2. Results and Discussion
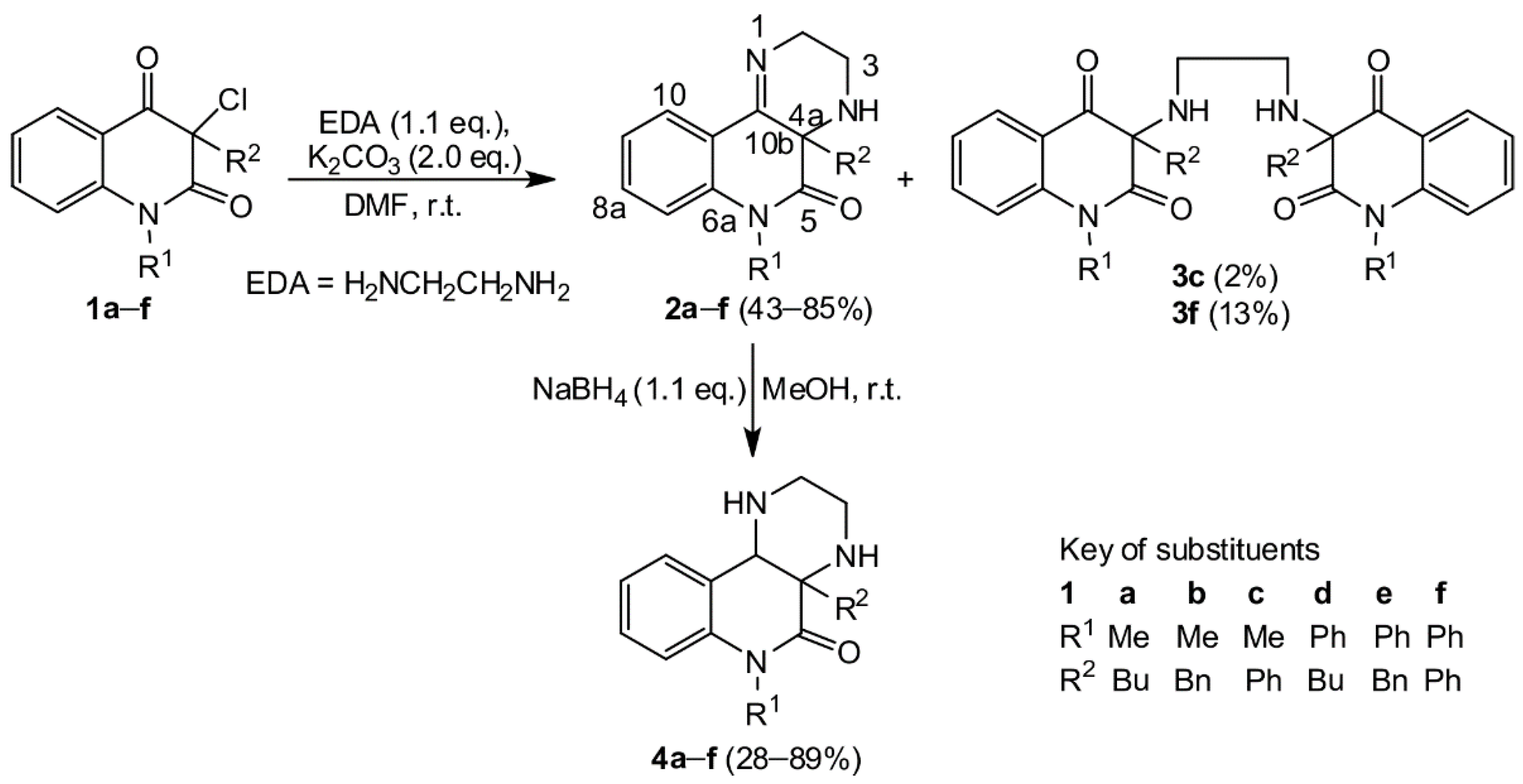
Our purpose was to study in detail the reaction connected with the isolation of a large quantity of minority compounds and to clarify the reaction mechanism. The reactions of 3-chloroquinolin-2,4-diones
1a–f with ethylene diamine were performed in DMF in the presence of powdered potassium carbonate. In a good yield, novel tricyclic pyrazino[2,3-
c]quinolin-5(6
H)-ones
2 were obtained (
Scheme 1). In just two cases, a small quantity of dimeric compounds
3c and
3f was produced via double alkylation of ethylene diamine with the chloroderivatives
1c and
1f. Their
1H and
13C NMR spectra exhibited two sets of signals according to the presence of two observable diastereoisomers. Reaction of compounds
2 with sodium borohydride confirmed the presence of the imine group and led to the expected dihydroderivatives
4 (
Scheme 1). Even though ethylene diamine is a strong base, we did not observe the formation of other compounds that would be products of a rearrangement analogous to rearrangement of 3-aminoquinolinediones. The NMR spectra and chemical shifts for the isolated compounds
2,
3, and
4 are presented in the
Supplementary Materials (see
Figures S1–S15 and Tables S1–S3, respectively). The reactions of compound
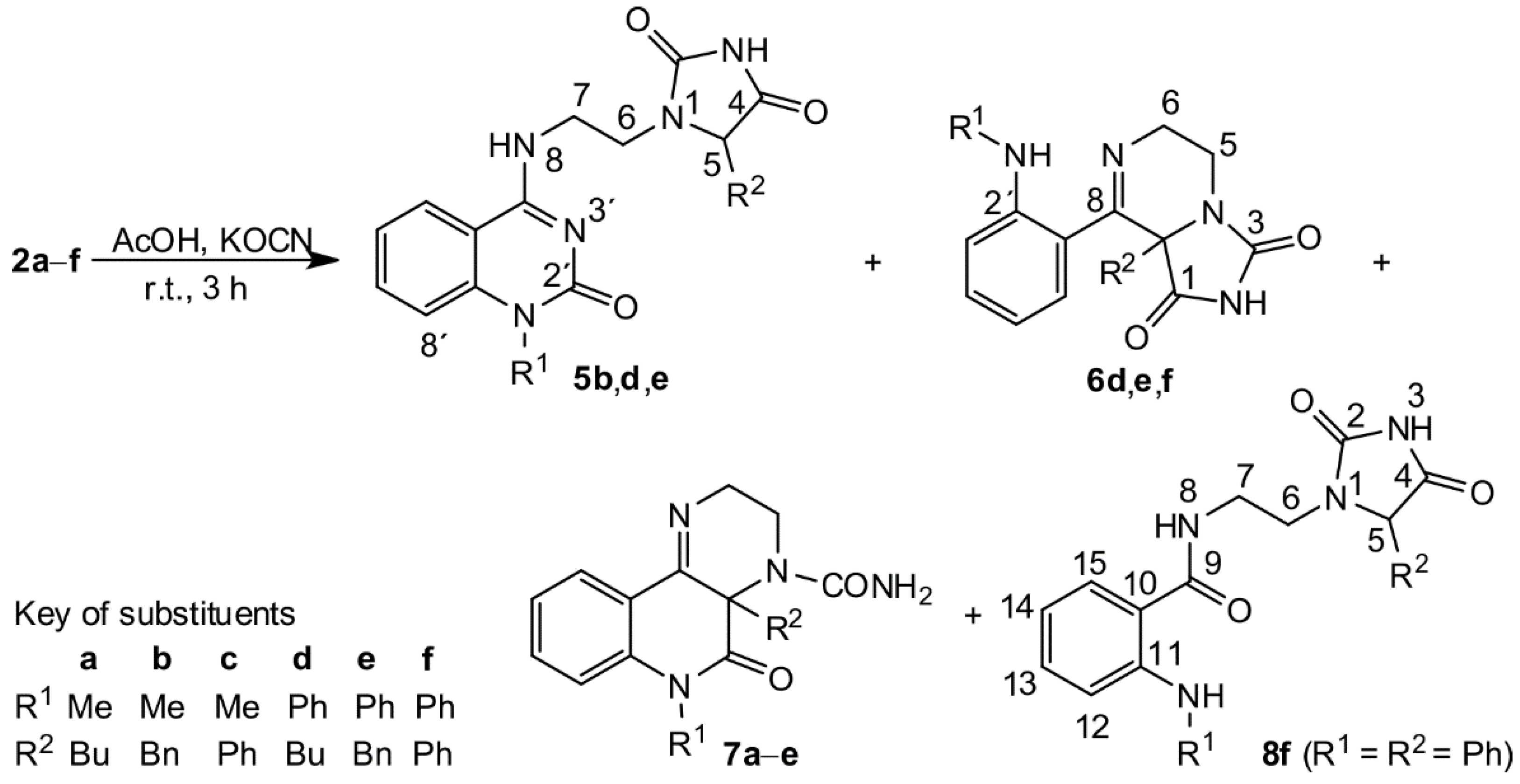
2 with potassium cyanate were carried out with a molar ratio 1:1.6 in a solution of acetic acid (
Scheme 2,
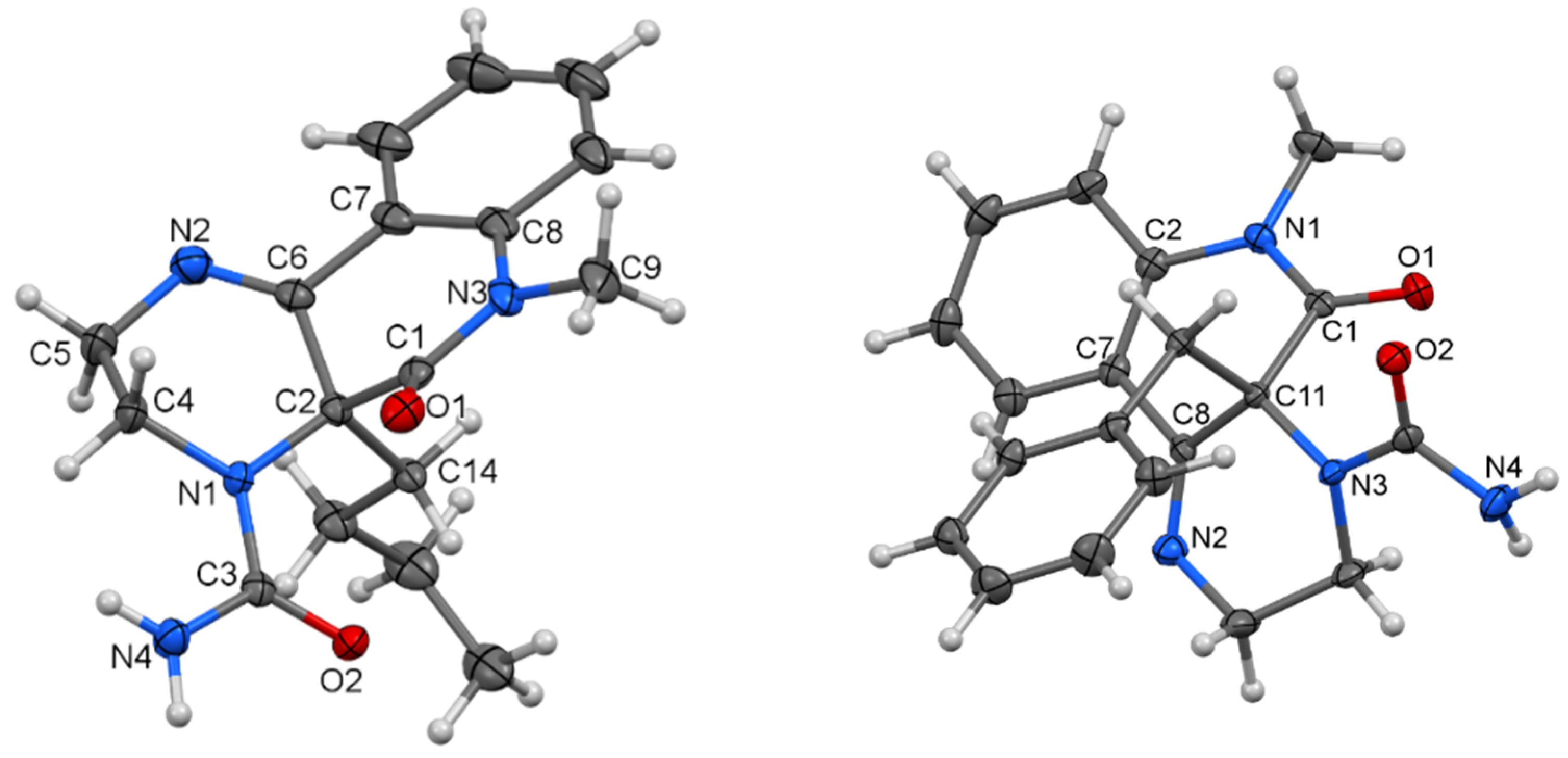
Table 1). Our first look at the IR and NMR spectra for the reaction products showed that at least three types of compounds were present. However, we were not able to determine the structure of the isolated compounds from their NMR spectra. Only a few isolated fragments were found, but it was impossible to determine how they were interconnected. Fortunately, after more unsuccessful experimentation, we managed to prepare a single crystal of the compound acquired from compound
2d. The structure of this compound (
5d) was established by X-ray diffraction analysis (
Figure 1). Although the structures of imidazolidine-2,4-dione (also a part of
5d skeleton) derivatives had been described crystallographically more than 170 times, derivatives with a longer hydrocarbon chain are absent from the literature. Moreover, the second part of the
5d molecule, a 1,2-dihydroquinazolin-2-one fragment, is scarcely reported [
31,
32].
In
5d (
Figure 1), the planes of the imidazolidine-2,4-dione and 1,2-dihydroquinazolin-2-one parts, which are separated by an iminoethane bridge, exhibit an interplanar angle of 26.16(9)°. The two molecules are interconnected by C=O···H–N bridges (see
Supplementary Materials, Figure S29).
The structure of
5d is surprising because its creation requires the scission of the C(2)–C(3) bond in the starting compound
2d. We did not observe such a reaction at any time. The transformation of quinolinedione to a quinazolinedione skeleton was previously observed only in cases where the starting compound was N-unsubstituted, allowing the formation of a useful isocyanate intermediate [
23].
Compound
5d consists of two bioactive moieties: 4-iminoquinazolin-2-one and substituted hydantoin. Several methods for the preparation of closely related quinazolin-4-ones [
33] and quinazoline-2,4-diones [
34] were recently described; however, none of them are remotely similar to the presented transformation. It must be pointed out that the reaction of compounds
2 with HNCO was carried out with a molar ratio 1:1.6 because we did not anticipate initially the reaction of compound
2 with more than one mole of isocyanic acid. Therefore, complete conversion of compounds
2 to
5 cannot be expected, but rather, only the formation of a mixture of products can proceed (
Table 1). Using an excess of KNCO, the composition of the reaction products changed (
Table 1), but at no time was the full conversion of
2 to
5 achieved.
Compounds
5b and
5e belong to the group of compounds produced by the reaction of
2 with two equivalents of HNCO that exhibited an absorption band at ca. 1770 cm
−1 in the IR spectrum characteristic of hydantoins [
35]. All their NMR data (see
Supplementary Materials,
Table S4 and Figures S16 and S18, respectively) are in the agreement with the proposed structure.
In addition to compound
5d, the next product was obtained from compound
2d. From ESI-MS and elemental analysis, it was determined that only one mole of HNCO was consumed. Its IR spectrum exhibited an absorption band at 1776 cm
–1, indicative of the presence of a hydantoin ring [
14], and a singlet at 11.2 ppm appeared in the
1H NMR spectrum pertaining to a NH proton in position 2 of the hydantoin moiety [
36]. The fragment Ar―NH―Ph was also found, which bears witness to the opening of the quinolinone ring in
2d.
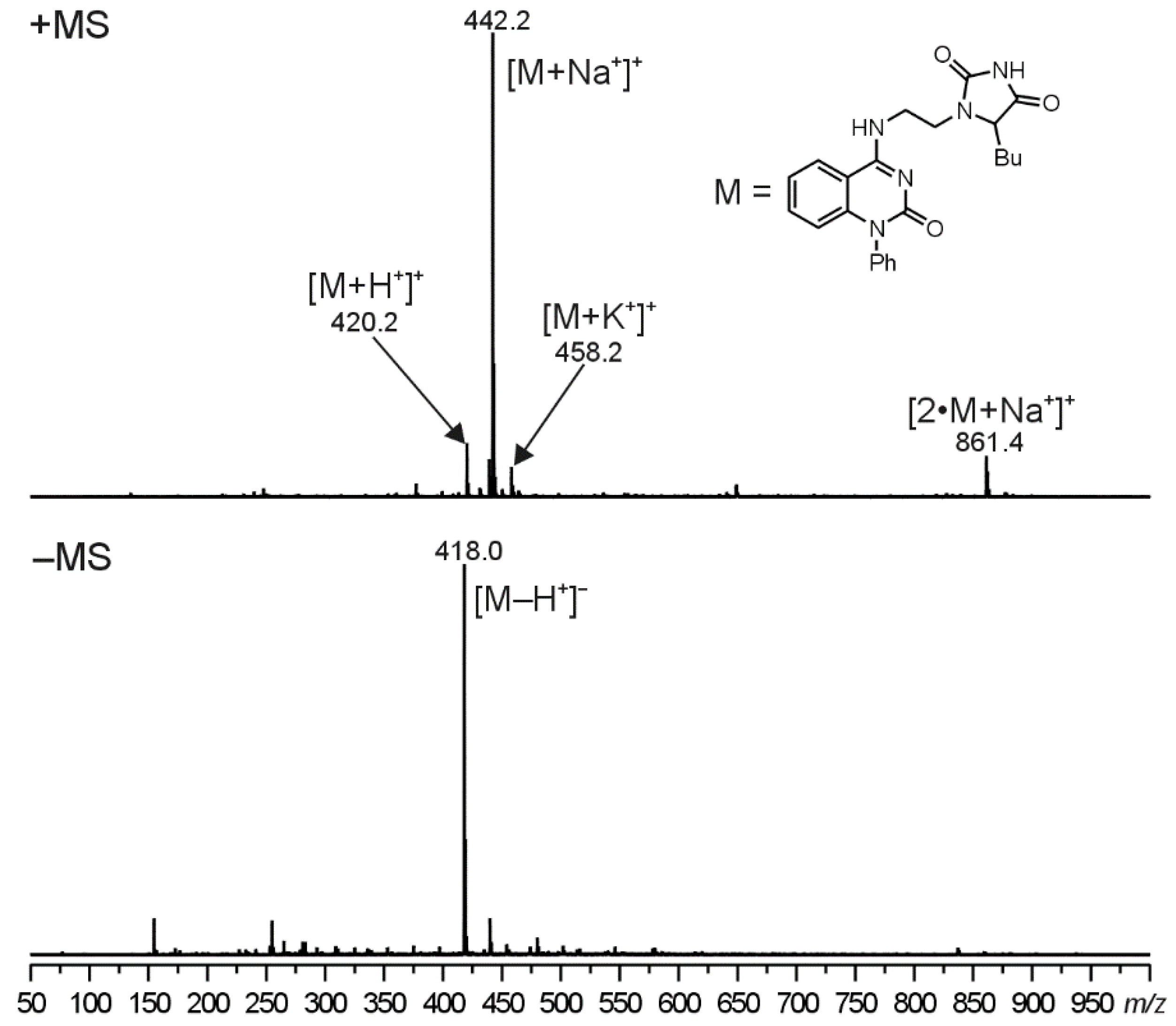
The molecular structure of compounds
5 were proved using ESI-MS/MS analyses. In the positive-ion first-order mass spectra, four singly charged ions were observed. The most abundant ion, assigned as a sodium adduct of the molecule ([M+Na
+]
+), was accompanied by two less intense signals at
m/
z corresponding to a protonated molecule ([M+H
+]
+) and a potassium adduct of the molecule ([M+K
+]
+). Moreover, a sodium adduct of the dimer ([2 M+Na
+]
+) was observed in the case of compounds
5. In the negative polarity mode, an ion assigned as a deprotonated molecule ([M+Na
+]
+) was formed. Illustrative ESI mass spectra for compound
5d can be seen in
Figure 2 (ESI-MS spectra for compounds
5b and
5e are given in the
Supplementary Materials, Figures S47 and S48, respectively).
Compound
6d represents the second structural group of products produced from the reaction of
2 with only one mole of isocyanic acid that exhibited an IR absorption band at
ca 1760 cm
–1. Compounds
6e and
6f also pertain to this group. All these compounds display an absorption band at
ca 1760 cm
–1 in the IR spectrum and a broad signal at
ca 11.1 ppm in their
1H NMR spectra. In their
13C NMR spectra (see
Supplementary Materials, Table S5 and Figures S19–S21, respectively), quaternary carbons signals appeared at
ca 68.9 ppm and, in their
15N NMR spectra, a signal adherent to the C=N group can be seen, much like that for the starting compound
2. Four nitrogen atoms were present in forenamed compounds. One belonged to a C=N group, the second was imidic, and the third pertained to a tertiary amino group. Therefore, the fourth nitrogen atom, which exhibited a singlet at
ca 8 ppm in its
1H NMR spectrum, must be part of Ar―NH―R
1 grouping. In both positive and negative ion ESI-MS spectra for compounds
6, the most abundant signal was observed at
m/z corresponding to a (de)protonated molecule (see
Supplementary Materials, Figures S49–S51).
The third product of the reaction of
2d with HCNO was a compound that did not have any IR absorption around 1760 cm
–1 and, therefore, did not contain a hydantoin ring. Its
1H and
13C NMR spectra (see
Supplementary Materials, Figure S25) were similar to
2d, but the presence of a CONH
2 group in the results suggest the structure of
7d. The reaction of this compound with an excess of HNCO (
Table 1) provided compound
5d, indicating that
7d is the first intermediate in the molecular rearrangement of
2d. It was found that compounds
7 resulted from all compounds
2 except
2f.
The molecular structure of compounds
7a (
Figure 3, left) and
7b (
Figure 3, right) were proven by X-ray analysis. The structures of
7a and
7b are characterized by the presence of substituted tricyclic systems where the π―electron conjugation is interrupted by the presence of a stereogenic center at C-2 (
7a) and or C-11 (
7b) as well as an ethylene bridge. The constitution of the tricyclic system in
7a is totally unknown. On the other hand, the characteristic interatomic distances and angles in both compounds that crystallize in achiral space groups
P2
1/
c and
P-1, respectively, are essentially the same as previously known structures with the same type of functional groups and atom hybridization [
37,
38].
Three molecules of
7a co-crystallize with two molecules of water to form an extensive system of H-bridges. In
7b, both optical isomers are interconnected by an NH···O=C bridging motif. Co-crystallized dichloromethane molecules occupy tunnels formed by the aromatic rings of the molecule. All the geometric parameters for all X-rayed structures are given in the
Supplementary Materials (Figures S28–S33, Tables S8–S16). Compounds
7c and
7d exhibited anomalous behavior in the form of very broad signals when their NMR spectra were measured in DMSO-
d6. Therefore, they were measured in CDCl
3.
As in the case of the above-mentioned compounds, the structures of compounds
7 were confirmed using mass spectrometry. Except commonly observed ions, such as protonated molecules, sodium and potassium adducts of the molecule, and/or sodium adducts of the dimer, we observed a singly charged signal in the positive-ion first-order ESI mass spectra that was assigned as a [M+H
+–HCNO]
+ ion. Its presence can be explained, according to tandem mass spectrometry experiments, as a result of in-source fragmentation. ESI mass spectra for compounds
7 are given in the
Supplementary Materials (see
Figures S52–S56).
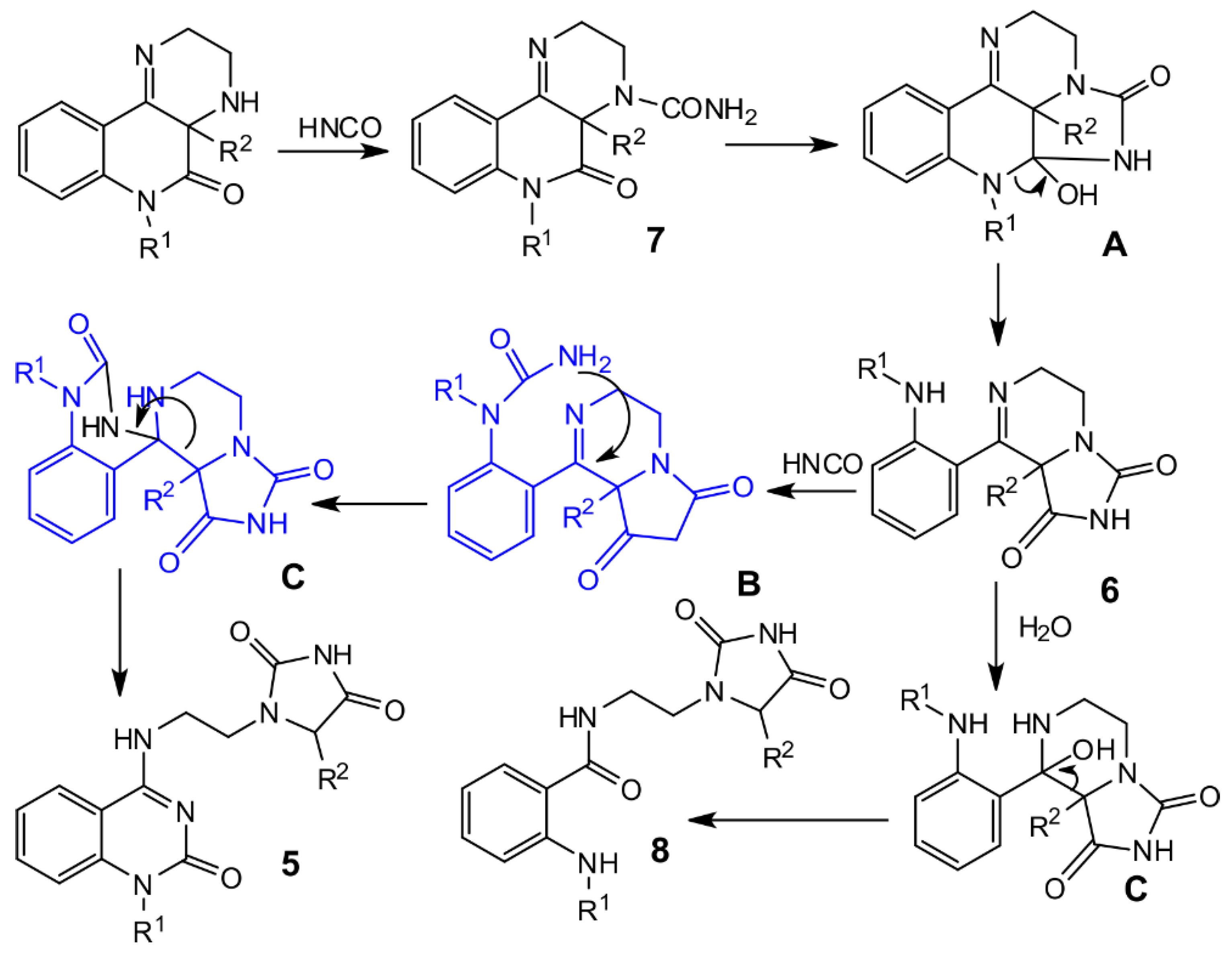
Compound
7 is primarily the product of the reaction between compound
2 and isocyanic acid, and therefore provides the starting compounds for the following molecular rearrangement to compounds
5 and
6. Our proposal for the reaction mechanism for rearrangement of compounds
2 is illustrated in
Scheme 3. We suppose that addition of compound
2 to isocyanic acid produces compound
7, which is subsequently changed to compound
6 via the intermediate
A. The reaction of compound
6 with isocyanic acid affords the intermediate
B, which undergoes retro-Claisen condensation for the formation of compounds
5.
One of the isolated products, prepared from
2f, was different from the compounds mentioned above. The fragment NCH
2CH
2N was present, but the compound did not contain the C=N group, and instead of a quaternary carbon atom, it contained a CHR group. The presence of an IR band at 1775 cm
–1 in the IR spectrum and 11.2 ppm in the
1H NMR spectrum indicated that the hydantoin ring must be present. In the molecule that pointed to the structure
8f, the amide group was found (see
Supplementary Materials, Table S7 and Figure S27). Not only IR and NMR, but also mass spectrometry provided clear evidence for the structure of compound
8f. Results for its ESI-MS analysis are given in
Supplementary Materials (see
Figure S57). The origin of this compound can be explained by the addition of water to compound
6f and following retro-Claisen condensation through intermediates
B and
C (
Scheme 3).
As mentioned in the introduction, some compounds bearing a quinoline or hydantoin moiety are known to possess a wide range of biological activities. However, there are only few examples of compounds possessing both of the above-mentioned structural motifs. For example, Kumar and co-workers published the synthesis of new series of 7-chloroquinoline-thiohydantoin derivatives with potent antimalarial activity [
39]. Quinoline and hydantoin derivatives are well-known for their anticancer activity, as recently described in several comprehensive reviews [
28,
40]. According to this fact, we decided to test the antiproliferative activity of compounds
5,
6, and
7 on two types of human tumor cell lines (K-562, chronic myelogenous leukemia and MV4;11, acute myelogenous leukemia). Moreover, the inhibitory potency of these compounds was assayed on two types of protein kinases, namely the recombinant heterodimeric complex CDK2/cyclin E and tyrosine-protein kinase ABL1. Unfortunately, no biological activity was observed for concentrations up to 10 µM.
3. Materials and Methods
3.1. General Data
Melting points were determined with a Kofler block. IR (KBr) spectra were recorded with a Smart OMNI-Transmission Nicolet iS10 spectrophotometer. The
1H,
13C, and
15N NMR spectra were recorded with a Bruker Avance III HD 500 spectrometer (500.13 MHz for
1H, 125.76 MHz for
13C, and 50.68 MHz for
15N) in DMSO-
d6.
1H and
13C chemical shifts are given on the
δ scale (ppm) and are referenced to internal TMS (
δ = 0.0).
15N chemical shifts were referred to external neat CH
3NO
2 in a co-axial capillary (
δ = 0.0). All 2D experiments (gradient-selected (gs)-COSY, gs-TOCSY, gs-HMQC, gs-HMQC-RELAY, gs-HMBC) were performed using the manufacturer’s software. Full-sets of diffraction data for
5d (yellow) and
7a and
7b (colorless) were collected at 150(2)K with a D8-Venture diffractometer (Bruker, Germany) equipped with Cu (Cu/K
α radiation; λ = 1.54178 Å) or Mo (Mo/K
α radiation; λ = 0.71073 Å) microfocus X-ray (IµS) sources, CMOS photon detector, and an Oxford Cryosystems cooling device was used for data collection. Experimental details are stated in Supporting Information. The frames were integrated with the Bruker SAINT Software package using a narrow-frame algorithm. Data were corrected for absorption effects using the Multi-Scan method (SADABS). The obtained data were treated by XT-version 2018/1 and SHELXL-2017/1 software implemented in an APEX3 v2016.5-0 (Bruker AXS) system [
41]. The positive-ion EI mass spectra were measured on a QP-2010 instrument (Shimadzu, Japan) within the mass range
m/
z = 50–600 using a direct inlet probe (DI). Samples were dissolved in dichloromethane (30 μg·mL
–1) and 10 μL of the solution was evaporated in a DI cuvette at 50 °C. The ion source temperature was 200 °C; the energy of electrons was 70 eV. Only signals exceeding a relative abundance of 5% are listed. The electrospray mass spectra (ESI-MS) were recorded using an amaZon X ion-trap mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with an electrospray ion source. All experiments were conducted in both positive and negative polarity mode. Individual samples (with a concentration of 500 ng·mL
–1) were infused into the ESI source as methanol/water (1/1,
v/
v) solutions via a syringe pump with a constant flow rate of 3 μL·min
–1. The other instrumental conditions were as follows:
m/
z range 50–1500, electrospray voltage of –4.2 kV (4.2 kV in negative polarity mode), capillary exit voltage of 140 V (–140 V in negative polarity mode), drying gas temperature of 220 °C, drying gas flow of 6.0 dm
3·min
–1, nebulizer pressure of 55.16 kPa. Nitrogen was used as the nebulizing and drying gases for all experiments. Tandem mass spectra were collected using collision-induced dissociation (CID) with He as the collision gas after isolating the required ions. Column chromatography was carried out on silica gel (Merck, grade 60, 70–230 mesh) using successive mixtures of chloroform/ethanol (in ratios from 99:1 to 8:2) (S1) or benzene/ethyl acetate (in ratios from 99:1 to 8:2) (S2). Reactions, the course of separation, and the purity of substances were monitored by TLC (elution systems: benzene/ethyl acetate (4:1) (S3), chloroform/ethanol (9:1 and 1:1) (S4 and S5), and chloroform/ethyl acetate (7:3) (S6)) on Alugram
® SIL G/UV
254 foils (Macherey-Nagel, Germany). Elemental analyses (C, H, N) were performed with an EA Flash EA 1112 Elemental Analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
3.2. General Procedure for the Reaction of Compounds 1 with Ethylene Diamine
To the solution of compound 1 (1 mmol) in DMF (9 mL), pulverized potassium carbonate (276 mg, 2 mmol) and ethylene diamine (EDA) (0.1 mL, 1.1 mmol) were added and the mixture was stirred at room temperature. The course of the reaction was monitored with TLC. After the spot corresponding to compound 1 faded away, the reaction mixture was diluted with water (20 mL). The deposited product was filtered with suction, dried and crystallized with an appropriate solvent. In cases where the crude product was oily or waxy, the solution was extracted with chloroform (3 × 20 mL). The collected extracts were dried, evaporated to dryness, and the residue was separated by chromatography on a silica gel column.
4a-Butyl-6-methyl-2,3,4,4a-tetrahydro-pyrazino[2,3-c]quinolin-5(6H)-one (2a)
Compound was prepared from 1a and EDA with 53% yield, reaction time 6 h. White solid, mp 111–113 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.2/3.82 and 3.49, C(3)H2 39.2/2.90 and 2.64, C(4a) 63.2, C(5) 171.7, C(6a) 139.1, C(7)H 114.9/7.21, C(8)H 131.4/7.49, C(9)H 123.3/7.18, C(10)H 125.1/7.65, C(10a) 123.9, C(10b) 162.7, C(1’(R1))H3 30.1/3.33, C(1’(R2))H2 39.0/1.45 and 1.31, C(2’(R2))H2 25.4/1.31 and 1.05, C(3’(R2))H2 22.0/1.05, C(4’(R2))H3 13.8/0.69 ppm. IR (cm–1) ν: 3346, 3042, 2959, 2936, 2872, 2825, 1676, 1645, 1602, 1462, 1431, 1410, 1367, 1347, 1315, 1297, 1279, 1265, 1229, 1192, 1125, 1109, 1056, 1044, 992, 961, 946, 840, 759, 740, 680, 654,627, 579, 532. ESI-MS (pos.) m/z (%): 565.2 [2·M + Na+]+ (7), 294.1 [M + K+]+ (19), 272.1 [M + Na+]+ (100), 216.0 [M + H+]+ (11). Anal. Calcd for C16H21N3O (271.36): C 70.82; H 7.80; N 15.49. Found: C 70.55; H 8.00; N 15.40.
6-Methyl-4a-benzyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2b)
Compound was prepared from 1b and EDA with 43% yield. Colorless solid, mp 102–106 °C (benzene/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.8/3.64 and 3.02, C(3)H2 38.8/2.88 and 2.45, C(4a) 64.1, C(5) 170.8, C(6a) 139.1, C(7)H 115.1/7.0,2 C(8)H 131.6/7.24, C(9)H 123.3/7.19, C(10)H 125.3/7.64, C(10a) 123.8, C(10b) 161.7, C(1’(R1))H3 30.1/3.35, C(1’(R2))H2 44.9/2.75 and 2.68, C(2’(R2)) 135.2, C(3’(R2))H 130.4/6.94, C(4’(R2))H 127.4/7.16, C(5’(R2))H 126.8/7.19 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −52.7, N(4) −351.7, N(6) −257.6 ppm. IR (cm–1) ν: 3329, 3066, 3028, 2942, 2905, 2838, 1668, 1633, 1603, 1497, 1472, 1455, 1438, 1416, 1357, 1339, 1298, 1270, 1226, 1202, 1159, 1129, 1076, 1062, 1047, 1010, 958, 903, 763, 699, 656, 620, 535, 504. ESI-MS (pos.) m/z (%): 633.2 [2·M + Na+]+ (6), 328.0 [M + Na+]+ (20), 306.0 [M + H+]+ (100), 214.9 [M + H+ − C7H7]+ (10). Anal. Calcd for C19H19N3O (305.37): C 74.73; H 6.27; N 13.76. Found: C 74.63; H 6.40; N 13.84.
6-Methyl-4a-phenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2c)
Compound was prepared from 1c with 54% yield beside 3c. White solid, mp 178–182 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.9/3.96 and 3.81, C(3)H2 37.8/2.71 and 2.65, C(4a) 65.6, C(5) 169.2, C(6a) 138.8, C(7)H 115.1/7.12, C(8)H 131.5/7.43, C(9)H 123.4/7.14, C(10)H 125.1/7.81, C(10a) 124.2, C(10b) 160.6, C(1’(R1))H3 30.1/3.39, C(1’(R2)) 139.9, C(2’(R2))H 126.9/7.18, C(3’(R2)) 128.5/7.29, C(4’(R2))H 127.9/7.24 ppm. IR (cm–1) ν: 3358, 2933, 2838, 1671, 1638, 1602, 1469, 1446, 1417, 1362, 1297, 1150, 1124, 1080, 991, 896, 845, 763, 744, 706, 692, 661, 625, 569, 536, 506. EI-MS m/z (%): 292 (21), 291 (M+, 100), 290 (20), 262 (23), 261 (47), 160 (12), 132 (14), 131 (20), 104 (18), 77 (16). ESI-MS (pos.) m/z (%): 605.2 [2·M + Na+]+ (42), 583.2 [2·M + H+]+ (16), 314.1 [M + Na+]+ (18), 292.1 [M + H+]+ (100). Anal. Calcd for C18H17N3O (291.35): C 74.20; H 5.88; N 14.42. Found: C 73.99; H 5.98; N 14.24.
4a-Butyl-6-phenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2d)
Compound was prepared from 1d and EDA with 85% yield. Colorless solid, mp 86–90 °C (hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.2/3.82 and 3.49, C(3)H2 39.2/2.90 and 2.64, C(4a) 63.2, C(5) 171.7, C(6a) 139.1, C(7)H 114.9/7.21, C(8)H 131.4/7.49, C(9)H 123.3/7.18, C(10)H 125.1/7.65, C(10a) 123.9, C(10b) 162.7, C(1’(R1)) 139.1, C(2’(R1))H overlap/7.25, C(3’(R1))H 130.2/7.57, C(4’(R1))H 128.7/7.50, C(1’(R2))H2 39.0/1.73 and 1.59, C(2’(R2))H2 25.4/1.38 and 1.15, C(3’(R2))H2 22.0/1.15, C(4’(R2))H3 13.9/0.77 ppm. IR (cm–1) ν: 3448, 3330, 2954, 2868, 1677, 1642, 1604, 1492, 1456, 1348, 1332, 1314, 1293, 1261, 1222, 1177, 1113, 1072, 999, 770, 697, 682, 656, 648, 610, 491. EI-MS: m/z (%) 334 (6), 333 (24), 291 (6), 290 (25), 277 (21), 276 (100), 275 (7), 262 (6), 77 (7), 57 (5). ESI-MS (pos.) m/z (%): 356.1 [M + Na+]+ (5), 334.2 [M + H+]+ (100), 278.1 [M + H+ – C4H8]+ (3). Anal. Calcd for C21H23N3O (333.43): C 75.65; H 6.95; N 12.60. Found: C 75.82; H 7.14; N 12.55.
4a-Benzyl-6-phenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2e)
Compound was prepared from 1e and EDA with 51% yield, reaction time 3 h. White solid, mp 161–164 °C (benzene/cyclohexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.8/3.73 and 3.10, C(3)H2 38.6/2.95 and 2.56, C(4a) 64.4, C(5) 170.9, C(6a) 140.0, C(7)H 116.2/6.34, C(8)H 131.2/7.35, C(9)H 123.4/7.13, C810)H 125.6/7.73, C(10a) 123.4, C(10b) 161.5, C(1’(R1)) 137.9, C(2’(R1))H overlapped signals, C(3’(R1))H 130.1/7.52, C(4’(R1))H 128.7/7.51, C(1’(R2))H2 44.7/3.06 and 2.91, C(2’(R2)) 135.2, C(3’(R2))H 130.6/7.10, C(4’(R2))H 127.5/7.22, C(5’(R2))H 126.9/7.22 ppm. IR (cm–1) ν: 3354, 2925, 2897, 2836, 1690, 1638, 1600, 1492, 1460, 1351, 1315, 1275, 1214, 1164, 1073, 1003, 959, 877, 782, 766, 741, 702, 657, 632, 598. EI-MS: m/z (%): 367 (14), 277 (21), 276 (100), 91 (8), 77 (5). ESI-MS (pos.) m/z (%): 757.3 [2·M + Na+]+ (5), 390.1 [M + Na+]+ (21), 368.1 [M + H+]+ (100), 277.0 [M + H+ – C7H7]+ (5). Anal. Calcd for C24H21N3O (367.44): C 78.45; H 5.76; N 11.44. Found: C 78.41; H 5.88; N 11.43.
4a,6-Diphenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2f)
Compound was prepared from 1f and EDA with 66% yield. Colorless solid, mp 156–160 °C (hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.9/3.97 and 3.94, C(3)H2 37.5/2.72 and 2.60, C(4a) 66.1, C(5) 169.4, C(6a) 139.7, C(7)H 116.9/6.09, C(8)H 131.2/7.16, C(9)H 123.5/7.08, C(10)H 125.5/7.83, C(10a) 123.7, C(10b) 160.2, C(1’(R1)) 137.8, C(2’(R1))H overlapped signals, C(3’(R1))H 130.3/7.56, C(4’(R1))H 129.5/7.52, C(1’(R2)) 139.4, C(2’(R2))H 127.0/7.26, C(3’(R2))H 129.5/7.35 C(4’(R2))H 128.7/7.28 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −53.0, N(4) −339.3, N(6) −234.3 ppm. IR (cm–1) ν: 3442, 2908, 2820, 1681, 1638, 1602, 1493, 1458, 1422, 1355, 1335, 1261, 1261, 1160, 1109, 995, 936, 893, 758, 740, 719, 700, 663, 627, 574, 516. EI-MS: m/z (%): 354 (26), 353 (100), 352 (16), 338 (6), 324 (13), 323 (26), 296 (5), 250 (7), 249 (5), 248 (5), 222 (7), 221 (9), 194 (14), 193 (6), 149 (6), 131 (8), 104 (11), 103 (6), 77 (17), 71 (5), 66 (5), 57 (9), 55 (6), 51 (7), 43 (10). ESI-MS (pos.) m/z (%): 354.1 [M + H+]+ (100). Anal. Calcd for C23H19N3O (353.42): C 78.16; H 5.42; N 11.89: Found: C 78.06; H 5.50; N 11.88.
3,3’-(Ethane-1,2-diyl)bis(azanediyl)bis(1-methyl-3-phenylquinoline)-2,4(1H,3H)-dione (3c)
Compound was prepared from 1c with 2% yield beside 2c. Yellowish solid, mp 198–210 °C (benzene-hexane). 1H and 13C chemical shifts in DMSO-d6: C(2) 171.32 and 171.23, C(3) 76.98 and 76.96, C(4) 193.27 and 192.72, C(4a) 120.71 and 120.60, C(5)H 127.48 and 127.39/7.75, C(6)H 123.29 and 123.27/7.17, C(7)H 136.41 and 136.39/7.69, C(8)H 115.99 and 115.96/7.39, C(8a) 142.3, NH 2.60, CH2 45.38 and 45.17/2.59 and 2.48, C(1’(R1)) 139.63 and 139.43 C(1’(R2)) 137.78 and 137.44, other C/H signals exist as broadened overlapped signals resonating at 126.9–131.2/7.12–7.8 ppm. IR (cm–1) ν: 3333, 3064, 3033, 2946, 2854, 1703, 1666, 1602, 1472, 1417, 1354, 1303, 1254, 1185, 1114, 1034, 994, 911, 864, 764, 699, 684, 637, 600, 533, 495. ESI-MS (pos.) m/z (%): 581.2 [M + Na+]+ (44), 559.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 575.1 [M – H+ + H2O]– (100), 557.1 [M – H+]– (37). Anal. Calcd for C34H30N4O4 (558.63): C 73.10; H 5.41; N 10.03. Found: C 73.41; H 5.68; N 9.95.
3,3’-(Ethane-1,2-diyl)bis(azanediyl)bis(1,3-diphenylquinoline)-2,4(1H,3H)-dione (3f)
Compound was prepared from 1f with 13% yield. Colorless solid, mp 262–268 °C (benzene/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2) 171.33 and 171.26, C(3) 77.23 and 77.19, C(4) 193.10 and 192.64, C(4a) 120.50 and 120.44, C(6)H 123.48 and 123.40, C(7)H 135.93/7.46, C(8)H 116.59 and 116.55/6.32, C(8a) 142.3, N 2.60, CH2 45.33 and 45.17/2.58 and 2.51, C(1’(R1))H3 30.03 and 30.00/3.53 and 3.50, C(1’(R2)) 137.91 and 137.86, C(2’(R2))H 126.69 and 126.64/7.31, C(3’(R2))H 128.84 and 128.79/7.28 C(4’(R2))H 128.66 and 128.62/7.28 ppm. IR (cm–1) ν: 3442, 3063, 2927, 2858, 1707, 1673, 1600, 1492, 1461, 1337, 1303, 1249, 1192, 1173, 1158, 1113, 1072, 1031, 1002, 981, 902, 820, 762, 747, 719, 703, 650, 609, 576, 539, 516. ESI-MS (pos.) m/z (%): 1387.5 [2·M + Na+]+ (11), 1365.4 [2·M + H+]+ (6), 721.2 [M + K+]+ (7), 705.3 [M + Na+]+ (31), 683.3 [M + H+]+ (100) Anal. Calcd for C44H34N4O4 (682.77): C 77.40; H 5.02; N 8.21. Found: C 76.98; H 5.13; N 8.36.
3.3. General Procedure for the Reduction of Compounds 2 with NaBH4
To the solution of compound 2 (1.5 mmol) in methanol (20 mL), NaBH4 (67 mg, 1.7 mmol) was added over 5 min. The mixture was stirred for 1.5–3 h at room temperature and then poured onto 20 mL of crushed ice. Hydrochloric acid (35%, 0.28 mL) was added, and after 5 min, 5% NaHCO3. The alkaline reaction mixture was extracted with chloroform (3 × 25 mL), dried and evaporated to dryness. The residue was crystallized with an appropriate solvent.
4a-Butyl-6-methyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4a)
Compound was prepared from 2a with 28% yield. Colorless solid, mp 145–149 °C (hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.4/2.90 and 2.68, C(3)H2 39.9/2.65 and 2.61, C(4a) 56.9, C(5) 171.6, C(6a) 138.1, C(7)H 114.1/7.06, C(8)H 127.6/7.27, C(9)H 123.4/7.27, C(10)H 122.7/7.06, C(10a) 127.4, C(10b)H 58.2/3.87, C(1’(R1))H3 29.3/3.24, C(2’(R1))H 129.2/7.29, C(3’(R1))H 130.1/7.50, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 22.6/1.96 and 0.57, C(2’(R2))H2 24.2/1.14 and 0.86, C(3’(R2))H2 22.4/1.05, C(4’(R2))H3 14.0/0.73 ppm. IR (cm–1) ν: 3369, 3064, 3040, 2951, 2928, 2862, 2801, 1666, 1601, 1497, 1470, 1443, 1418, 1357, 1294, 1275, 1233, 1203, 1156, 1124, 1040, 983, 958, 887, 874, 847, 824, 758, 684, 632, 593, 548, 537. ESI-MS (pos.) m/z (%): 547.2 [2·M + H+]+ (7), 274.1 [M + H+]+ (100). Anal. Calcd for C16H23N3O (273.37): C 70.30; H 8.48; N 15.37. Found: C 70.53; H 8.34; N 15.23.
4a-Benzyl-6-methyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4b)
Compound was prepared from 2b with 30% yield. Colorless solid, mp 207–210 °C (ethanol). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.5/3.11 and 3.03, C(3)H2 40.1/2.77 and 2.71, C(4a) 58.6, C(5) 170.4, C(6a) 138.2, C(7)H 114.3/7.13, C(8)H 127.8/7.37, C(9)H 123.5/7.32, C(10)H 122.8/7.13, C(10a) 127.3, C(10b)H 58.4/3.98, C(1’(R1))H3 29.3/3.24, C(1’(R2))H2 29.7/3.21 and 2.04, C(2’(R2)) 136.7, C(3’(R2))H 129.7/6.80, C(4’(R2))H 127.9/7.15, C(5’(R2))H 126.1/7.15 ppm. IR (cm–1) ν: 3295, 3070, 3024, 2969, 2940, 2898, 2835, 2814, 2769, 2721, 1665, 1604, 1504, 1479, 1470, 1459, 1422, 1367, 1336, 1318, 1284, 1238, 1145, 1132, 1118, 1082, 1050, 991, 973, 862, 827, 764, 730, 702, 691, 662, 643, 504. ESI-MS (pos.) m/z (%): 637.2 [2·M + Na+]+ (4), 330.1 [M + Na+]+ (9), 308.1 [M + H+]+ (100). Anal. Calcd for C19H21N3O (307.39): C 74.69; H 6.89; N 13.69. Found: C 74.57; H 7.05; N 13.61.
6-Methyl-4a-phenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4c)
Compound was prepared from 2c with 89% yield. Colorless solid, mp 216–218 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 46.1/2.99 and 2.87, C(3)H2 40.4/2.60 and 2.32, C(4a) 59.7, C(5) 170.4, C(6a) 138.0, C(7)H 114.7/6.95, C(8)H 127.4/7.20, C(9)H 123.1/7.13, C(10)H 122.8/7.49, C(10a) 12714, C(10b)H 58.0/4.25, C(1’(R1))H3 29.8/3.23, C(1’(R2)) 137.4, C(2’(R2))H 129.1/7.460.86, C(3’(R2)) 127.3/7.05, C(4’(R2))H 126.7/7.05 ppm. IR (cm–1) ν: 3067, 2954, 2922, 2802, 1668, 1601, 1495, 1470, 1448, 1412, 1355, 1306, 1271, 1155, 1140, 1117, 1042, 981, 951, 816, 771, 756, 719, 705, 679, 694, 600, 543. ESI-MS (pos.) m/z (%): 587.2 [2·M + H+]+ (7), 316.0 [M + Na+]+ (8), 294.1 [M + H+]+ (100). Anal. Calcd for C18H19N3O (293.36): C 73.69; H 6.53; N 14.32. Found: C 73.60; H 6.70; N 14.32.
4a-Butyl-6-phenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4d)
Compound was prepared from 2d with 62% yield. Colorless solid, mp 120–124 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.4/2.96 and 2.74, C(3)H2 39.7/2.73 and 2.66, C(4a) 57.5, C(5) 171.8, C(6a) 138.6, C(7)H 115.5/6.15, C(8)H 127.4/7.07, C(9)H 123.9/7.04, C(10)H 123.0/7.44, C(10a) 127.1, C(10b)H 58.2/4.15, C(1’(R1)) 139.2, C(2’(R1))H 129.2/7.15, C(3’(R1))H 130.0/7.54, C(4’(R1))H 128.2/7.44, C(1’(R2))H2 22.7/2.08 and 0.89, C(2’(R2))H2 24.3/1.26 and 1.02, C(3’(R2))H2 22.5/1.18, C(4’(R2))H3 14.1/0.80 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −344.6, N(4) −354.2, N(6) −235.0 ppm. IR (cm–1) ν: 3251, 3206, 3064, 2958, 2932, 2872, 1709, 1666, 1604, 1494, 1461, 1405, 1379, 1353, 1300, 1266, 1201, 1158, 1141, 1105, 1048, 929, 872, 838, 757, 696, 667, 564. ESI-MS (pos.) m/z (%): 671.3 [2·M + H+]+ (11), 358.1 [M + Na+]+ (5), 336.1 [M + H+]+ (100). Anal. Calcd for C20H21N3O (335.40): C 71.62; H 6.31; N 12.53. Found: C 71.79; H 6.48; N 12.43.
4a-Benzyl-6-phenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4e)
Compound was prepared from 2e with 80% yield. Colorless solid, mp 182–184 °C (benzene/hexane) 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.5/3.08 and 2.82, C(3)H2 40.4/3.15 and 2.74, C(4a) 58.8, C(5) 170.4, C(6a) 138.5, C(7)H 116.1/6.29, C(8)H 127.5/7.14, C(9)H 123.8/7.14, C(10)H 123.1/7.44, C(10a) 127.4, C(10b)H 58.6/4.27, C(1’(R1)) 139.2, C(2’(R1))H 129.1/7.16, C(3’(R1))H 129.8/7.52, C(4’(R1))H 128.0/7.43, C(1’(R2))H2 29.8/3.36 and 2.27, C(2’(R2)) 136.7, C(3’(R2))H 129.9/7.04, C(4’(R2))H 128.1/7.26, C(5’(R2))H 126.2/7.16 ppm. IR (cm–1) ν: 3287, 3268, 3059, 3019, 2913, 2851, 1690, 1603, 1489, 1449, 1347, 1335, 1289, 1277, 1233, 1193, 1153, 1123, 1080, 1029, 897, 951, 899, 874, 755, 724, 695, 639, 595, 556. ESI-MS (pos.) m/z (%): 761.3 [2·M + Na+]+ (5), 739.3 [2·M + H+]+ (18), 392.1 [M + Na+]+ (10), 370.1 [M + H+]+ (100). Anal. Calcd for C24H23N3O (369.46): C 78.02; H 6.27; N 11.37. Found: C 77.97; H 6.25; N 11.28.
4a,6-Diphenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4f)
Compound was prepared from 2f with 80% yield. Colorless solid, mp 174–179 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.5/3.09 and 2.96, C(3)H2 40.1/2.70 and 2.43, C(4a) 60.3, C(5) 170.2, C(6a) 138.2, C(7)H 115.8/6.01, C(8)H 127.1/6.98, C(9)H 123.3/7.11, C(10)H 123.2/7.53, C(10a) 127.8, C(10b)H 57.5/4.55, C(1’(R1)) 138.2, C(2’(R1))H 128.7/7.08, C(3’(R1))H 129.9/7.52, C(4’(R1))H 128.2/7.43, C(1’(R2)) 136.9, C(2’(R2))H 129.3/7.64, C(3’(R2))H 127.5/7.16, C(4’(R2))H 127.1/7.16 ppm. IR (cm–1) ν: 3261, 2943, 2904, 2851, 1674, 1604, 1467, 1452, 1346, 1291, 1144, 1072, 772, 755, 717, 698, 648, 586, 550. ESI-MS (pos.) m/z (%): 356.2 [M + H+]+ (100). Anal. Calcd for C23H21N3O (355.43): C 77.72; H 5.96; N 11.82. Found: C 77.59; H 6.00; N 11.69.
3.4. General Procedure for the Reaction of Compounds 2 with Isocyanic Acid
To the solution of 2 (1.5 mmol) in acetic acid (4.5 mL), potassium cyanate (0.195 g, 2.4 mmol) was added, and the mixture was stirred for 3 h at room temperature. The mixture was poured onto crushed ice (20 mL) and extracted with chloroform (5 × 15 mL). The collected extracts were dried and evaporated to dryness. The residue was chromatographed on a silica gel column.
Compound was prepared from 2b with 18% yield beside 7b. Colorless solid, mp 209–215 °C (ethyl acetate). 1H and 13C chemical shifts in DMSO-d6: C(2) 156.5, C(4) 173.8, C(5)H 60.6/4.55, C(6)H2 38.7/3.61, C(7)H2 38.1/3.85 and 3.17, C(2’) 155.2, C(4’) 160.0, C(4a’) 109.8, C(5’)H 123.7/7.95, C(6’)H 121.0/7.20, C(7’)H 133.9/7.67, C(8’)H 114.4/7.34, C(8a’) 142.8, C(1’(R1))H3 30.0/3.43, C(2’(R1))H3 30.0/3.43 C(3’(R1))H3 30.0/3.43 C(4’(R1))H3, 30.0/3.43, C(1’(R2))H2 33.4/3.14 and 3.01, C(2’(R2)) 135.4, C(3’(R2))H 128.2/7.10, C(4’(R2))H 129.4/7.20, C(5’(R2))H 126.7/7.20 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −286.8, N(3)H n.o./10.53 1J(15N, 1H) 95.2 Hz, N(8)H −286.8/8.37 1J(15N, 1H) 88.5 Hz, N(1’) −262.9, N(3’) −171.2 ppm. IR (cm–1) ν: 3400, 3129, 3030, 2939, 2746, 1761, 1709, 1619, 1597, 1565, 1543, 1497, 1456, 1419, 1352, 1329, 1263, 1234, 1173, 1138, 1127, 1095, 1036, 1005, 946, 872, 851, 768, 749, 702, 681, 650, 621, 594, 535. ESI-MS (pos.) m/z (%): 805.3 [2·M + Na+]+ (8), 430.1 [M + K+]+ (5), 414.1 [M + Na+]+ (100), 392.1 [M + H+]+ (22). ESI-MS (neg.) m/z (%): 390.0 [M – H+]– (100). Anal. Calcd for C21H21N5O3 (391.16): C 64.44; H 5.41; N 17.89. Found: C 64.63; H 5.70; N 17.89.
Compound was prepared from 2d in 17% yield beside 6d and 7d. Yellowish solid, mp 247–250 °C (benzene/cyclohexane). 1H and 13C chemical shifts in DMSO-d6: C(2) 156.9, C(4) 174.6, C(5)H 59.9/4.26, C(6)H2 38.8/3.67, C(7)H2 38.6/3.82 and 3.24, C(2’) 154.6, C(4’) 160.6, C(4a’) 109.4, C(5’)H 123.7/8.04, C(6’)H 121.4/7.19, C(7’)H 133.6/7.45, C(8’)H 115.0/6.40, C(8a’) 147.7, C(1’(R1)) 138.2, C(2’(R1))H 128.3/7.26 C(3’(R1))H 129.9/7.58, C(4’(R1))H 128.3/7.49, C(1’(R2))H2 27.4/1.76 and 1.73, C(2’(R2))H2 135.4, C(3’(R2)) H2 21.9/1.20, C(4’(R2)) H3 13.8/0.79 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −286.4, N(3)H n.o./10.78 1J(15N, 1H) 90.8 Hz, N(8)H −285.0/8.58 1J(15N, 1H) 92.4 Hz, N(1’) −241.2, N(3’) −171.4 ppm. IR (cm–1) ν: 3335, 3063, 2956, 2871, 1771, 1703, 1640, 1599, 1565, 1537, 1492, 1453, 1418, 1390, 1354, 1327, 1263, 1230, 1183, 1156, 1138, 1113, 1086, 1070, 973, 877, 813, 764, 748, 704, 675, 654, 613, 562, 547, 510. ESI-MS (pos.) m/z (%): 861.4 [2·M + Na+]+ (8), 458.2 [M + K+]+ (6), 442.2 [M + Na+]+ (100), 420.2 [M + H+]+ (11). ESI-MS (neg.) m/z (%): 418.0 [M – H+]– (100). Anal. Calcd for C23H25N5O3 (419.48): C 65.85; H 6.01; N 16.70. Found: C 65.74; H 6.07; N 16.57. Using the excess of KOCN (3 equiv.), 17% of 5d, 8% of 6d and 31% of 7d was obtained. Using the excess of KOCN (3 equiv.), compound 5d was prepared in 57% yield from 6d and in 33% yield from 7d.
Compound was prepared from 2e with 22% yield beside 6e and 7e. Colorless solid, mp 181–192 °C (ethyl acetate). After recrystallization from ethanol, the mp increased to 280–283 °C without any change in its IR spectrum. 1H and 13C chemical shifts in DMSO-d6: C(2) 156.6, C(4) 173.8, C(5)H 60.6/4.60, C(6)H2 38.8/3.69, C(7)H2 38.2/3.82 and 3.20, C(2’) 154.5, C(4’) 160.6, C(4a’) 109.4, C(5’)H 123.6/8.02, C(6’)H 121.3/7.21, C(7’)H 133.5/7.49, C(8’)H 115.0/6.41, C(8a’) 143.7, C(1’(R1)) 138.2, C(2’(R1))H 128.2/7.24, C(3’(R1))H 129.9/7.59, C(4’(R1))H 128.2/7.50, C(1’(R2))H2 30.9/3.20 and 3.05, C(2’(R2)) 135.4, C(3’(R2))H 128.2/7.14, C(4’(R2))H 129.4/7.28, C(5’(R2))H 126.7/7.22 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −287.6, N(3)H −232.2/10.58 1J(15N, 1H) 94.6 Hz, N(8)H −285.0/8.56 1J(15N, 1H) 92.7 Hz, N(1’) −241.1, N(3’) −171.7 ppm. IR (cm–1) ν: 3331, 3065, 2936, 1770, 1712, 1653, 1641, 1615, 1600, 1538, 1488, 1454, 1423, 1355, 1330, 1225, 1184, 1156, 1131, 1084, 1030, 775, 753, 707, 675, 622, 541, 509. ESI-MS (pos.) m/z (%): 929.4 [2·M + Na+]+ (4), 492.2 [M + K+]+ (11), 476.2 [M + Na+]+ (100), 454.2 [M + H+]+ (16). ESI-MS (neg.) m/z (%): 452.0 [M – H+]– (100). Anal. Calcd for C26H23N5O3 (453.49): C 68.86; H 5.11; N 15.44. Found: C 68.67; H 5.56; N 15.22. Using the excess of KOCN (4 equiv.), 50% of 5e, 6% of 6e and 29% of 7e was prepared from 2e.
8a-Butyl-8-[2-(phenylamino)phenyl]-5,6-dihydroimidazo[1,5-a]pyrazine-1,3(2H,8aH)-dione (6d)
Compound was prepared from 2d with 13% yield. Colorless solid, mp 187–190 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(1) 170.6, C(3) 156.2, C(5)H2 46.4/3.82, C(6)H2 33.9/3.82 and 3.15, C(8) 163.1, C(8a) 68.0, C(1’) 128.3, C(2’) 141.7, C(3’)H 117.9/7.10, C(4’)H 128.7/7.19, C(5’)H 119.7/6.86, C(6’)H 129.3/7.10, C(1’(R1)) 143.6, C(2’(R1))H 118.6/7.02, C(3’(R1))H 128.7/7.19, C(4’(R1))H 120.3/6.83, C(1’(R2))H2 32.7/1.94, C(2’(R2))H2 24.7/1.15 and 0.98, C(3’(R2)) H2 21.7/1.15, C(4’(R2)) H3 13.7/0.74 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(2)H n.o./11.01, N(4)H −290.8, N(7) −53.7, N(2’)H −295.9/7.15 1J(15N, 1H) 90.3 Hz. IR (cm–1) ν: 3302, 3046, 2958, 2871, 2732, 1776, 1719, 1640, 1593, 1508, 1455, 1419, 1303, 1127, 1115, 1070, 1021, 912, 890, 859, 756, 699, 678, 627, 578, 534, 499. ESI-MS (pos.) m/z (%): 399.2 [M + Na+]+ (12), 377.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 375.0 [M – H+]– (100). Anal. Calcd for C22H24N4O2 (376.45): C 70.19; H 6.43; N 14.88. Found: C 70.30; H 6.58; N 14.53.
8a-Benzyl-8-[2-(phenylamino)phenyl]-5,6-dihydroimidazo[1,5-a]pyrazine-1,3(2H,8aH)-dione (6e)
Compound was prepared from 2e with 14% yield. Colorless solid, mp 227–230 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(1) 169.9, C(3) 155.8, C(5)H2 46.6/3.93 and 3.86, C(6)H2 34.0/3.78 and 3.42, C(8) 162.9, C(8a) 68.3, C(1’) 128.2, C(2’) 142.1, C(3’)H 117.7/7.12, C(4’)H 128.9/7.22, C(5’)H 120.1/6.93, C(6’)H 129.4/7.22,C(1’(R1)) 143.5, C(2’(R1))H 119.1/7.02, C(3’(R1))H 128.7/7.21, C(4’(R1))H 120.5/6.83, C(1’(R2))H2 38.5/3.26 and 3.36, C(2’(R2)) 133.8, C(3’(R2))H 131.2/7.02, C(4’(R2))H 128.3/7.20, C(5’(R2))H 127.1/7.22 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(2)H n.o./10.68, N(4)H −291.4, N(7) −50.6, N(2’)H −295.6/7.37 1J(15N, 1H) 90.4 Hz. IR (cm–1) ν: 3323, 3032, 2932, 2731, 1770, 1720, 1645, 1595, 1510, 1496, 1478, 1454, 1411, 1302, 1137, 1067, 1039, 929, 753, 700, 689, 663, 597, 560, 535, 491. ESI-MS (pos.) m/z (%): 433.2 [M + Na+]+ (7), 411.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 409.0 [M – H+]– (100). Anal. Calcd for C25H22N4O2 (410.47): C 73.15; H 5.40; N 13.65. Found: C 73.12; H 5.55; N 13.81. Using an excess of KOCN (3 equiv.), 6% of 6e, 29% of 7e, and 30% of 5e was obtained.
8a-Phenyl-8-[2-(phenylamino)phenyl]-5,6-dihydroimidazo[1,5-a]pyrazine-1,3(2H,8aH)-dione (6f)
Compound was prepared from 2f and KOCN (3 equiv.) with 20% yield beside 8f. Colorless solid, mp 190–192 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(1) 170.3, C(3) 155.4, C(5)H2 45.5/4.03 and 3.55, C(6)H2 36.2/3.48 and 3.34, C(8) 165.0, C(8a) 68.9, C(1’) 125.3, C(2’) 138.8, C(3’)H 118.9/7.19, C(4’)H 130.8/7.23, C(5’)H 119.4/6.82, C(6’)H 131.6/7.25,C(1’(R1)) 142.8, C(2’(R1))H 118.9/7.01, C(3’(R1))H 128.9/7.22, C(4’(R1))H 120.9/6.89, C(1’(R2)) 137.3, C(2’(R2))H 126.5/7.39, C(3’(R2))H 128.9/7.42, C(4’(R2))H 130.0/7.42 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(2)H n.o./11.05, N(4)H −285.5, N(7) −58.2, N(2’)H −292.2/8.85 1J(15N, 1H) 87.2 Hz. IR (cm–1) ν: 3181, 3061, 2925, 2849, 2740, 1775, 1718, 1594, 1570, 1497, 1451, 1310, 1220, 1167, 1124, 1070, 1031, 963, 913, 889, 853, 751, 696, 594, 549. ESI-MS (pos.) m/z (%): 815.2 [2·M + Na+]+ (4), 419.1 [M + Na+]+ (18), 397.1 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 813.2 [2·M – 2·H + Na+]− (33), 395.0 [M – H+]– (100). Anal. Calcd for C24H20N4O2 (396.43): C 72.71; H 5.08; N 14.13. Found: C 72.29; H 5.06; N 14.08.
4a-Butyl-6-methyl-5-oxo-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carboxamide (7a)
Compound was prepared from 2a and KOCN (1.4 equiv.) with 47% yield. Using an excess of KNCO (4 equiv.), 7a was prepared with 57% yield. Colorless solid, mp 155–158 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 n.o/3.94, C(3)H2 40.1/3.80 and 3.49, CO 159.9, C(4a) n.o., C(5) 168.5, C(6a) 139.3, C(7)H 114.8/7.20, C(8)H 131.7/7.51, C(9)H 122.8/7.13, C(10)H 125.2/7.74, C(10a) 124.6, C(10b) 162.5, C(1’(R1))H3 30.3/3.31, C(1’(R2))H2 30.3/1.58, C(2’(R2))H2 25.2/1.19 and 0.85, C(3’(R2))H2 21.7/1.03, C(4’(R2))H3 13.7/0.66 ppm. IR (cm–1) ν: 3350. 3193, 2954, 2856, 1684, 1644, 1621, 1603, 1461, 1402, 1358, 1310, 1255, 1223, 1138, 1059, 1041, 1008, 992, 966, 941, 862, 750, 727, 699, 676, 595, 559. ESI-MS (pos.) m/z (%): 651.3 [2·M + Na+]+ (19), 353.0 [M + K+]+ (6), 337.1 [M + Na+]+ (77), 315.1 [M + H+]+ (100), 272.0 [M + H+ – HCNO]+ (16). ESI-MS (neg.) m/z (%): 312.9 [M – H+]– (100). Anal. Calcd for C17H22N4O2 (314.38): C 64.95; H 7.05; N 17.82. Found: C 64.75; H 7.22; N 17.71.
4a-Benzyl-6-methyl-5-oxo-2,3,5,6-tetrahydropyrazino[2,3-c]quinolone-4-(4aH)-carbox- amide (7b)
Compound was prepared from 2b with 63% yield beside 5b. Colorless solid, mp 146–151 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 48.6/3.66, C(3)H2 40.4/3.61 and 2.74, CO 160.3, C(4a) 69.2, C(5) 167.8, C(6a) 139.6, C(7)H 115.1/7.20, C(8)H 131.9/7.53, C(9)H 123.0/7.20, C(10)H 125.9/7.75, C(10a) 122.9, C(10b) 161.1 C(1’(R1))H3 30.7/3.35, C(1’(R2))H2 39.7/2.60 and 2.32, C(2’(R2)) 135.4, C(3’(R2))H 129.8/6.91, C(4’(R2))H 127.5/7.20, C(5’(R2))H 129.8/7.20 ppm. IR (cm–1) ν: 3447, 2942, 1686, 1663, 1647, 1602, 1493, 1470, 1430, 1360, 1297, 1270, 1224, 1138, 1059, 1039, 1011, 973, 951, 919, 875, 760, 704, 620, 556, 504. ESI-MS (pos.) m/z (%): 719.3 [2·M + Na+]+ (18), 387.1 [M + K+]+ (12), 371.1 [M + Na+]+ (85), 349.1 [M + H+]+ (100), 306.1 [M + H+ – HCNO]+ (32). Anal. Calcd for C 68.95; H 5.73; N 16.08. Found: C 68.81; H 5.92; N 15.82.
6-Methyl-5-oxo-4a-phenyl-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carbox- amide (7c)
Compound was prepared from 2c with 32% yield. Using the excess of KOCN (4 equiv.), 7c was prepared in 68% yield. Yellowish solid, mp 209–211 °C (chloroform). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.4/4.21, C(3)H2 40.1/3.48 and 2.77, CO 160.7, C(4a) 68.8, C(5) 171.5, C(6a) 137.5, C(7)H 116.4/6.42, C(8)H 131.4/7.27, C(9)H 124.2/7.17, C(10)H 126.2/7.96, C(10a) 123.1, C(10b) 161.3, C(1’(R1)) 139.2, C(2’(R1))H 129.2/7.29, C(3’(R1))H 130.1/7.50, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 34.8/3.19 and 1.95, C(2’(R2))H2 25.2/1.36 and 1.06, C(3’(R2))H2 22.1/1.21, C(4’(R2))H3 13.7/0.81 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −54.1, N(4) −296.1, NH2 −301.9/5.21 1J(15N, 1H) 83.6 Hz. IR (cm–1) ν: 3422, 3352, 3305, 3247, 3197, 3062, 2953, 1689, 1663, 1626, 1601, 1471, 1406, 1361, 1297, 1170, 1127, 1079, 1029, 1010, 933, 903, 886, 821, 762, 696, 633, 554. ESI-MS (pos.) m/z (%): 691.2 [2·M + Na+]+ (9), 373.0 [M + K+]+ (17), 357.0 [M + Na+]+ (100), 335.1 [M + H+]+ (84), 292.0 [M + H+ – HCNO]+ (13). Anal. Calcd for C19H18N4O2 (334.37): C 68.25; H 5.43; N 16.76. Found: C 68.21; H 5.50; N 16.79.
4a-Butyl-5-oxo-6-phenyl-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carboxamide(7d)
Compound was prepared from 2d with 38% yield beside 5d and 6d. Colorless solid, mp 144–152 °C (benzene/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.4/4.21, C(3)H2 40.1/3.48 and 2.77, CO 160.7, C(4a) 68.8, C(5) 171.5, C(6a) 137.5, C(7)H 116.4/6.42, C(8)H 131.4/7.27, C(9)H 124.2/7.17, C(10)H 126.2/7.96, C(10a) 123.1, C(10b) 161.3, C(1’(R1)) 139.2, C(2’(R1))H 129.2/7.29, C(3’(R1))H 130.1/7.50, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 34.8/3.19 and 1.95, C(2’(R2))H2 25.2/1.36 and 1.06, C(3’(R2))H2 22.1/1.21, C(4’(R2))H3 13.7/0.81 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −54.1, N(4) −296.1, NH2 −301.9/5.21 1J(15N, 1H) 83.6 Hz. IR (cm–1) ν: 3434, 3398, 3215, 2955, 2851, 1700, 1662, 1645, 1607, 1489, 1459, 1428, 1350, 1330, 1313, 1301, 1257, 1217, 1173, 1163, 1131, 1052, 1030, 1009, 955, 877, 801, 769, 756, 701, 679, 646, 603, 570, 511, 490. ESI-MS (pos.) m/z (%): 775.4 [2·M + Na+]+ (8), 415.2 [M + K+]+ (10), 399.2 [M + Na+]+ (84), 377.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 375.0 [M – H+]– (100). Anal. Calcd for C22H24N4O2 (376.45): C 70.19; H 6.43; N 14.89. Found: C 70.51; H 6.41; N 14.52.
4a-Benzyl-5-oxo-6-phenyl-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carbox- amide (7e)
Compound was prepared from 2e with 49% yield beside 5e and 6e. Colorless solid, mp 197–200 °C (benzene/cyclohexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 48.5/3.66, C(3)H2 40.4/3.74 and 2.74, CO 160.3, C(4a) 69.2, C(5) 167.7, C(6a) 138.4, C(7)H 115.9/6.24, C(8)H 131.5/7.32, C(9)H 123.0/7.19, C(10)H 127.0/7.84, C(10a) 122.5, C(10b) 161.4, C(1’(R1)) 139.2, C(2’(R1))H 129.2 and 128.2/7.19, C(3’(R1))H 130.1/7.56, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 34.8/3.19 and 1.95, C(2’(R2))H2 25.2/1.36 and 1.06, C(3’(R2))H2 22.1/1.21, C(4’(R2))H3 13.7/0.81 ppm. IR (cm–1) ν: 3427, 3196, 3063, 2938, 2850, 1703, 1666, 1602, 1492, 1460, 1420, 1359, 1336, 1310, 1298, 1267, 1217, 1179, 1074, 1046, 1032, 877, 845, 792, 754, 704, 641, 606, 575, 560, 544, 497. ESI-MS (pos.) m/z (%): 843.4 [2·M + Na+]+ (6), 449.1 [M + K+]+ (16), 433.2 [M + Na+]+ (85), 411.2 [M + H+]+ (100), 368.2 [M + H+ – HCNO]+ (6). ESI-MS (neg.) m/z (%): 409.0 [M – H+]– (100). Anal. Calcd for C25H22N4O2 (410.46): C 73.15; H 5.40; N 13.65. Found: C 73.28; H 5.92; N 13.51.
Compound was prepared from 2f with 18% yield beside 6f. Colorless solid, mp 160–165 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2) 158.8, C(4) 172.9, C(5)H2 64.3/2.22, C(6)H2 36.6/3.38 and 3.31, C(7)H2 39.6/3.77, C(9) 169.1, C(10) 119.0, C(11) 144.0, C(12)H 114.9/7.29, C(13)H 131.9/7.32, C(14)H 118.1/6.83, C(15) 128.9/7.57, C(1’(R2)) 141.5, C(2’(R2))H 119.4/7.15, C(3’(R2))H 129.4/7.30, C(4’(R2))H 121.7/6.97, C(1’(R2)) 133.8, C(2’(R2))H 127.6/7.16, C(3’(R2))H 129.1/7.36, C(4’(R2))H 128.8/7.36 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −284.5, N(3)H −235.5./11.03 1J(15N, 1H) 94.6 Hz, N(8)H −269.0/9.44 1J(15N, 1H) 91.5 Hz, N(11)H −292.2/8.68 1J(15N, 1H) 89.5 Hz. IR (cm–1) ν: 3390, 3353, 3228, 3068, 2939, 2740, 1775, 1722, 1629, 1589, 1512, 1447, 1421, 1383, 1329, 1310, 1282, 1222, 1167, 1157, 1120, 1078, 1053, 1027, 963, 942, 895, 841, 751, 701, 628, 580, 516. ESI-MS (pos.) m/z (%): 851.3 [2·M + Na+]+ (11), 453.2 [M + K+]+ (26), 437.2 [M + Na+]+ (100), 415.2 [M + H+]+ (31). ESI-MS (neg.) m/z (%): 413.0 [M – H+]– (100). Anal. Calcd for C24H22N4O3 (414.46): C 69.55; H 5.35; N 13.52. Found: C 69.99; H 5.78; N 13.17.
3.5. CDK and ABL Inhibition Assay
CDK2/cyclin E and ABL1 activity was assayed as previously described [
42,
43]. Briefly, the kinase was assayed with [γ-
33P]ATP and suitable peptide substrates in a reaction buffer (60 mM HEPES-NaOH, pH 7.5, 3 mM MgCl
2, 3 mM MnCl
2, 3 μM Na-orthovanadate, 1.2 mM DTT, 2.5 μg/50 μL PEG
20.000). The reactions were stopped by adding 5 µL of 3% aq. H
3PO
4. Aliquots were spotted onto P-81 phosphocellulose, washed with 0.5% aq. H
3PO
4 and air-dried. Kinase inhibition was quantified using an FLA-7000 digital image analyzer. The concentration of the test compound required to reduce kinase activity by 50% was determined from a dose-response curves and reported as the IC
50 value.
3.6. In Vitro Cytotoxicity
Cell lines K562 and MV4;11 were obtained from the European Collection of Cell Cultures. The cell lines were cultivated in Dulbecco’s Modified Eagle medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C in 5% CO2. For the viability assays, cells were seeded into 96-well plates (5000 cells per well), and after the preincubation period, were treated in triplicate with six different doses of each compound for 72 h. After treatment, a resazurin (Sigma-Aldrich) solution was added for four hours, and the fluorescence of resorufin formed in live cells was measured at 544 nm/590 nm (excitation/emission) using a Fluoroskan Ascent microplate reader (Labsystems). The IC50 value, the drug concentration that was lethal for 50% of the cells, was calculated from the dose–response curve.