Probiotics Function in Preventing Atopic Dermatitis in Children
Abstract
:1. Introduction
Methods
2. Atopic Dermatitis
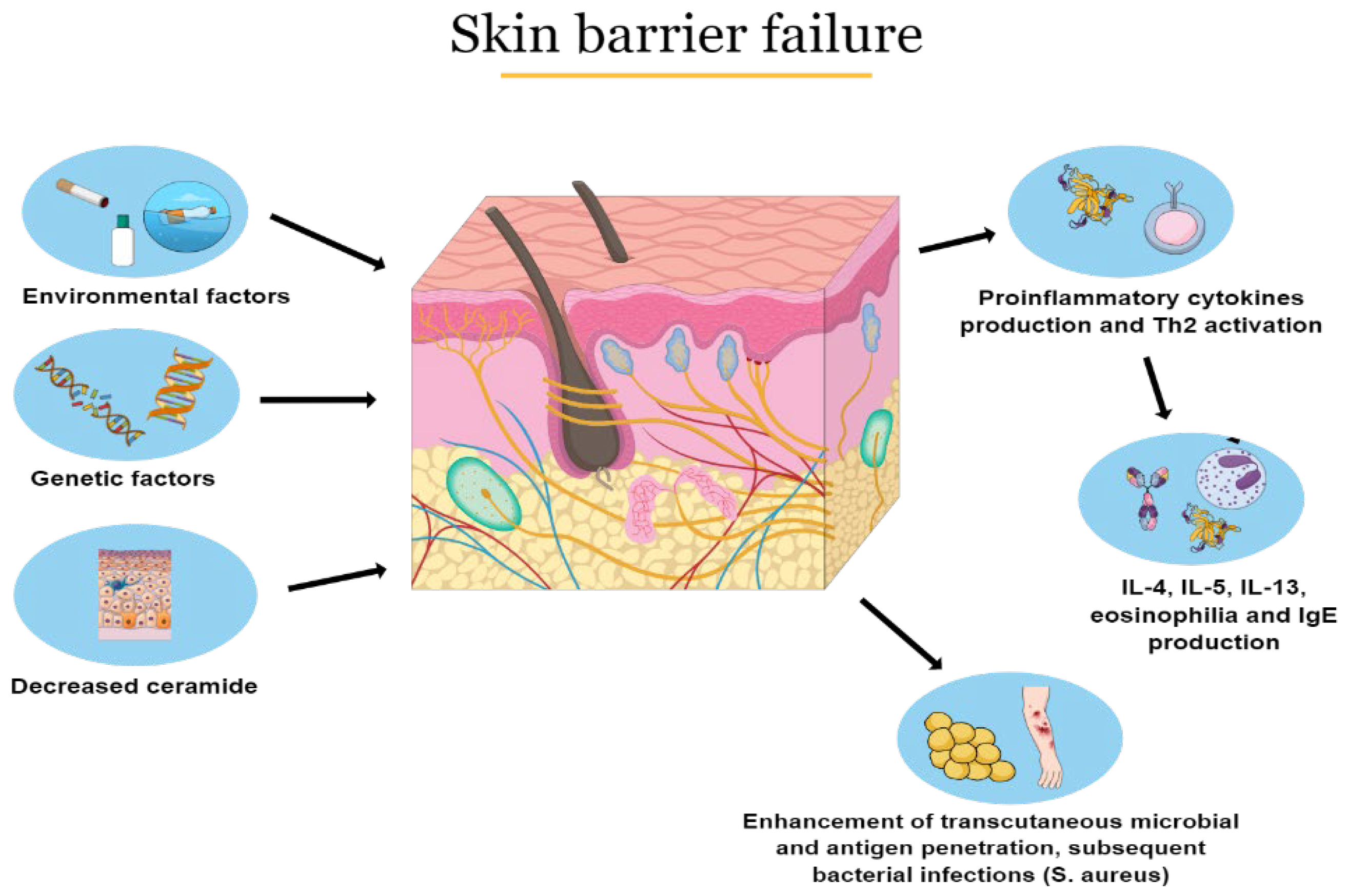
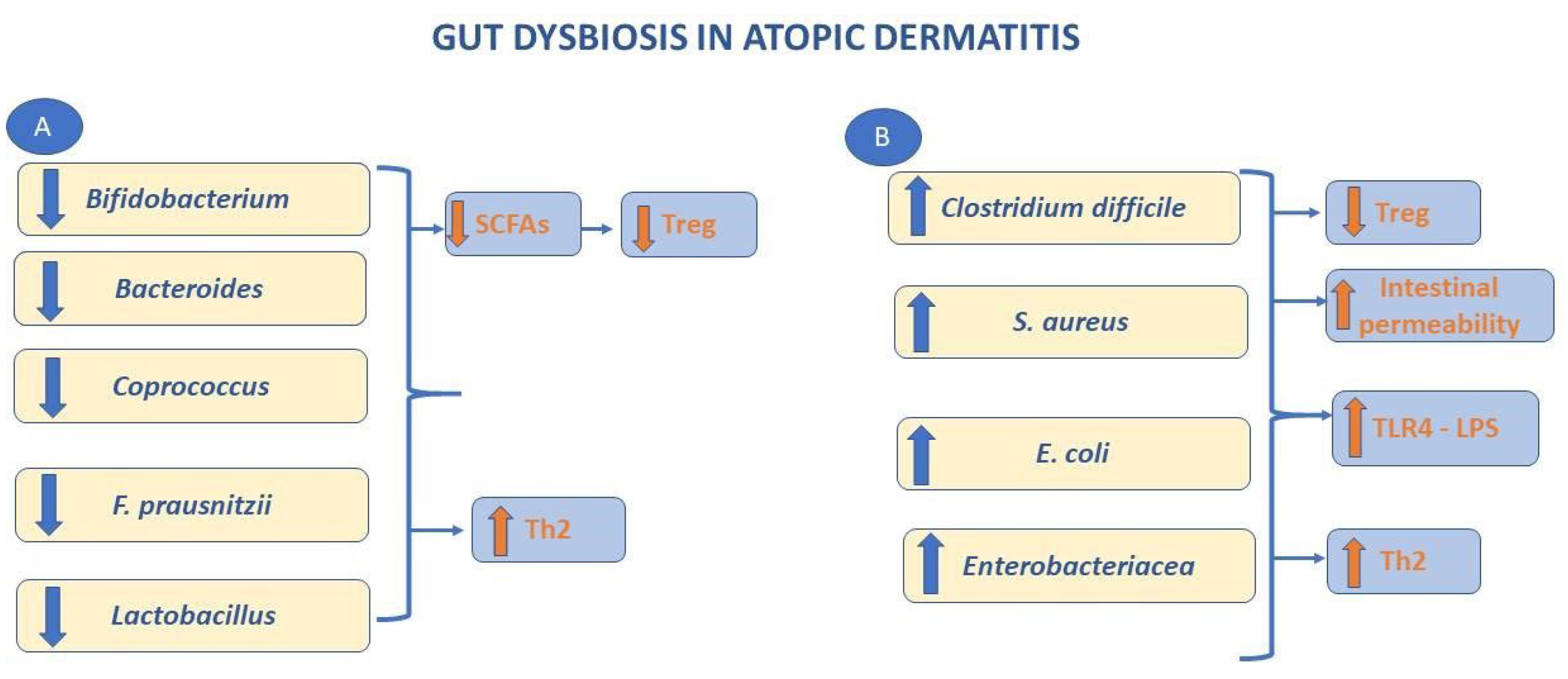
2.1. Pathophysiology
2.2. Clinical Aspects and Diagnosis
2.3. Treatment
3. Probiotics
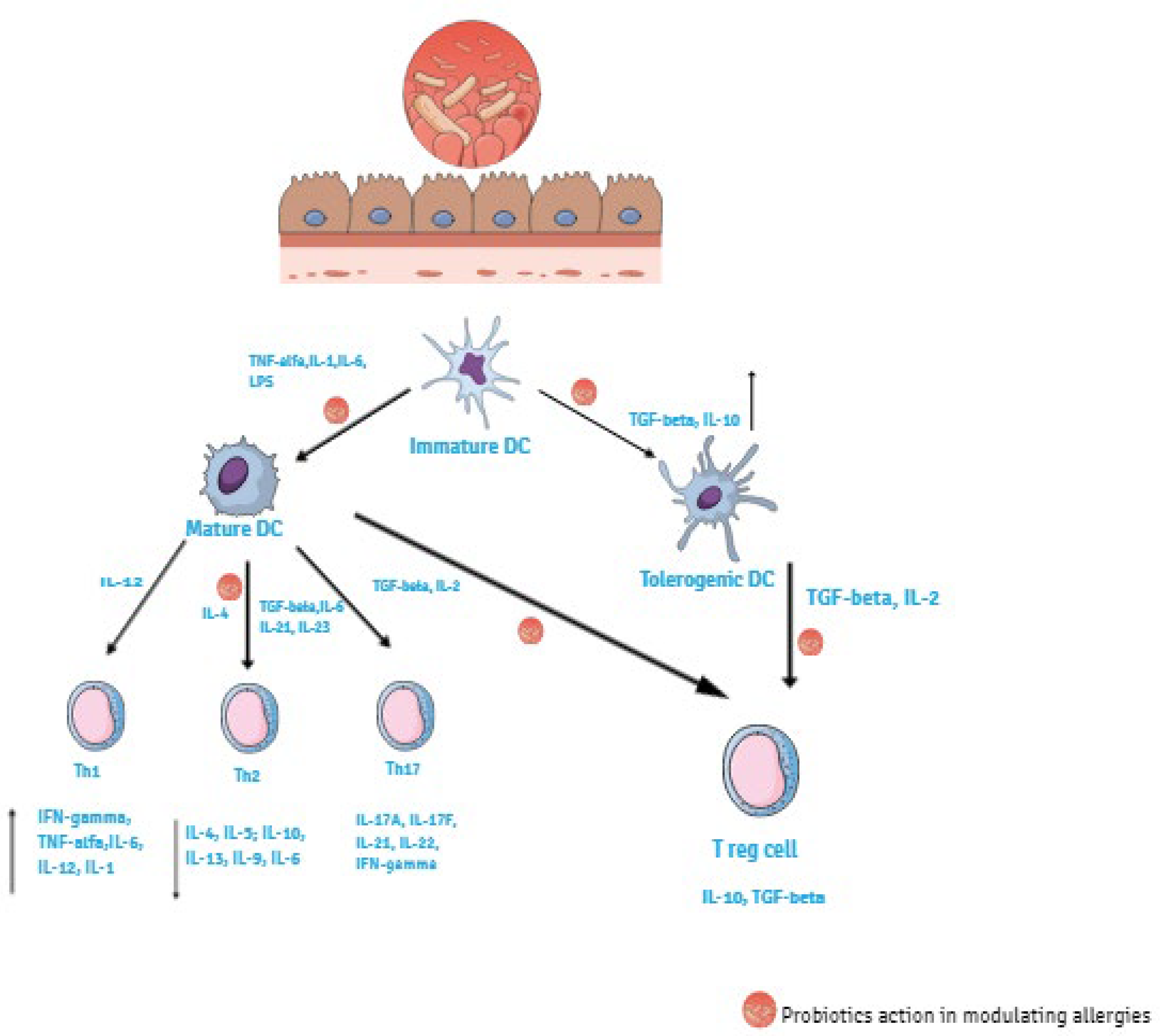
3.1. Enhancement of Barrier Function
3.2. Suppression of Pathogens
4. Probiotics Function on Preventing AD
5. Human Studies
5.1. Monostrain
5.2. Multistrain
5.3. Review and Meta-Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antony, K.M.; Ma, J.; Mitchell, K.B.; Racusin, D.A.; Versalovic, J.; Aagaard, K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am. J. Obstet. Gynecol. 2015, 212, 653.e1–653.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumbaugh, D.E.; Arruda, J.; Robbins, K.; Ir, D.; Santorico, S.A.; Robertson, C.E.; Frank, D.N. Mode of Delivery Determines Neonatal Pharyngeal Bacterial Composition and Early Intestinal Colonization. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 320–328. [Google Scholar] [CrossRef]
- Francino, M.P. Birth Mode-Related Differences in Gut Microbiota Colonization and Immune System Development. Ann. Nutr. Metab. 2018, 73 (Suppl. 3), 12–16. [Google Scholar] [CrossRef]
- Cerdó, T.; Diéguez, E.; Campoy, C. Early nutrition and gut microbiome: Interrelationship between bacterial metabolism, immune system, brain structure, and neurodevelopment. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E617–E630. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.-Q.; Liu, F.; Wu, J.-Y. Human milk oligosaccharides and infant gut microbiota: Molecular structures, utilization strategies and immune function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef]
- Hu, T.; Dong, Y.; Yang, C.; Zhao, M.; He, Q. Pathogenesis of Children’s Allergic Diseases: Refocusing the Role of the Gut Microbiota. Front. Physiol. 2021, 12, 749544. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef]
- Sjögren, Y.M.; Jenmalm, M.C.; Böttcher, M.F.; Björkstén, B.; Sverremark-Ekström, E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy 2009, 39, 518–526. [Google Scholar] [CrossRef] [Green Version]
- West, C.E.; Jenmalm, M.C.; Prescott, S.L. The gut microbiota and its role in the development of allergic disease: A wider perspective. Clin. Exp. Allergy 2015, 45, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, E.; Cinicola, B.; Carello, R.; Caimmi, S.; Brindisi, G.; De Castro, G.; Zicari, A.M.; Tosca, M.A.; Manti, S.; Martelli, A.; et al. Atopic Dermatitis. Acta Biomed. 2020, 15, e2020011. [Google Scholar]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 8–16. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz, M.; Alexis, A.F.; Beck, L.A.; Block, J.; Eichenfield, L.F.; Fonacier, L.; Guttman-Yassky, E.; Paller, A.S.; Pariser, D.; Silverberg, J.I.; et al. Expert Perspectives on Management of Moderate-to-Severe Atopic Dermatitis: A Multidisciplinary Consensus Addressing Current and Emerging Therapies. J. Allergy Clin. Immunol. Pract. 2017, 5, 1519–1531. [Google Scholar] [CrossRef]
- Elakovská, J.; Bukač, R.; Vaňková, J.; Andrýs, C. The relation between the sensitization to molecular components of inhalant allergens and food reactions in patients suffering from atopic dermatitis. Food Agric. Immunol. 2021, 32, 33–53. [Google Scholar] [CrossRef]
- Čelakovská, J.; Krcmova, I.; Bukac, J.; Vaneckova, J. Sensitivity and specificity of specific IgE, skin prick test and atopy patch test in examination of food allergy. Food Agric. Immunol. 2017, 28, 238–247. [Google Scholar] [CrossRef]
- Kantor, R.; Silverberg, J.I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp. Dermatol. 2018, 27, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Irvine, A.; McLean, W.H.I.; Leung, D.Y. Filaggrin Mutations Associated with Skin and Allergic Diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Villarreal, M.; Stewart, S.; Choi, J.; Ganguli-Indra, G.; Babineau, D.; Philpot, C.; David, G.; Yoshida, T.; Boguniewicz, M.; et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br. J. Dermatol. 2017, 177, e125–e127. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018, 67, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Makino, Y.; Nagata, M.; Furuta, J.; Enomoto, H.; Hirota, T.; Tamari, M.; Noguchi, E. A rare variant in CYP27A1 and its association with atopic dermatitis with high serum total IgE. Allergy 2016, 71, 1486–1489. [Google Scholar] [CrossRef]
- Bjerre, R.; Bandier, J.; Skov, L.; Engstrand, L.; Johansen, J.D. The role of the skin microbiome in atopic dermatitis: A systematic review. Br. J. Dermatol. 2017, 177, 1272–1278. [Google Scholar] [CrossRef]
- Brauweiler, A.M.; Hall, C.F.; Goleva, E.; Leung, D.Y. Staphylococcus aureus Lipoteichoic Acid Inhibits Keratinocyte Differentiation through a p63-Mediated Pathway. J. Investig. Dermatol. 2017, 137, 2030–2033. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut Microbiota, Probiotics, and Their Interactions in Prevention and Treatment of Atopic Dermatitis: A Review. Front. Immunol. 2021, 12, 720393. [Google Scholar] [CrossRef]
- Paller, A.; Jaworski, J.C.; Simpson, E.L.; Boguniewicz, M.; Russell, J.J.; Block, J.K.; Tofte, S.; Dunn, J.D.; Feldman, S.R.; Clark, A.R.; et al. Major Comorbidities of Atopic Dermatitis: Beyond Allergic Disorders. Am. J. Clin. Dermatol. 2018, 19, 821–838. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Schmitt, J.; Langan, S.; Williams, H.C. What are the best outcome measurements for atopic eczema? A systematic review. J. Allergy Clin. Immunol. 2007, 120, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Vyas, J.; Finlay, A. Counting the Burden: Atopic Dermatitis and Health-related Quality of Life. Acta Derm. Venereol. 2020, 100, adv00161. [Google Scholar] [CrossRef] [PubMed]
- Pucci, N.; Novembre, E.; Cammarata, M.G.; Bernardini, R.; Monaco, M.G.; Calogero, C.; Vierucci, A. Scoring atopic dermatitis in infants and young children: Distinctive features of the SCORAD index. Allergy 2005, 60, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Tuchinda, P.; Nitiyarom, R.; Chunharas, A.; Chantaphakul, H.; Aunhachoke, K.; Chularojanamontri, L.; Rajatanavin, N.; Jirapongsananuruk, O.; Vichyanond, P.; et al. Clinical practice guidelines for the diagnosis and management of atopic dermatitis. Asian Pac. J. Allergy Immunol. 2021, 39, 145–155. [Google Scholar] [CrossRef]
- Licari, A.; Castagnoli, R.; Marseglia, A.; Olivero, F.; Votto, M.; Ciprandi, G.; Marseglia, G.L. Dupilumab to Treat Type 2 Inflammatory Diseases in Children and Adolescents. Pediatr. Drugs 2020, 22, 295–310. [Google Scholar] [CrossRef]
- Fanfaret, I.S.; Boda, D.; Ion, L.M.; Hosseyni, D.; Leru, P.; Ali, S.; Corcea, S.; Bumbacea, R. Probiotics and prebiotics in atopic dermatitis: Pros and cons (Review). Exp. Ther. Med. 2021, 22, 1376. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Health Organization. Guidelines for Evaluation of Probiotics in Food; World Health Organization: London, ON, Canada, 2002. [Google Scholar]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; De Foy, J.-M.P.; Dequenne, I.; De Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Borchers, A.T.; Selmi, C.; Meyers, F.J.; Keen, C.L.; Gershwin, M.E. Probiotics and immunity. J. Gastroenterol. 2009, 44, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Derrien, M.; Levenez, F.; Brazeilles, R.; Ballal, S.A.; Kim, J.; Degivry, M.-C.; Quéré, G.; Garault, P.; Vlieg, J.E.T.V.H.; et al. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 2016, 10, 2235–2245. [Google Scholar] [CrossRef] [Green Version]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslami, M.; Bahar, A.; Keikha, M.; Karbalaei, M.; Kobyliak, N.; Yousefi, B. Probiotics function and modulation of the immune system in allergic diseases. Allergol. Immunopathol. 2020, 48, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal. Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Delcenserie, V.; Marte, L.; Lamourex, M.; Amiot, J.; Boutin, Y.; Roy, D. Immunomodulator effects of probiotics in the intestinal tract. Curr. Issue Mol. Biol. 2008, 10, 37–54. [Google Scholar]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Ricciardi-Castagnoli, P.; Granucci, F. Opinion: Interpretation of the complexity of innate immune responses by functional genomics. Nat. Rev. Immunol. 2002, 2, 881–889. [Google Scholar] [CrossRef]
- Bodera, P.; Chcialowski, A. Immunomodulatory effect of probiotic bacteria. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 58–64. [Google Scholar] [CrossRef]
- Hajavi, J.; Esmaeili, S.; Varasteh, A.; Vazini, H.; Atabati, H.; Mardani, F.; Momtazi-Borojeni, A.A.; Hashemi, M.; Sankian, M.; Sahebkar, A. The immunomodulatory role of probiotics in allergy therapy. J. Cell. Physiol. 2019, 234, 2386–2398. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, C.; Yu, D.; Liu, Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy 2021, 76, 456–470. [Google Scholar] [CrossRef]
- Siegmund, K.; Rückert, B.; Ouaked, N.; Bürgler, S.; Speiser, A.; Akdis, C.A. Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells. J. Immunol. 2009, 182, 2124–2130. [Google Scholar] [CrossRef] [Green Version]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.-Y. Retinoic Acid Imprints Gut-Homing Specificity on T Cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H. FOXP3 and its role in immune system. Adv. Exp. Med. Biol. 2009, 665, 17–29. [Google Scholar] [PubMed]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.-E.; De Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umetsu, D.T.; DeKruyff, R.H. The regulation of allergy and asthma. Immunol. Rev. 2006, 212, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Feleszko, W.; Jaworska, J.; Rha, R.D.; Steinhausen, S.; Avagyan, A.; Jaudszus, A. Probiotic-induced suppression of allergic sensitizazion and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin. Exp. Allergy 2007, 37, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Foligne, B.; Zoumpopoulou, G.; Dewulf, J.; Ben Younes, A.; Chareyre, F.; Sirard, J.-C.; Pot, B.; Grangette, C. A Key Role of Dendritic Cells in Probiotic Functionality. PLoS ONE 2007, 2, e313. [Google Scholar] [CrossRef]
- Boirivant, M.; Strober, W. The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 2007, 23, 679–692. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chaun Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Bakdash, G.; Vogelpoel, L.T.C.; van Capel, T.M.M.; Kapsenberg, M.L.; De Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the Molecular Mechanisms Underlying the Protective Effects of Microbial SCFAs on Intestinal Tolerance and Food Allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, H.; Tracey, K.J. Regulation of macrophage activation and inflammation by spermine: A new chapter in an old story. Crit. Care Med. 2000, 28, N60–N66. [Google Scholar] [CrossRef]
- Rodriguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Boyle, R.J.; Ismail, I.H.; Kivivuori, S.; Licciardi, P.V.; Robins-Browne, R.M.; Mah, L.-J.; Axelrad, C.; Moore, S.; Donath, S.; Carlin, J.B.; et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: A randomized controlled trial. Allergy 2011, 66, 509–516. [Google Scholar] [CrossRef]
- Wickens, K.; Black, P.; Stanley, T.V.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G.; Crane, J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef]
- Enomoto, T.; Sowa, M.; Nishimori, K. Effects of Bifidobacterial supplementation to pregnant woman and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol. Int. 2014, 63, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Cabana, M.D.; McKean, M.; Caughey, A.B.; Fong, L.; Lynch, S.; Wong, A.; Leong, R.; Boushey, H.A.; Hilton, J.F. Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. Pediatrics 2017, 140, e20163000. [Google Scholar] [CrossRef] [Green Version]
- Wickens, K.; Barthow, C.; Mitchell, E.A.; Kang, J.; van Zyl, N.; Purdie, G.; Stanley, T.; Fitzharris, P.; Murphy, R.; Crane, J. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr. Allergy Immunol. 2018, 29, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Dotterud, C.K.; Storrø, O.; Johnsen, R. Probiotics in pregnant woman to prevent allergic disease: A randomized, double-blind trial. Br. J. Dermatol. 2010, 163, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Randi, J.; Bratsæter, A.L.; Magnus, M.C. Probiotic milk consumption in pregnancy and infancy and the subsequent childhood allergic disease. J. Allergy Clin. Immunol. 2014, 133, 165–171. [Google Scholar]
- Allen, S.J.; Jordan, S.; Storey, M. Probiotic in the prevention of eczema: A randomized controlled trial. Arch. Dis. Child. 2014, 99, 1014–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, M.R.; Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.M.; Laursen, R.P.; Bruun, S.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Høst, A.; Larnkjaer, A. Probiotics in late infancy reduce the incidence of eczema: A randomized controlled trial. Pediatr. Allergy Immunol. 2019, 30, 335–340. [Google Scholar] [CrossRef]
- Doege, K.; Grajecki, D.; Zyriax, B.C.; Detinkina, E.; Zu Eulenburg, C.; Buhling, K.J. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—A metanalysis. Br. J. Nutr. 2012, 107, 1–6. [Google Scholar] [CrossRef]
- Pelucchi, C.; Chatenoud, L.; Turati, F.; Galeone, C.; Moja, L.; Bach, J.-F.; La Vecchia, C. Probiotics Supplementation During Pregnancy or Infancy for the Prevention of Atopic Dermatitis: A meta-analysis. Epidemiology 2012, 23, 402–414. [Google Scholar] [CrossRef]
- Mansfield, J.A.; Bergin, S.W.; Cooper, J.R.; Olsen, C.H. Comparative Probiotic Strain Efficacy in the Prevention of Eczema in Infants and Children: A Systematic Review and Meta-Analysis. Mil. Med. 2014, 179, 580–592. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Wang, L.; Yang, L.; Tao, S.; Xia, R.; Fan, W. Long-term effect of early-life supplementation with probiotics on preventing atopic dermatitis: A meta-analysis. J. Dermatol. Treat. 2015, 26, 537–540. [Google Scholar] [CrossRef]
- Zuccotti, G.V.; Meneghin, F.; Aceti, A.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Gori, D.; Indrio, F.; Maggio, L.; et al. Probiotics for prevention of atopic diseases in infants: Systematic review and meta-analysis. Allergy 2015, 70, 1356–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiocchi, A.; Pawankar, R.; Cuello Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Spigler, M.; Terracciano, L.; et al. World Allergy Organization-McMaster University Giodelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuello-Garcia, C.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, L.; Land, B.V.; Sprikkelman, A.B.; Garssen, J. Role of Microbial Modulation in Management of Atopic Dermatitis in Children. Nutrients 2017, 9, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, G.; Im, S.-H. Probiotics as a Potential Immunomodulating Pharmabiotics in Allergic Diseases: Current Status and Future Prospects. Allergy Asthma Immunol. Res. 2018, 10, 575–590. [Google Scholar] [CrossRef]
- Li, L.; Han, Z.; Niu, X.; Zhang, G.; Jia, Y.; Zhang, S.; He, C. Probiotic supplementation for prevention of atopic dermatitis in infants and children: A sistematic review and meta-analysis. Am. J. Clin. Dermatol. 2019, 20, 367–377. [Google Scholar] [CrossRef]
- Pedersen, E.; Skov, L.; Thyssen, J.; Jensen, P. Role of the Gut Microbiota in Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2019, 99, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Tu, R.; Hu, Y.; He, T.; Zhang, W.; Gu, L.; Liu, H. Probiotics supplement for the prevention of eczema in children: Study protocol for a meta-analysis and systematic review. Medicine 2019, 98, e16957. [Google Scholar] [CrossRef]
- Amalia, N.; Orchard, D.; Francis, K.L.; King, E. Systematic review and meta-analysis on the use of probiotic supplementation in pregnant mother, breastfeeding mother and infant for the prevention of atopic dermatitis in children. Australas. J. Dermatol. 2020, 61, e158–e173. [Google Scholar] [CrossRef]
- Jiang, W.; Ni, B.; Liu, Z.; Liu, X.; Xie, W.; Wu, I.X.Y.; Li, X. The Role of Probiotics in the Prevention and Treatment of Atopic Dermatitis in Children: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pediatr. Drugs 2020, 22, 535–549. [Google Scholar] [CrossRef]
- De Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; Du Toit, G.; et al. Preventing food allergy in infancy and childhood: Systematic review of randomised controlled trials. Pediatr. Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Recto, M.S.T.; Castor, M.A.R.; Casis-Hao, R.J.; Nano, A.L.M. Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Luo, J.; Liu, H.; Xi, Y.; Lin, Q. Can Mixed Strains of Lactobacillus and Bifidobacterium Reduce Eczema in Infants under Three Years of Age? A Meta-Analysis. Nutrients 2021, 13, 1461. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Nationality | Study Design | Sample Size at Baseline | Sample Size at Follow-Up | Probiotics | Period of Administration | Follow-Up | Results |
|---|---|---|---|---|---|---|---|
| Dotterud et al. 2010, Norway [73] | RCT | AG: 138 mothers CG: 140 mothers | AG: 42 children CG: 58 children | Probiotic milk containing Lactobacillus rhamnosus GG-5, Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. Lactis Bb-12 | From 36 weeks of gestation to 3 months postnatally during breastfeeding | 2 years | Reduction of incidence of AD at 2 years of age in children of mothers receiving probiotic milk (OR 0.51, 95% CI 0.30–0.87, p = 0.013) |
| Boyle et al. 2011, Australia [68] | RCT | AG: 125 mothers CG: 125 mothers | AG: 108 infants CG: 102 infants | Lactobacillus rhamnosus GG | From 36 weeks of gestation to delivery | 12 months | No statistically significant difference between the active group and the placebo group on the cumulative incidence of AD (34% probiotic, 39% placebo; RR 0.88; 95% CI 0.63, 1.22) or IgE-associated AD (18% probiotic, 19% placebo; RR 0.94; 95% CI 0.53, 1.68) |
| Wickens et al. 2012, New Zealand [69] | DBRCT | AG: 157 infants (HN001 group) AG: 158 infants (HN019 group) CG: 150 infants | AG: 136 infants (HN001 group) AG: 146 infants (HN019 group) CG: 143 infants | Lactobacillus rhamnosus HN001 Bifidobacterium animalis subsp. Lactis HN019 | From 35 weeks of gestation to 6 months of age after birth in mothers if breastfeeding. From birth to 2 years in all infants | 4 years | The cumulative prevalence of AD was significantly reduced in the group of infants receiving HN001 (HR 0.57, 95% CI 0.39–0.83) |
| Enomoto et al. 2014, Japan [70] | Open trial | AG: 130 pregnant woman and their infants CG: 36 mothers-infant pairs | AG: 94 infants CG: 31 infants | Bifidobacterium breve M-16V and Bifidobacterium longum BB536 | From 1 month prior to delivery to pregnant woman to 6 months after birth to infants. | 18 months | After 18 months of follow-up, a lower incidence of AD in the probiotic group (OR: 0.304 [95% CI: 0.105–0.892]) |
| Randi et al. 2014, Norway [74] | Cohort study | NA | AG: 15,042 infants CG:25572 infants | Probiotic milk containing Lactobacillus acidophilus La-5, Bifidobacterium subsp lactis BB12, Lactobacillus rhamnosus | From gestation in pregnant woman to 6 months after birth in infants | 6 months | Consumption of probiotic milk products was related to a reduced incidence of AD in children (RR 0.94 [95% CI, 0.89–0.99]) |
| Allen et al. 2014, UK [75] | RCT | AG: 220 mothers CG: 234 mothers | AG: 187 children CG: 191 children | Lactobacillus salivarius CUL61, Lactobacillus paracasei CUL08, Bifidobacterium animalis subsp lactis CUL34 and Bifidobacterium bifidum CUL20 | From 36 weeks of gestation to 6 months of age in children | 2 years | The probiotic seemed to prevent atopic sensitization to common food allergen but not to prevent AD in infants (OR 1.07 [ 95% CI 0.72 to 1.6]) |
| Simpson et al. 2015, Norway. [76] | DBRCT | AG: 211 pregnant women CG: 204 pregnant women | AG: 81 children CG: 82 children | Probiotic milk containing Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. Lactis BB-12 | From 36 week gestation until 3 months postpartum in mothers | 6 years | Perinatal maternal probiotic supplementation is effective in reducing the cumulative incidence of AD in children (OR 0.64 [95% CI 0.39–1.07, p = 0.086]) |
| Cabana et al., 2017, California [71] | DBRCT | AG: 92 infants CG: 92 infants | NA | Lactobacillus rhamnosus GG | First 6 months of life | 6 years | Early monostrain probiotic supplementation does not prevent the development of AD at 2 years of age (HR of 0.95 (95% CI, 0.59–1.53) |
| Wickens et al. 2018, New Zealand [72] | DBRCT | AG: 157 infants (HN001 group) CG: 158 infants (HN019 group) CG: 159 infants | AG: 97 children (HN001 group) AG: 104 children (HN019 group) CG: 97 children | Lactobacillus rhamnosus HN001 (HN001) or Bifidobacterium lactis HN019 (HN019) | From 35 weeks of gestation to 6 months’ post-partum in breastfeeding mothers; from birth to 2 years of age in infants | 11 years | Lactobacillus rhamnosus HN001 significantly protected against the development of AD for at least the first decade of life (RR 0.46, 95% CI 0.25–0.86, p = 0.015) |
| Schmidt at al. 2018, Denmark [77] | DBRCT | AG: 144 infants CG: 146 infants | AG: 119 infants CG: 122 infants | Lactobacillus rhamnosus and Bifidobacterium animalis subsp lactis | From a mean age of 10 months for 6 months | 6 months | Protective role of probiotics on the development of AD (RR 0.37, 95% CI 0.14–0.98; p = 0.036). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anania, C.; Brindisi, G.; Martinelli, I.; Bonucci, E.; D’Orsi, M.; Ialongo, S.; Nyffenegger, A.; Raso, T.; Spatuzzo, M.; De Castro, G.; et al. Probiotics Function in Preventing Atopic Dermatitis in Children. Int. J. Mol. Sci. 2022, 23, 5409. https://doi.org/10.3390/ijms23105409
Anania C, Brindisi G, Martinelli I, Bonucci E, D’Orsi M, Ialongo S, Nyffenegger A, Raso T, Spatuzzo M, De Castro G, et al. Probiotics Function in Preventing Atopic Dermatitis in Children. International Journal of Molecular Sciences. 2022; 23(10):5409. https://doi.org/10.3390/ijms23105409
Chicago/Turabian StyleAnania, Caterina, Giulia Brindisi, Ivana Martinelli, Edoardo Bonucci, Miriam D’Orsi, Sara Ialongo, Anna Nyffenegger, Tonia Raso, Mattia Spatuzzo, Giovanna De Castro, and et al. 2022. "Probiotics Function in Preventing Atopic Dermatitis in Children" International Journal of Molecular Sciences 23, no. 10: 5409. https://doi.org/10.3390/ijms23105409
APA StyleAnania, C., Brindisi, G., Martinelli, I., Bonucci, E., D’Orsi, M., Ialongo, S., Nyffenegger, A., Raso, T., Spatuzzo, M., De Castro, G., Zicari, A. M., Carraro, C., Piccioni, M. G., & Olivero, F. (2022). Probiotics Function in Preventing Atopic Dermatitis in Children. International Journal of Molecular Sciences, 23(10), 5409. https://doi.org/10.3390/ijms23105409







