ahmad, R.; Ma, X.-M. Osthole ameliorates estrogen deficiency-induced cognitive impairment in female mice. Front. Pharmacol. 2021, 12, 641909. [Google Scholar] [CrossRef] [PubMed]
- Szegő, É.M.; Csorba, A.; Janáky, T.; Kékesi, K.A.; Ábrahám, I.M.; Mórotz, G.M.; Penke, B.; Palkovits, M.; Murvai, Ü.; Kellermayer, M.S. Effects of estrogen on beta-amyloid-induced cholinergic cell death in the nucleus basalis magnocellularis. Neuroendocrinology 2011, 93, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.M.; Dohanich, G.P. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J. Neurosci. 2001, 21, 6949–6956. [Google Scholar] [CrossRef] [Green Version]
- Rashidy-Pour, A.; Bavarsad, K.; Miladi-Gorji, H.; Seraj, Z.; Vafaei, A.A. Voluntary exercise and estradiol reverse ovariectomy-induced spatial learning and memory deficits and reduction in hippocampal brain-derived neurotrophic factor in rats. Pharmacol. Biochem. Behav. 2019, 187, 172819. [Google Scholar] [CrossRef]
- Habibi, P.; Babri, S.; Ahmadiasl, N.; Yousefi, H. Effects of genistein and swimming exercise on spatial memory and expression of microRNA 132, BDNF, and IGF-1 genes in the hippocampus of ovariectomized rats. Iran. J. Basic Med. Sci. 2017, 20, 856. [Google Scholar]
- Chae, C.-H.; Kim, H.-T. Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochem. Int. 2009, 55, 208–213. [Google Scholar] [CrossRef]
- Toran-Allerand, C.D.; Miranda, R.C.; Bentham, W.D.; Sohrabji, F.; Brown, T.J.; Hochberg, R.B.; Maclusky, N.J. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc. Natl. Acad. Sci. USA 1992, 89, 4668–4672. [Google Scholar] [CrossRef] [Green Version]
- Bora, S.H.; Liu, Z.; Kecojevic, A.; Merchenthaler, I.; Koliatsos, V.E. Direct, complex effects of estrogens on basal forebrain cholinergic neurons. Exp. Neurol. 2005, 194, 506–522. [Google Scholar] [CrossRef]
- McMillan, P.; Singer, C.; Dorsa, D. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J. Neurosci. 1996, 16, 1860–1865. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Lee, S.H.; Bae, D.K.; Yang, Y.-H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.-K.; Hong, J.T.; Shin, I.S.; et al. Transplantation of Human Adipose Tissue-Derived Mesenchymal Stem Cells Restores the Neurobehavioral Disorders of Rats with Neonatal Hypoxic-Ischemic Encephalopathy. Cell Med. 2013, 5, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madziar, B.; Lopez-Coviella, I.; Zemelko, V.; Berse, B. Regulation of cholinergic gene expression by nerve growth factor depends on the phosphatidylinositol-3’-kinase pathway. J. Neurochem. 2005, 92, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dai, F.-r.; Du, X.-p.; Yang, Q.-d.; Zhang, X.; Chen, Y. Infusion of BDNF into the nucleus accumbens of aged rats improves cognition and structural synaptic plasticity through PI3K-ILK-Akt signaling. Behav. Brain Res. 2012, 231, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Scott-Solomon, E.; Kuruvilla, R. Mechanisms of neurotrophin trafficking via Trk receptors. Mol. Cell. Neurosci. 2018, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Ginsberg, S.D.; Ikonomovic, M.D.; Dekosky, S.T. Human cholinergic basal forebrain: Chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 2003, 26, 233–242. [Google Scholar] [CrossRef]
- Miller, F.; Kaplan, D. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell. Mol. Life Sci. CMLS 2001, 58, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Colicos, M.A.; Barker, P.A.; Kennedy, T.E. p75 Neurotrophin Receptor Expression Is Induced in Apoptotic Neurons After Seizure. J. Neurosci. 1999, 19, 6887–6896. [Google Scholar] [CrossRef] [Green Version]
- Qu, N.; Wang, L.; Liu, Z.C.; Tian, Q.; Zhang, Q. Oestrogen receptor alpha agonist improved long-term ovariectomy-induced spatial cognition deficit in young rats. Int. J. Neuropsychopharmacol. 2013, 16, 1071–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Lee, H.J.; Joo, S.S.; Bae, D.K.; Yang, G.; Yang, Y.H.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.B.; et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp. Neurol. 2012, 234, 521–526. [Google Scholar] [CrossRef]
- MacLennan, A.H.; MacLennan, A.; Wenzel, S.; Chambers, H.M.; Eckert, K. Continuous low-dose oestrogen and progestogen hormone replacement therapy: A randomised trial. Med. J. Aust. 1993, 159, 102–106. [Google Scholar] [CrossRef]
- Clisham, P.R.; de Ziegler, D.; Lozano, K.; Judd, H.L. Comparison of continuous versus sequential estrogen and progestin therapy in postmenopausal women. Obs. Gynecol. 1991, 77, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Hawthorn, R.J.; Spowart, K.; Walsh, D.; Hart, D.M. The endometrial status of women on long-term continuous combined hormone replacement therapy. Br. J. Obs. Gynaecol. 1991, 98, 939–940. [Google Scholar] [CrossRef]
- Williams, S.R.; Frenchek, B.; Speroff, T.; Speroff, L. A study of combined continuous ethinyl estradiol and norethindrone acetate for postmenopausal hormone replacement. Am. J. Obs. Gynecol. 1990, 162, 438–446. [Google Scholar] [CrossRef]
- Luciano, A.A.; Turksoy, R.N.; Carleo, J.; Hendrix, J.W. Clinical and metabolic responses of menopausal women to sequential versus continuous estrogen and progestin replacement therapy. Obs. Gynecol. 1988, 71, 39–43. [Google Scholar]
- Yon, J.-M.; Kim, Y.-B.; Park, D. The Ethanol Fraction of White Rose Petal Extract Abrogates Excitotoxicity-Induced Neuronal Damage In Vivo and In Vitro through Inhibition of Oxidative Stress and Proinflammation. Nutrients 2018, 10, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene Name | Accession No. | Mouse Primer | |
|---|---|---|---|
| Forward | Reverse | ||
| CHT | NM_022025.4 | TTCCAGATTCAGGCAGTAGACG | GGGAGGGAAACTCCTATCTTGT |
| ChAT | NM_009891.2 | TGGGTCTCTGAATACTGGCTGA | GGGCTAGAGTTGACTGGCAGG |
| VAChT | NM_021712.3 | CCCTTTTGATGGCTGTGA | GGGCTAGGGTACTCATTAGA |
| GAPdH | XM_039103226 | GTCGGTGTGAACGGATTTGG | CCACTTTGTCACAAGAGAAGGCA |
| Epitope | Company | Cat. Number | Dilution | 2° Ab (IgG) |
|---|---|---|---|---|
| BDNF | Abcam | Ab226843 | 1:1000 | Rb |
| NGF | Abcam | Ab6199 | 1:1000 | Rb |
| TrkA | ThermoFisher | Pa5-98018 | 1:1000 | Rb |
| TrkB | Abcam | Ab18987 | 1:2000 | Rb |
| P75NTR | Abcam | Ab52987 | 1:1000 | Rb |
| p-PI3K | Cell signaling | #4228 | 1:1000 | Rb |
| p-AKT | Cell signaling | #9271 | 1:1000 | Rb |
| AKT | Cell Signaling | #9272 | 1:1000 | Rb |
| Synapsin | Abcam | Ab64581 | 1:1000 | Rb |
| Actin | Cell Signal | #5125 | 1:1000 | Rb |
| ER α | Abcam | Ab66102 | 1:100 | Ms |
| ER β | Abcam | Ab288 | 1:100 | Ms |
| CHT1 | Abcam | Ab154186 | 1:1000 | Rb |
| ChAT | Abcam | Ab178850 | 1:1000 | Rb |
| VAChT | Synaptic systems | #139103 | 1:500 | Rb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, E.-J.; Choi, Y.; Park, D. Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF. Int. J. Mol. Sci. 2022, 23, 5560. https://doi.org/10.3390/ijms23105560
Yoon E-J, Choi Y, Park D. Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF. International Journal of Molecular Sciences. 2022; 23(10):5560. https://doi.org/10.3390/ijms23105560
Chicago/Turabian StyleYoon, Eun-Jung, Yunseo Choi, and Dongsun Park. 2022. "Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF" International Journal of Molecular Sciences 23, no. 10: 5560. https://doi.org/10.3390/ijms23105560
APA StyleYoon, E.-J., Choi, Y., & Park, D. (2022). Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF. International Journal of Molecular Sciences, 23(10), 5560. https://doi.org/10.3390/ijms23105560






