Nitric Oxide Mobilizes Intracellular Zn2+ via the GC/cGMP/PKG Signaling Pathway and Stimulates Adipocyte Differentiation
Abstract
1. Introduction
2. Results
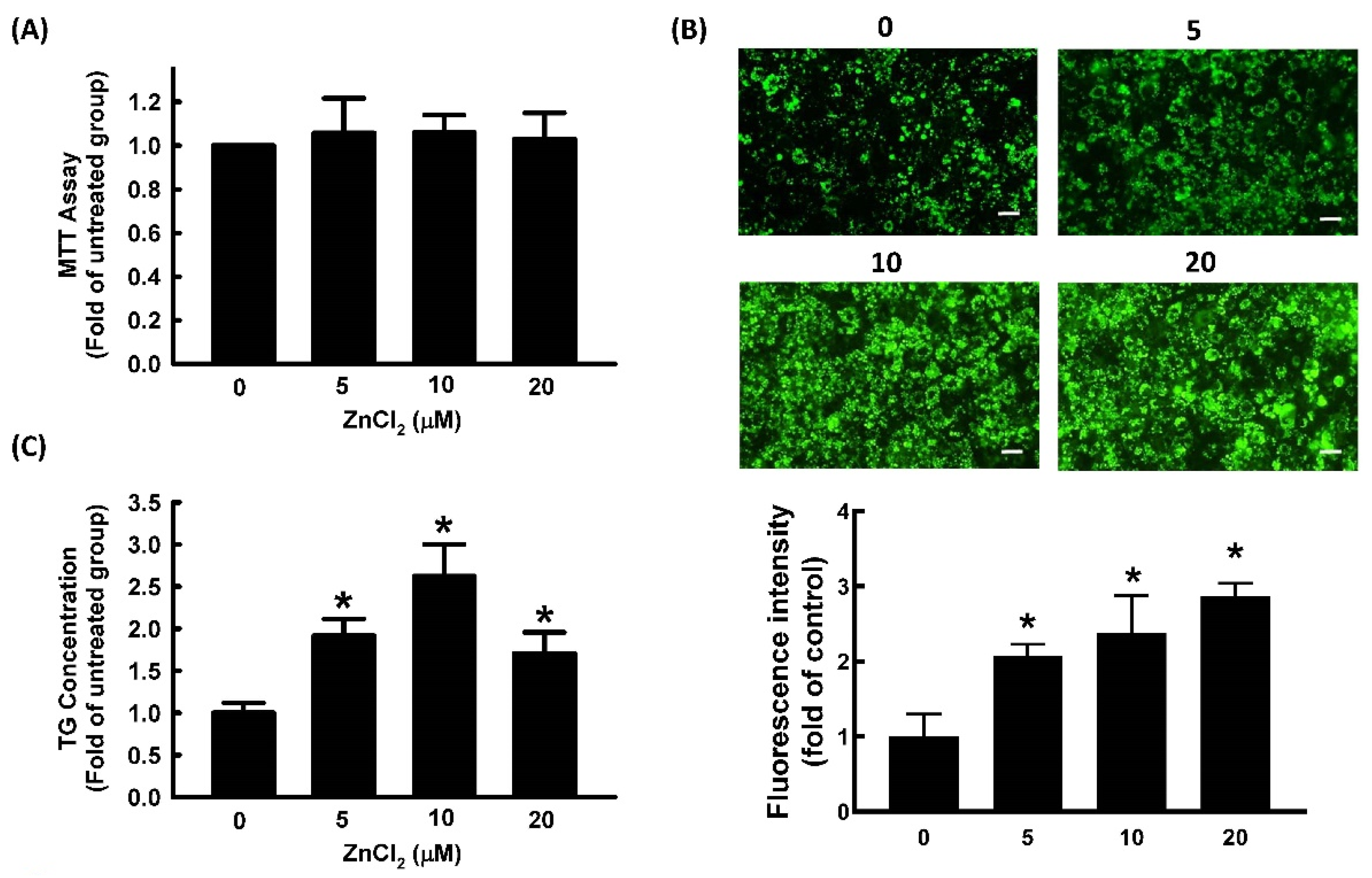
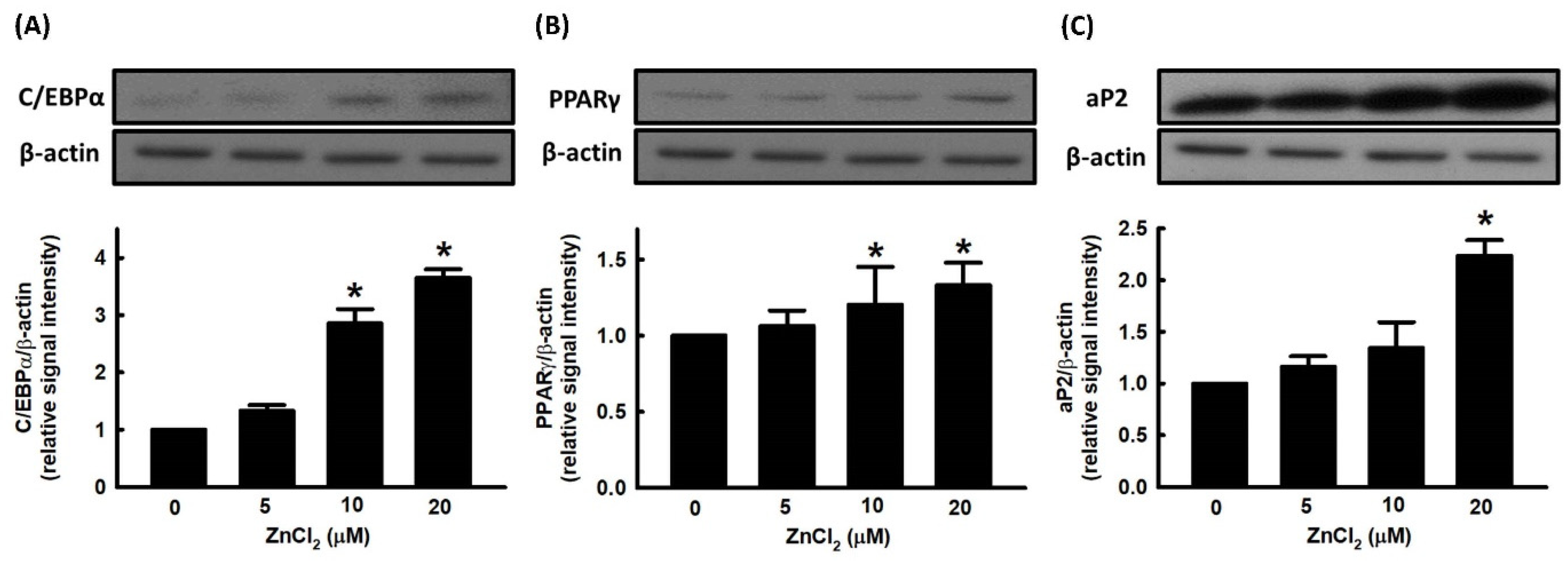
2.1. Effect of ZnCl2 on Cell Viability and Adipocyte Differentiation
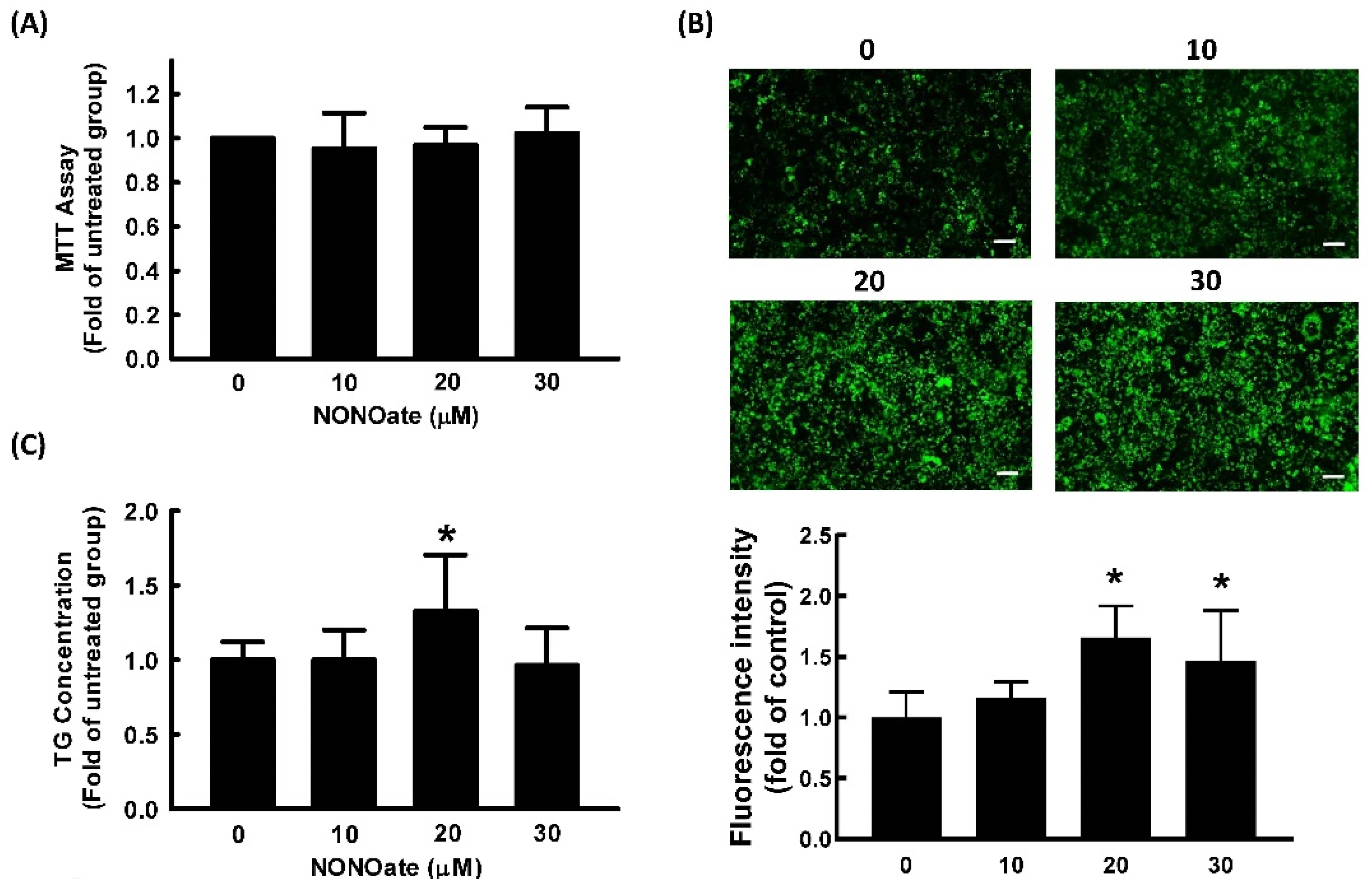
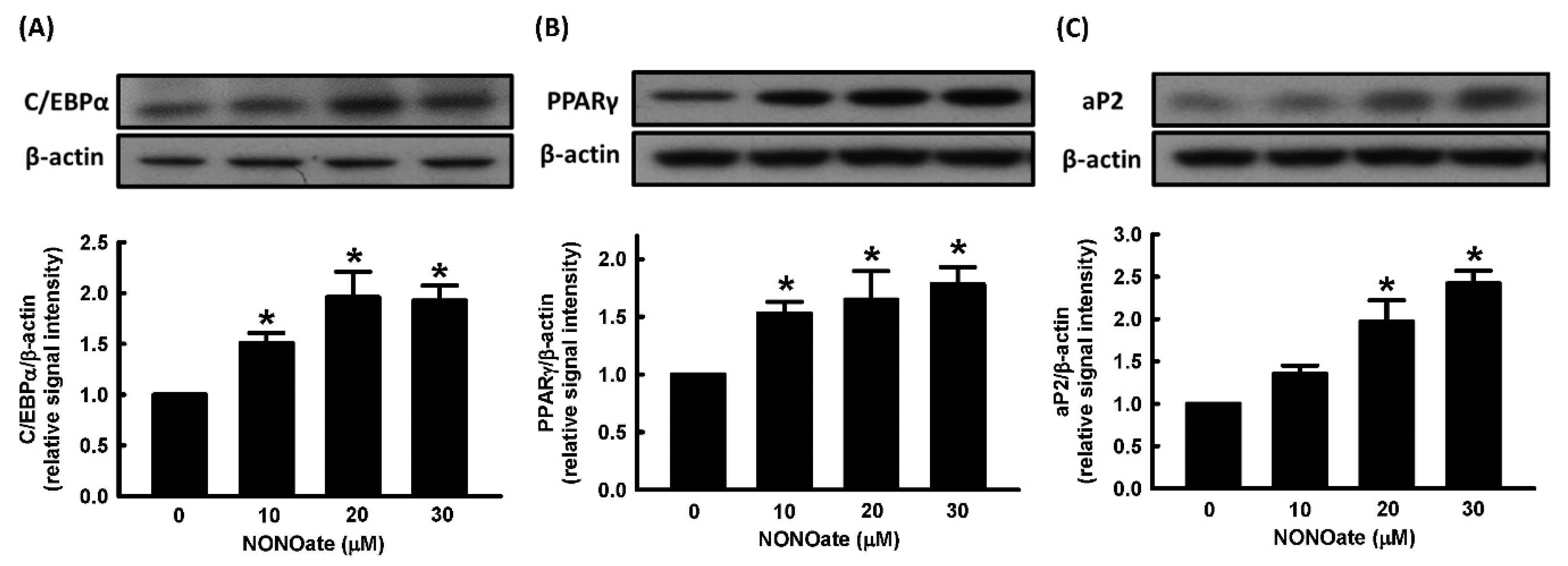
2.2. Effect of NO on Cell Viability and Adipocyte Differentiation
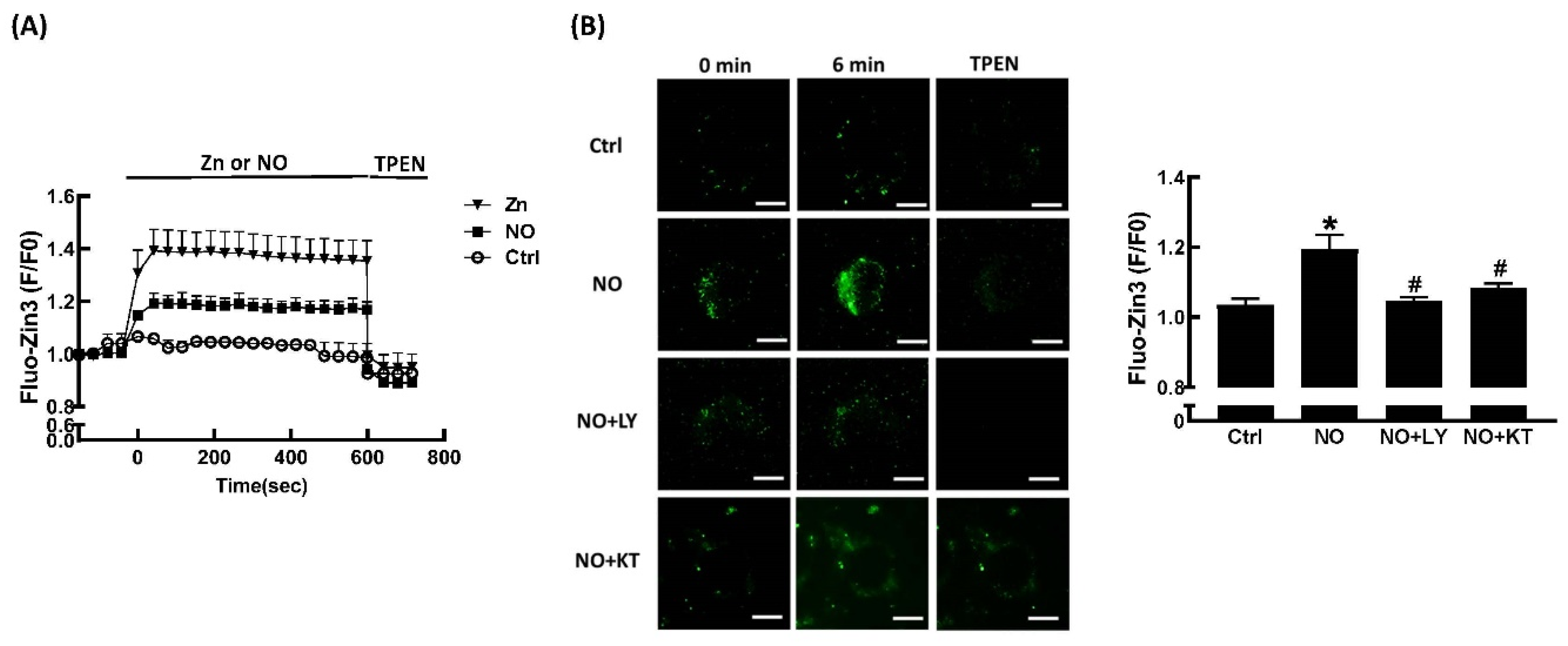
2.3. Effects of ZnCl2 and NO on Intracellular Zn2+ Mobilization
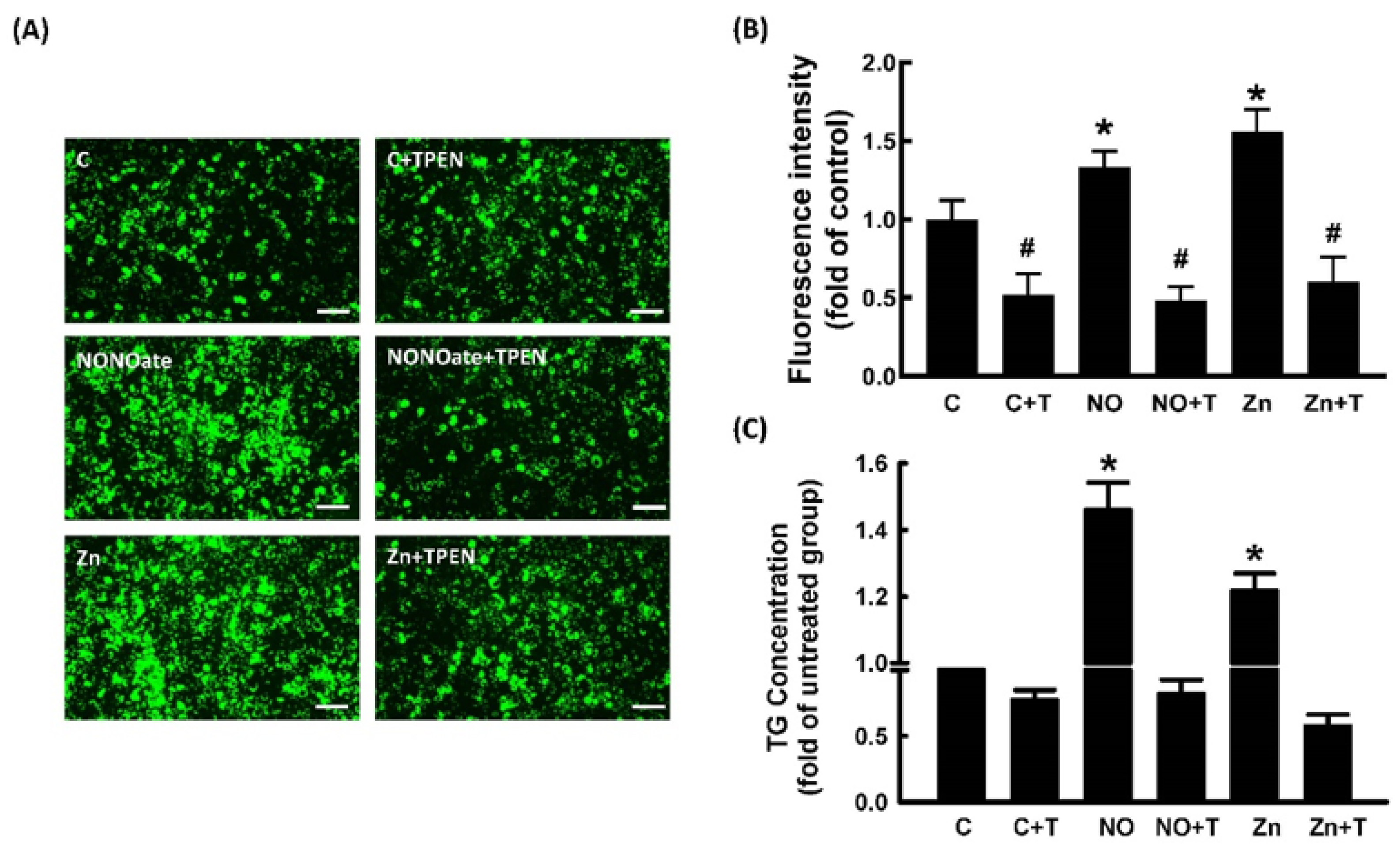
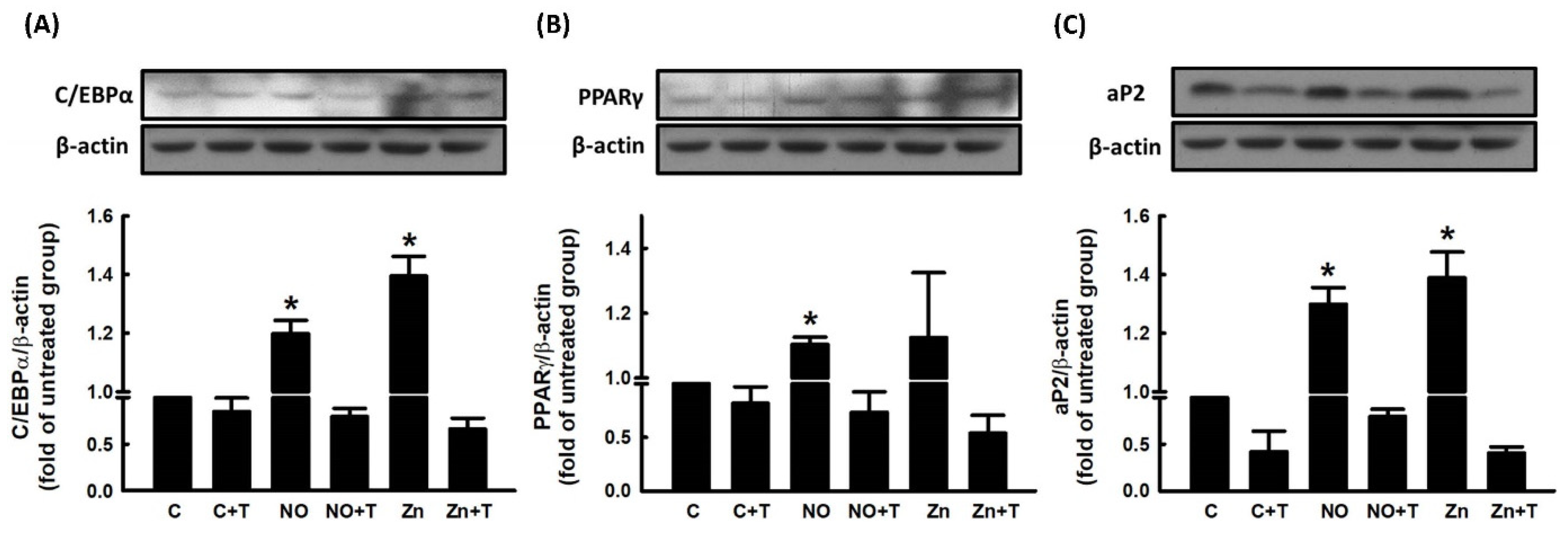
2.4. Role of Zn2+ in NO-Stimulated Adipocyte Differentiation
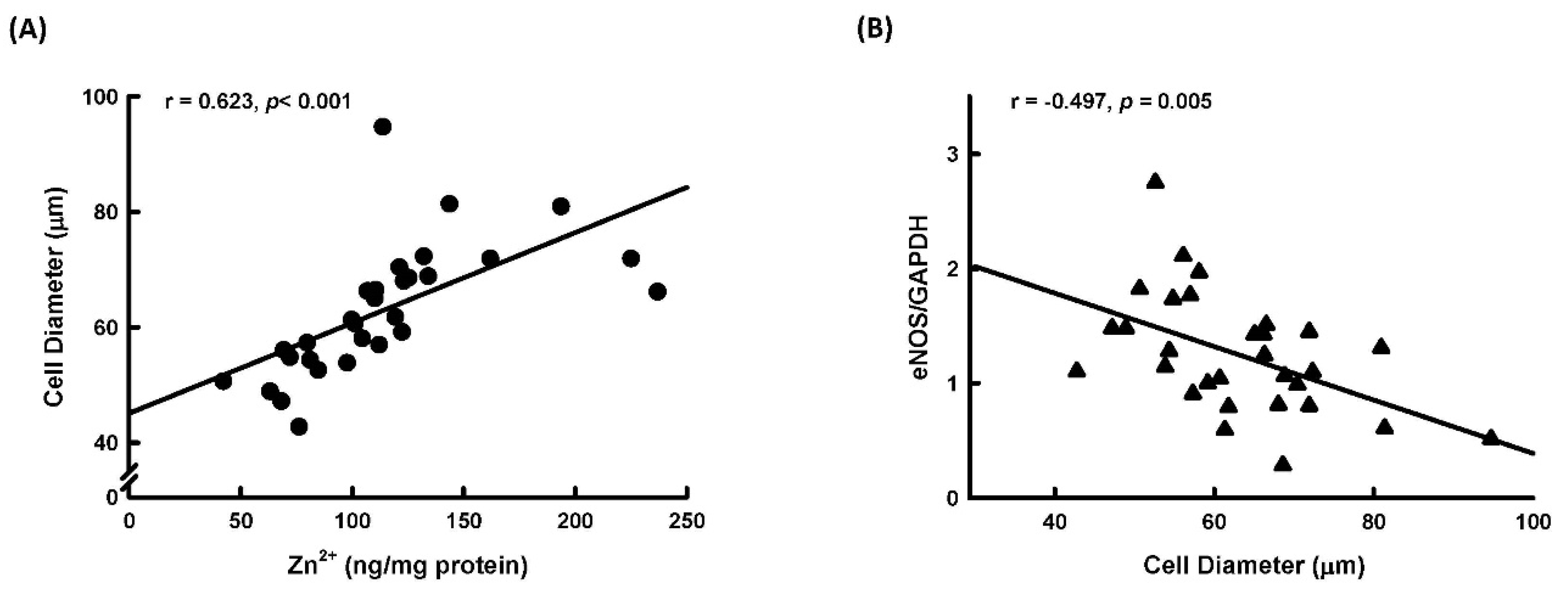
2.5. Correlation between Adipocyte Size, Zn2+ Level, and NOS Expression in Human Adipose Tissue
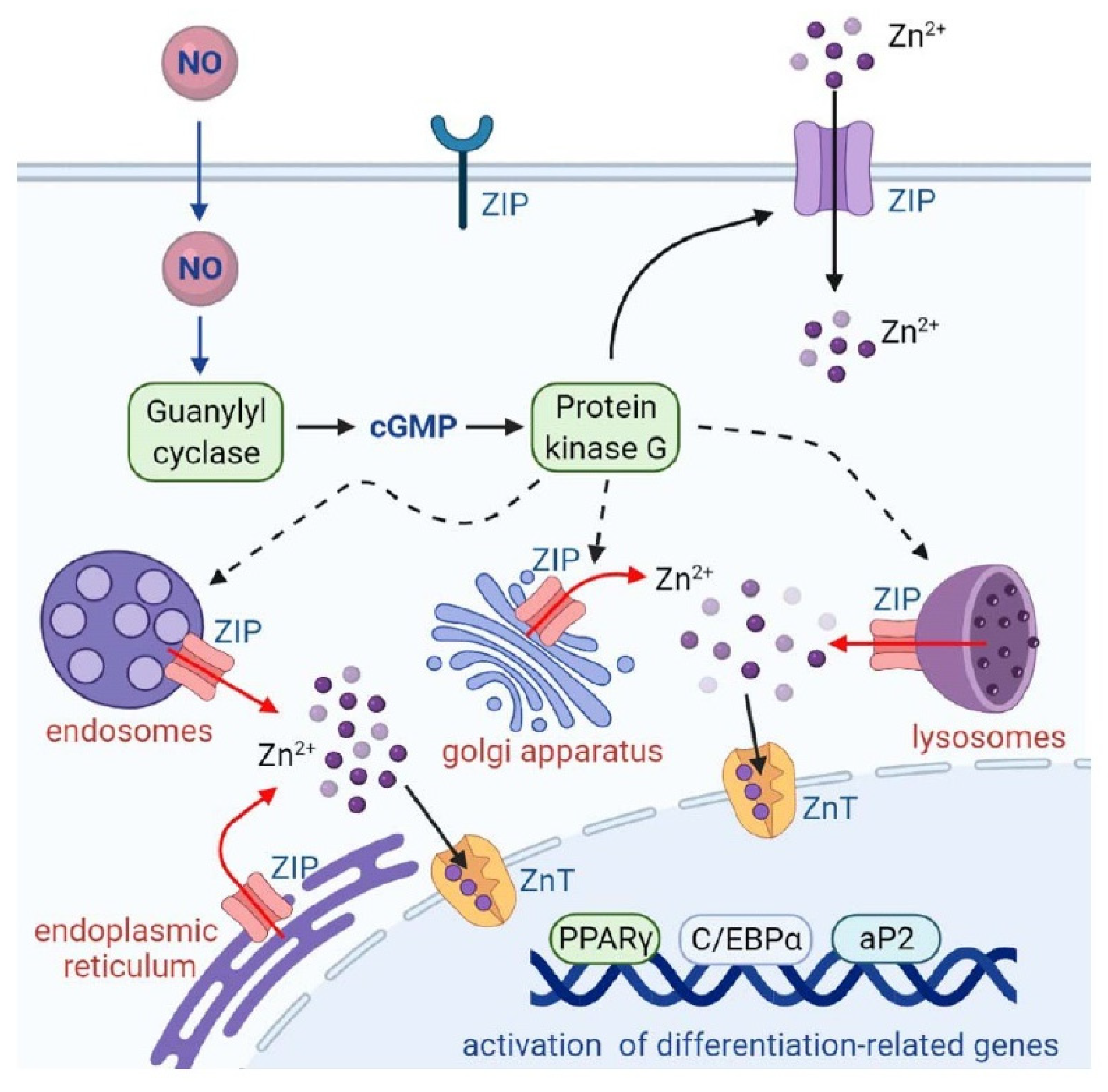
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Experimental Design
4.3. 3T3-L1 Fibroblast Cell Culture and Differentiation Conditions
4.4. BODIPY 493/503 Staining
4.5. Measurement of Triglyceride
4.6. Immunoblot Analysis
4.7. MTT Assay
4.8. Live-cell Imaging of Intracellular Zinc
4.9. Collection of Human Subcutaneous Adipose Tissues
4.10. Real-time Polymerase Chain Reaction (RT-PCR)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 3 May 2022).
- Nurdiantami, Y.; Watanabe, K.; Tanaka, E.; Pradono, J.; Anme, T. Association of general and central obesity with hypertension. Clin. Nutr. 2018, 37, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; De Schutter, A.; Milani, R.V. Healthy obese versus unhealthy lean: The obesity paradox. Nat. Rev. Endocrinol. 2015, 11, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; De Schutter, A.; Parto, P.; Jahangir, E.; Kokkinos, P.; Ortega, F.B.; Arena, R.; Milani, R.V. Obesity and prevalence of cardiovascular diseases and prognosis-the obesity paradox updated. Prog. Cardiovasc. Dis. 2016, 58, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Hammer, R.E.; Richardson, J.A.; Ikemoto, S.; Bashmakov, Y.; Goldstein, J.L.; Brown, M.S. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: Model for congenital generalized lipodystrophy. Genes Dev. 1998, 12, 3182–3194. [Google Scholar] [CrossRef]
- Prasad, A.S.; Halsted, J.A.; Nadimi, M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am. J. Med. 1961, 31, 532–546. [Google Scholar] [CrossRef]
- Di Martino, G.; Matera, M.G.; De Martino, B.; Vacca, C.; Di Martino, S.; Rossi, F. Relationship between zinc and obesity. J. Med. 1993, 24, 177–183. [Google Scholar]
- Gu, K.; Xiang, W.; Zhang, Y.; Sun, K.; Jiang, X. The association between serum zinc level and overweight/obesity: A meta-analysis. Eur. J. Nutr. 2019, 58, 2971–2982. [Google Scholar] [CrossRef]
- Rios-Lugo, M.J.; Madrigal-Arellano, C.; Gaytan-Hernandez, D.; Hernandez-Mendoza, H.; Romero-Guzman, E.T. Association of serum zinc levels in overweight and obesity. Biol. Trace Elem. Res. 2020, 198, 51–57. [Google Scholar] [CrossRef]
- Suliburska, J.; Cofta, S.; Gajewska, E.; Kalmus, G.; Sobieska, M.; Samborski, W.; Krejpcio, Z.; Drzymala-Czyz, S.; Bogdanski, P. The evaluation of selected serum mineral concentrations and their association with insulin resistance in obese adolescents. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2396–2400. [Google Scholar]
- Yerlikaya, F.H.; Toker, A.; Aribas, A. Serum trace elements in obese women with or without diabetes. Indian J. Med. Res. 2013, 137, 339–345. [Google Scholar]
- Garcia, O.P.; Ronquillo, D.; del Carmen Caamano, M.; Martinez, G.; Camacho, M.; Lopez, V.; Rosado, J.L. Zinc, iron and vitamins A, C and e are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients 2013, 5, 5012–5030. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Paik, H.Y.; Kim, J.; Chung, J. The alteration of zinc transporter gene expression is associated with inflammatory markers in obese women. Biol. Trace Elem. Res. 2014, 158, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ennes, D.F.F.; de Sousa, L.V.B.; Mello, S.N.R.; Franciscato, C.S.M.; do Nascimento, M.D. Biomarkers of metabolic syndrome and its relationship with the zinc nutritional status in obese women. Nutr. Hosp. 2011, 26, 650–654. [Google Scholar] [CrossRef]
- Tascilar, M.E.; Ozgen, I.T.; Abaci, A.; Serdar, M.; Aykut, O. Trace elements in obese Turkish children. Biol. Trace Elem. Res. 2011, 143, 188–195. [Google Scholar] [CrossRef]
- Weisstaub, G.; Hertrampf, E.; Lopez de Romana, D.; Salazar, G.; Bugueno, C.; Castillo-Duran, C. Plasma zinc concentration, body composition and physical activity in obese preschool children. Biol. Trace Elem. Res. 2007, 118, 167–174. [Google Scholar] [CrossRef]
- Tominaga, K.; Kagata, T.; Johmura, Y.; Hishida, T.; Nishizuka, M.; Imagawa, M. SLC39A14, a LZT protein, is induced in adipogenesis and transports zinc. FEBS J. 2005, 272, 1590–1599. [Google Scholar] [CrossRef]
- Troche, C.; Aydemir, T.B.; Cousins, R.J. Zinc transporter Slc39a14 regulates inflammatory signaling associated with hypertrophic adiposity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E258–E268. [Google Scholar] [CrossRef]
- Engeli, S.; Janke, J.; Gorzelniak, K.; Bohnke, J.; Ghose, N.; Lindschau, C.; Luft, F.C.; Sharma, A.M. Regulation of the nitric oxide system in human adipose tissue. J. Lipid Res. 2004, 45, 1640–1648. [Google Scholar] [CrossRef]
- Choi, J.W.; Pai, S.H.; Kim, S.K.; Ito, M.; Park, C.S.; Cha, Y.N. Increases in nitric oxide concentrations correlate strongly with body fat in obese humans. Clin. Chem. 2001, 47, 1106–1109. [Google Scholar] [CrossRef]
- Yan, H.; Aziz, E.; Shillabeer, G.; Wong, A.; Shanghavi, D.; Kermouni, A.; Abdel-Hafez, M.; Lau, D.C. Nitric oxide promotes differentiation of rat white preadipocytes in culture. J. Lipid Res. 2002, 43, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.C.; Chang, C.L.; Chuang, T.Y.; Huang, S.W.; Kwok, C.F.; Ho, L.T. Insulin sensitivity and resistin expression in nitric oxide-deficient rats. Diabetologia 2006, 49, 3017–3026. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cuajungco, M.P.; Lees, G.J. Nitric oxide generators produce accumulation of chelatable zinc in hippocampal neuronal perikarya. Brain Res. 1998, 799, 118–129. [Google Scholar] [CrossRef]
- Pearce, L.L.; Wasserloos, K.; St Croix, C.M.; Gandley, R.; Levitan, E.S.; Pitt, B.R. Metallothionein, nitric oxide and zinc homeostasis in vascular endothelial cells. J. Nutr. 2000, 130, 1467S–1470S. [Google Scholar] [CrossRef] [PubMed]
- Berendji, D.; Kolb-Bachofen, V.; Meyer, K.L.; Grapenthin, O.; Weber, H.; Wahn, V.; Kroncke, K.D. Nitric oxide mediates intracytoplasmic and intranuclear zinc release. FEBS Lett. 1997, 405, 37–41. [Google Scholar] [CrossRef]
- Yang, D.M.; Huang, C.C.; Chang, Y.F. Combinatorial roles of mitochondria and cGMP/PKG pathway in the generation of neuronal free Zn2+ under the presence of nitric oxide. J. Chin. Med. Assoc. 2020, 83, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef]
- Thompson, A.J.; Mander, P.K.; Brown, G.C. The NO donor DETA-NONOate reversibly activates an inward current in neurones and is not mediated by the released nitric oxide. Br. J. Pharmacol. 2009, 158, 1338–1343. [Google Scholar] [CrossRef]
- Jang, Y.; Wang, H.; Xi, J.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. NO mobilizes intracellular Zn2+ via cGMP/PKG signaling pathway and prevents mitochondrial oxidant damage in cardiomyocytes. Cardiovasc. Res. 2007, 75, 426–433. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Yuce, K. Zinc Transporter Proteins. Neurochem. Res. 2018, 43, 517–530. [Google Scholar] [CrossRef]
- Meerarani, P.; Reiterer, G.; Toborek, M.; Hennig, B. Zinc modulates PPARgamma signaling and activation of porcine endothelial cells. J. Nutr. 2003, 133, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, L.; Zhou, X.; Du, M.; Jiang, Z.; Hausman, G.J.; Bergen, W.G.; Zan, L.; Dodson, M.V. Emerging roles of zinc finger proteins in regulating adipogenesis. Cell. Mol. Life Sci. 2013, 70, 4569–4584. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Tran, T.T.; Truong, L.T.N.; Mai, H.H.; Nguyen, T.T. Overcharging of the zinc ion in the structure of the zinc-finger protein is needed for DNA binding stability. Biochemistry 2020, 59, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.H.; Kao, L.S.; Liu, P.S.; Huang, C.C.; Yang, D.M.; Pan, C.Y. Dopamine elevates intracellular zinc concentration in cultured rat embryonic cortical neurons through the cAMP-nitric oxide signaling cascade. Mol. Cell. Neurosci. 2017, 82, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.J.; Leelavanichkul, K.; Bauer, E.; Cao, R.; Wilson, A.; Wasserloos, K.J.; Watkins, S.C.; Pitt, B.R.; St Croix, C.M. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ. Res. 2008, 102, 1575–1583. [Google Scholar] [CrossRef]
- Razny, U.; Kiec-Wilk, B.; Wator, L.; Polus, A.; Dyduch, G.; Solnica, B.; Malecki, M.; Tomaszewska, R.; Cooke, J.P.; Dembinska-Kiec, A. Increased nitric oxide availability attenuates high fat diet metabolic alterations and gene expression associated with insulin resistance. Cardiovasc. Diabetol. 2011, 10, 68. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Cummins, T.D.; Tang, Y.; Hellmann, J.; Holden, C.R.; Harbeson, M.A.; Chen, Y.; Patel, R.P.; Spite, M.; Bhatnagar, A.; et al. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ. Res. 2012, 111, 1176–1189. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Huang, P.L. Endothelial nitric oxide synthase knockdown in human stem cells impacts mitochondrial biogenesis and adipogenesis: Live-cell real-time fluorescence imaging. J. Clin. Med. 2021, 10, 631. [Google Scholar] [CrossRef]
- Jang, J.E.; Ko, M.S.; Yun, J.Y.; Kim, M.O.; Kim, J.H.; Park, H.S.; Kim, A.R.; Kim, H.J.; Kim, B.J.; Ahn, Y.E.; et al. Nitric oxide produced by macrophages inhibits adipocyte differentiation and promotes profibrogenic responses in preadipocytes to induce adipose tissue fibrosis. Diabetes 2016, 65, 2516–2528. [Google Scholar] [CrossRef]
- Yang, S.; Guo, L.; Su, Y.; Wen, J.; Du, J.; Li, X.; Liu, Y.; Feng, J.; Xie, Y.; Bai, Y.; et al. Nitric oxide balances osteoblast and adipocyte lineage differentiation via the JNK/MAPK signaling pathway in periodontal ligament stem cells. Stem Cell Res. Ther. 2018, 9, 118. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fukada, T.; Bhin, J.; Suzuki, L.; Tsuzuki, T.; Takamine, Y.; Bin, B.H.; Yoshihara, T.; Ichinoseki-Sekine, N.; Naito, H.; et al. Zinc transporter ZIP13 suppresses beige adipocyte biogenesis and energy expenditure by regulating C/EBP-beta expression. PLoS Genet. 2017, 13, e1006950. [Google Scholar] [CrossRef] [PubMed]
- Nasab, H.; Rajabi, S.; Eghbalian, M.; Malakootian, M.; Hashemi, M.; Mahmoudi-Moghaddam, H. Association of As, Pb, Cr, and Zn urinary heavy metals levels with predictive indicators of cardiovascular disease and obesity in children and adolescents. Chemosphere 2022, 294, 133664. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.C.; Chen, K.H.; Wang, P.H.; Hwang, J.L.; Seow, K.M. Endocannabinoid system activation may be associated with insulin resistance in women with polycystic ovary syndrome. Fertil. Steril. 2015, 104, 200–206. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Chen, L.-K.; Huang, T.-Y.; Yang, D.-M.; Liu, S.-Y.; Tsai, P.-J.; Chen, T.-H.; Lin, H.-F.; Juan, C.-C. Nitric Oxide Mobilizes Intracellular Zn2+ via the GC/cGMP/PKG Signaling Pathway and Stimulates Adipocyte Differentiation. Int. J. Mol. Sci. 2022, 23, 5488. https://doi.org/10.3390/ijms23105488
Chen C-W, Chen L-K, Huang T-Y, Yang D-M, Liu S-Y, Tsai P-J, Chen T-H, Lin H-F, Juan C-C. Nitric Oxide Mobilizes Intracellular Zn2+ via the GC/cGMP/PKG Signaling Pathway and Stimulates Adipocyte Differentiation. International Journal of Molecular Sciences. 2022; 23(10):5488. https://doi.org/10.3390/ijms23105488
Chicago/Turabian StyleChen, Chien-Wei, Luen-Kui Chen, Tai-Ying Huang, De-Ming Yang, Shui-Yu Liu, Pei-Jiun Tsai, Tien-Hua Chen, Heng-Fu Lin, and Chi-Chang Juan. 2022. "Nitric Oxide Mobilizes Intracellular Zn2+ via the GC/cGMP/PKG Signaling Pathway and Stimulates Adipocyte Differentiation" International Journal of Molecular Sciences 23, no. 10: 5488. https://doi.org/10.3390/ijms23105488
APA StyleChen, C.-W., Chen, L.-K., Huang, T.-Y., Yang, D.-M., Liu, S.-Y., Tsai, P.-J., Chen, T.-H., Lin, H.-F., & Juan, C.-C. (2022). Nitric Oxide Mobilizes Intracellular Zn2+ via the GC/cGMP/PKG Signaling Pathway and Stimulates Adipocyte Differentiation. International Journal of Molecular Sciences, 23(10), 5488. https://doi.org/10.3390/ijms23105488





