Nanoarchitectonics for Biodegradable Superabsorbent Based on Carboxymethyl Starch and Chitosan Cross-Linked with Vanillin
Abstract
1. Introduction
2. Results and Discussion
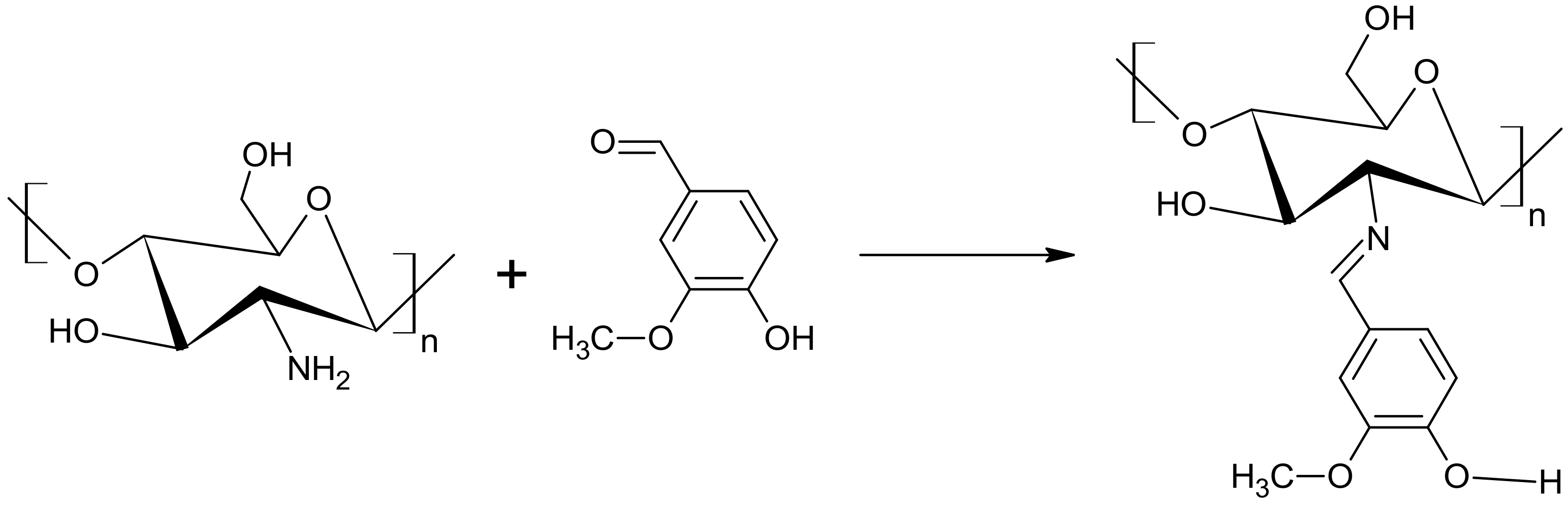
2.1. Chemical Structure of the Products
2.2. Infrared Spectra Discussion
2.3. Thermal Analysis
2.4. Scanning Electron Microscopy
2.5. Swelling Properties
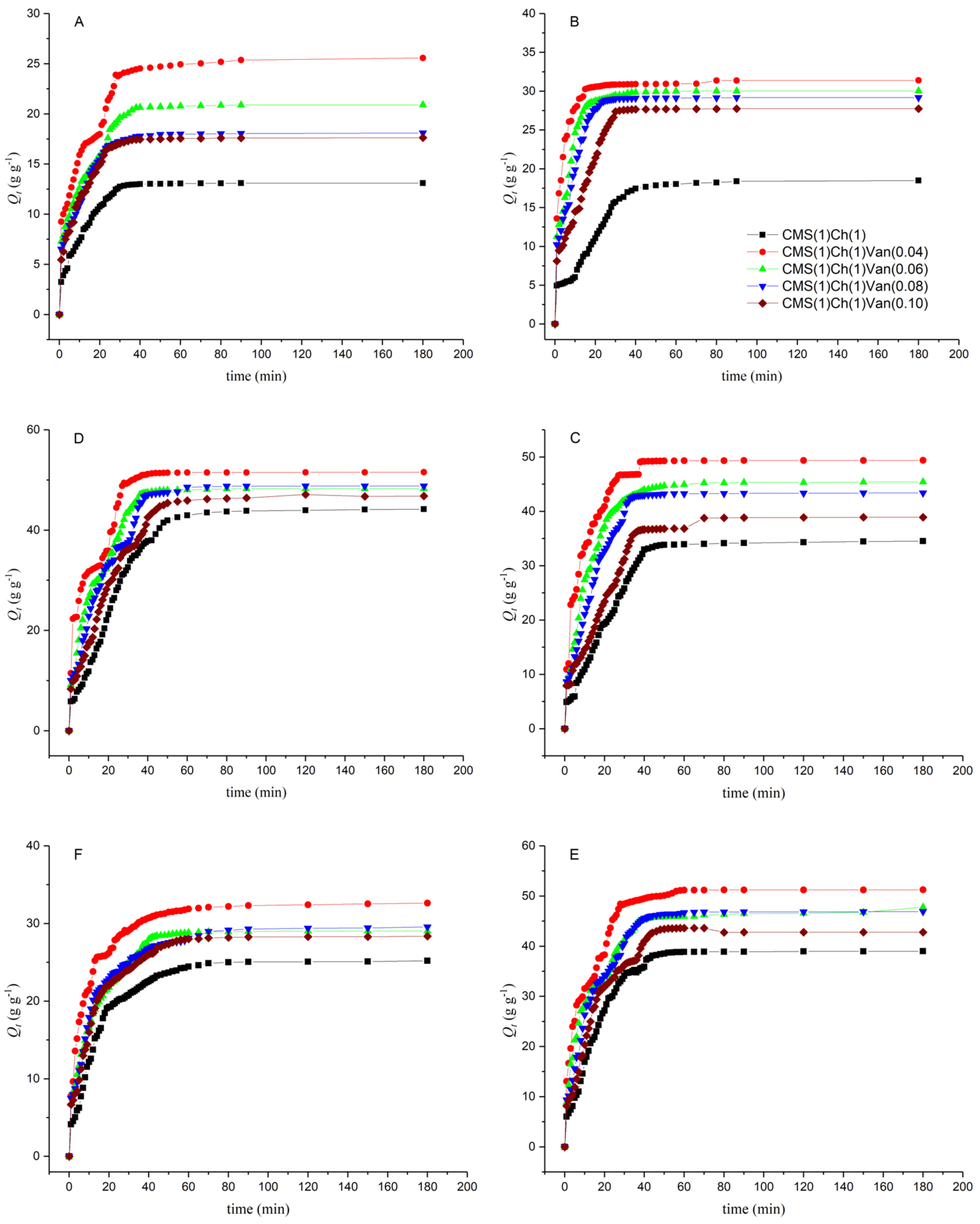
2.5.1. Swelling Properties in Deionized Water Depend on the Amount of Crosslinker Used
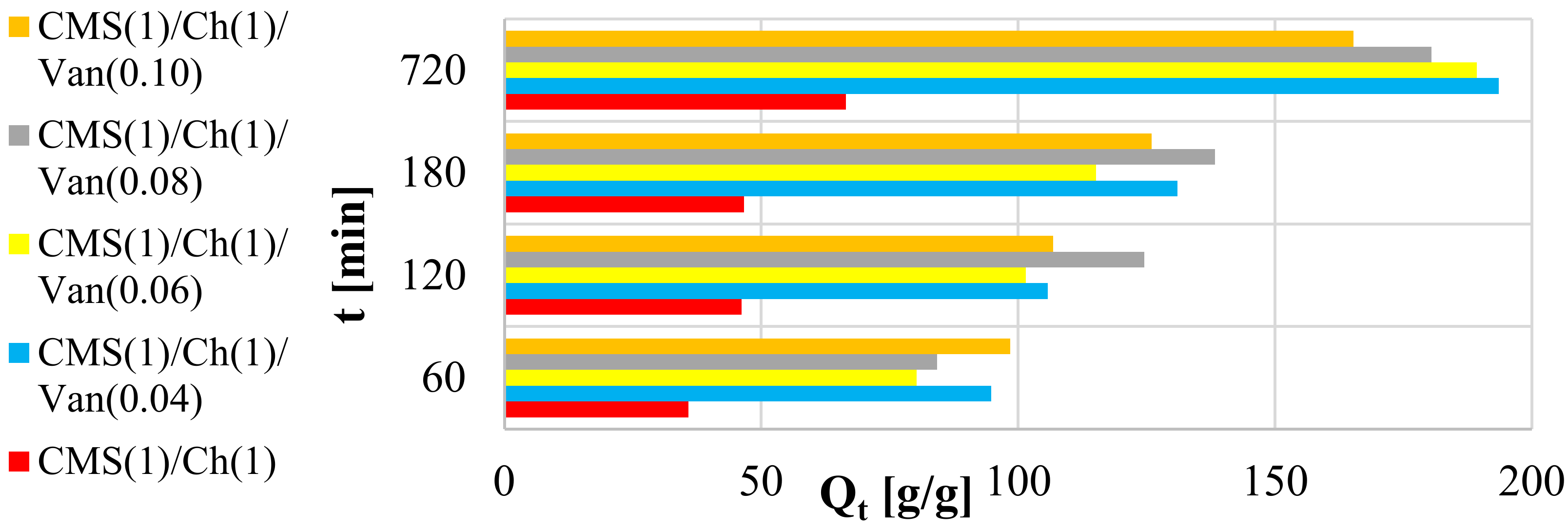
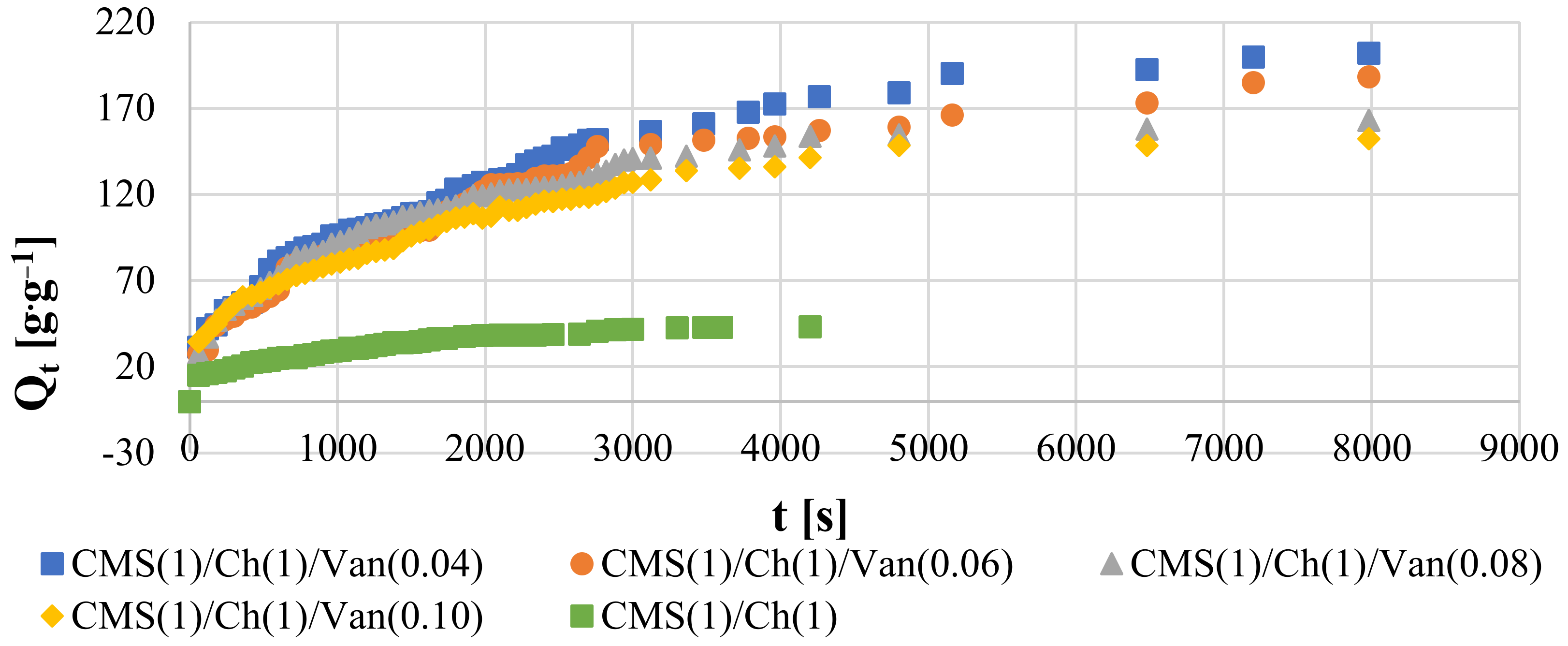
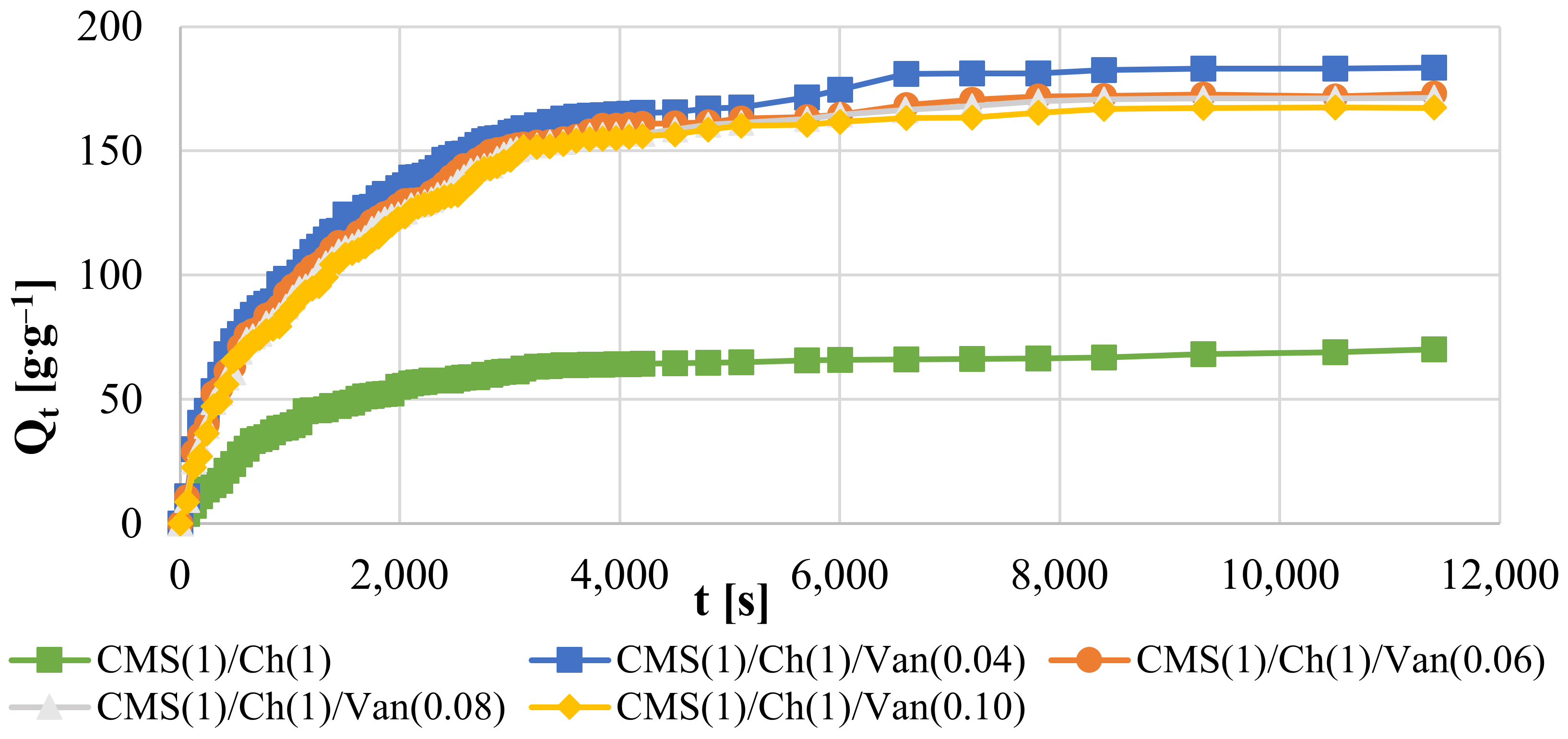
2.5.2. Swelling Kinetics at Room Temperature
2.5.3. Thermoresponsive Properties
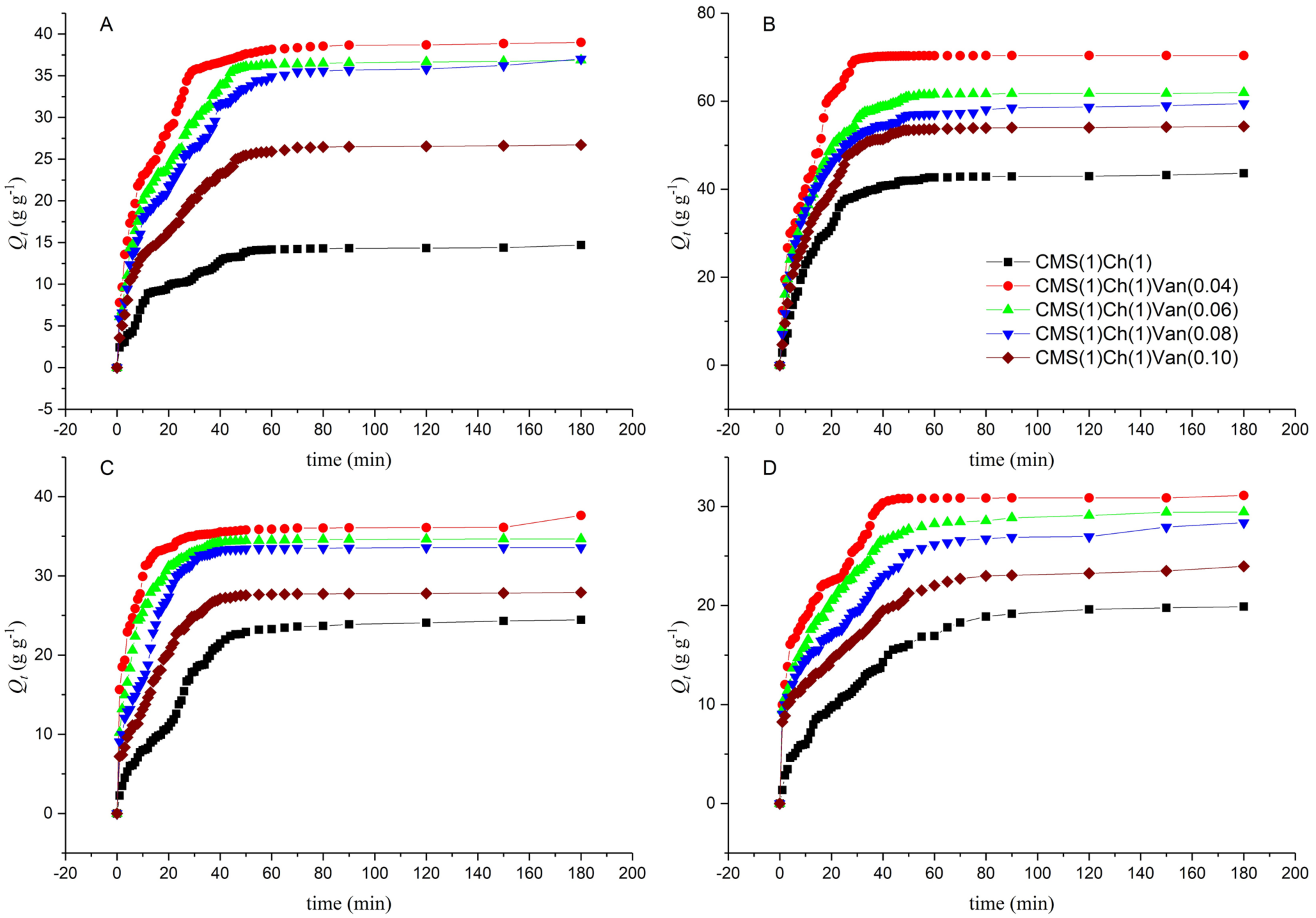
2.5.4. Swelling Behavior in Saline Solutions
2.5.5. Effect of pH on Equilibrium Swelling
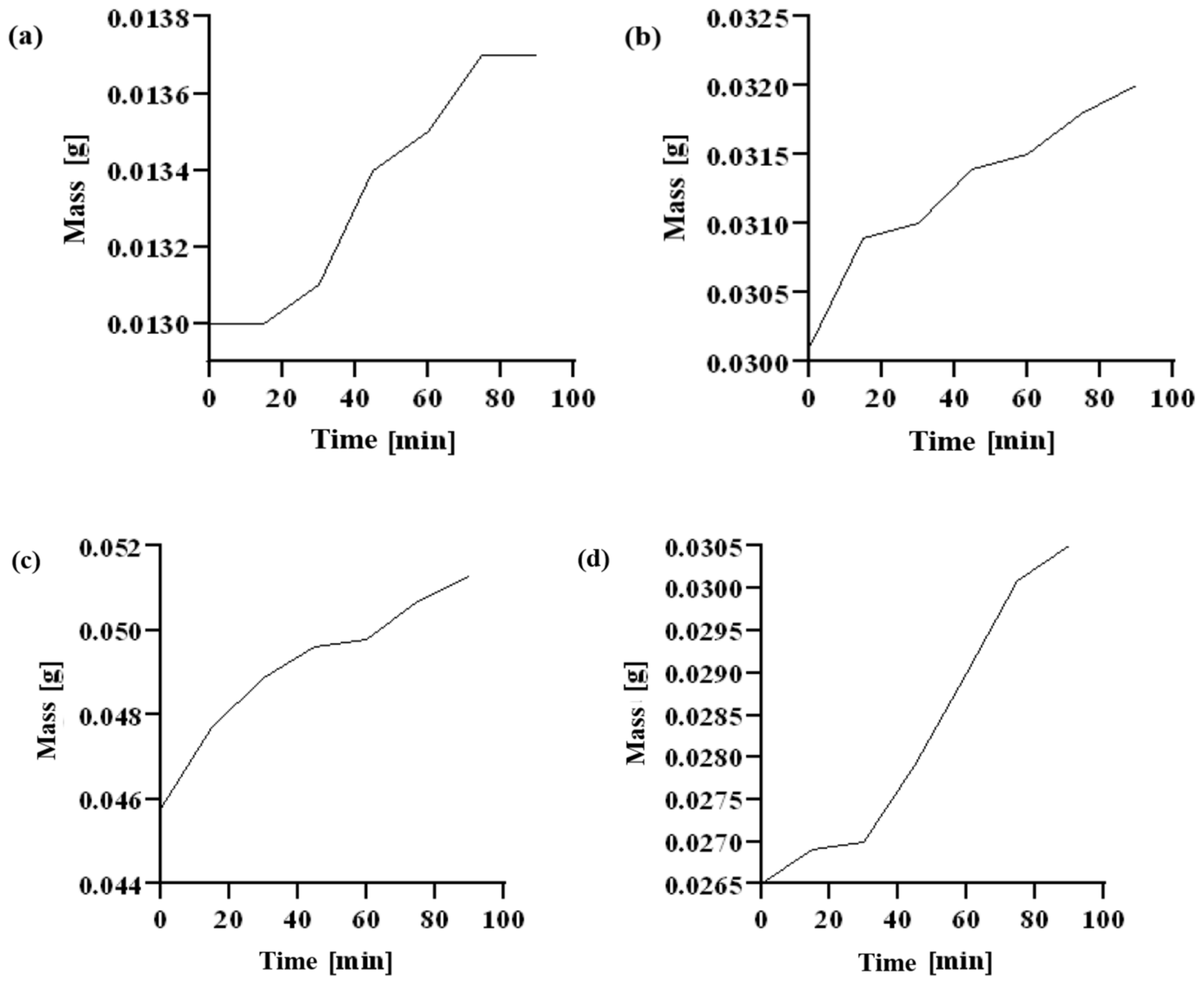
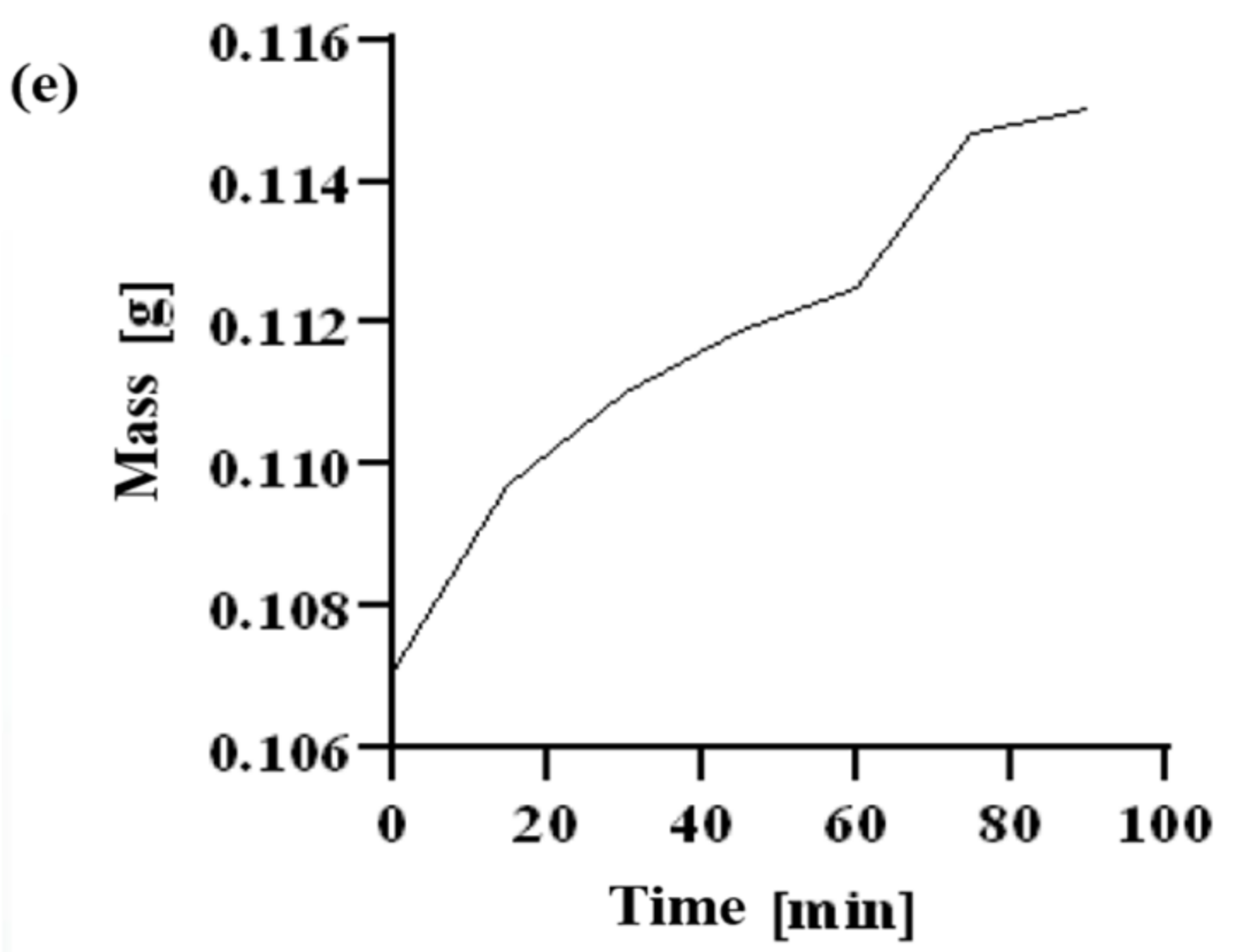
2.6. Dehydration Test
2.7. The Pro-Ecological Aspect
2.7.1. Composting Process
2.7.2. Ozone Aging
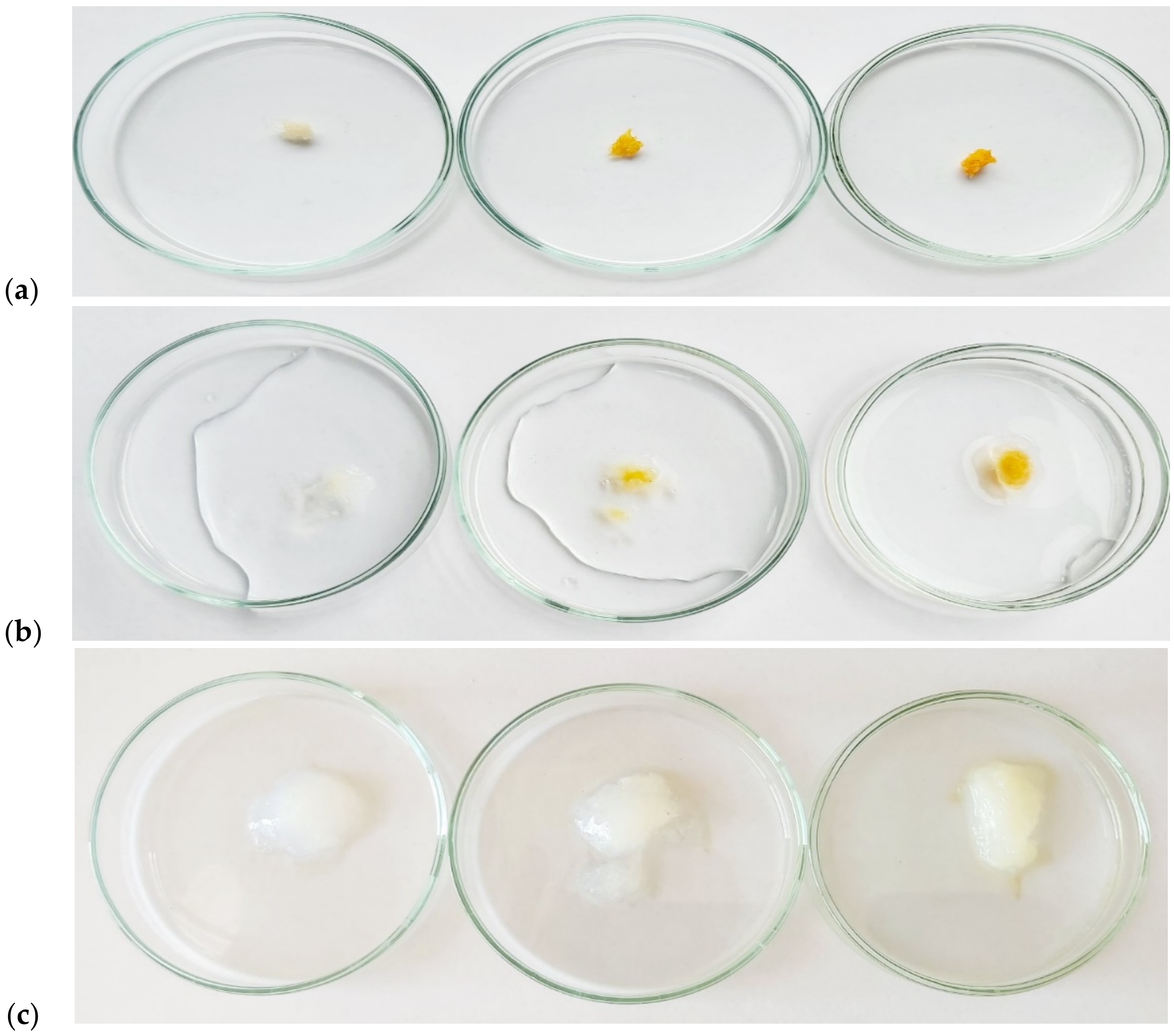
2.7.3. Deep and Surface Water Absorption
2.7.4. Biological Tests—Sowing Plants from the Organization for Economic Co-Operation and Development (OECD) Group
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Carboxymethyl Starch (CMS)
3.3. Preparation of a Superabsorbent Polymer Composed of CMS and Chitosan (Ch)
3.4. Characteristics of the Obtained Superabsorbent Polymers
3.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.2. Thermal Analysis
3.4.3. Scanning Electron Microscopy
3.4.4. Swelling Properties
3.4.5. Swelling Dynamics
3.4.6. Dehydration Tests
3.4.7. Statistical Assessment of the Data
3.4.8. The Pro-Ecological Aspect
Composting Process
Ozone Aging
Deep and Surface Water Absorption
Biological Tests—Sowing Plants from the Organization for Economic Co-Operation and Development (OECD) Group
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent polymers: A review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’ derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Raffa, P.; Picchioni, F.; Koning, C.E. Superabsorbent Polymers: From long-established, microplastics generating systems, to sustainable, biodegradable and future proof alternatives. Prog. Polym. Sci. 2022, 125, 101475. [Google Scholar] [CrossRef]
- Masuda, F.; Nishida, K.; Nakamura, A. Water Absorbing Starch Resins. U.S. Patent 4,076,663, 28 February 1978. [Google Scholar]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, S.; Zhang, Y.; Rempel, C.; Liu, Q. Treatments of protein for biopolymer production in view of processability and physical properties: A review. J. Appl. Polym. Sci. 2016, 133, 1–13. [Google Scholar] [CrossRef]
- Younes, B. Classification, characterization, and the production processes of biopolymers used in the textiles industry. J. Text. Inst. 2017, 108, 674–682. [Google Scholar] [CrossRef]
- Li, X.M.; Wu, Z.Z.; Zhang, B.; Pan, Y.; Meng, R.; Chen, H.Q. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2019, 293, 197–203. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in chemical modifications of starches and their applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Spychaj, T. Novel hydrophilic carboxymethyl starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2015, 128, 82–89. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Saini, P.; Dhawan, S.K. Synthesis of carboxymethyl potato starch and comparison of optimal reaction conditions from different sources. Polym. Adv. Technol. 2008, 19, 1185–1192. [Google Scholar] [CrossRef]
- Assaad, E.; Mateescu, M.A. The influence of protonation ratio on properties of carboxymethyl starch excipient at various substitution degrees: Structural insights and drug release kinetics. Int. J. Pharm. 2010, 394, 75–84. [Google Scholar] [CrossRef]
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and high substituted carboxymethyl starch: Synthesis, characterization and application. Starch-Stärke 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Tatongjai, J.; Lumdubwong, N. Physicochemical properties and textile utilization of low- and moderate-substituted carboxymethyl rice starches with various amylose content. Carbohydr. Polym. 2010, 81, 377–384. [Google Scholar] [CrossRef]
- Spychaj, T.; Zdanowicz, M.; Kujawa, J.; Schmidt, B. Carboxymethyl starch with high degree of substitution: Synthesis, properties and application. Polimery 2013, 58, 501–511. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Qu, G. Study on synthesis and properties of modified starch binder for foundry. J. Mater. Processing Technol. 2007, 183, 407–411. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Z.; Li, L. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef]
- Marroquin, J.B.; Rhee, K.Y.; Park, S.J. Chitosan nanocomposite films: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Acta Biomaterialia Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-kruszkowska, E. The role of a deep eutectic solvent in changes of physicochemical and antioxidative properties of chitosan-based films. Carbohydr. Polym. 2020, 255, 117527. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR study on cellulose with the varied moisture contents: Insight into the supramolecular structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Zou, Q.; Li, J.; Li, Y. Preparation and characterization of vanillin-crosslinked chitosan therapeutic bioactive microcarriers. Int. J. Biol. Macromol. 2015, 79, 736–747. [Google Scholar] [CrossRef]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-based blends for biomedical applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, P. Chemical modifications of polysaccharides. In Chemical and Functional Properties of Food Saccharides; Hindawi Publishing Corporation: London, UK, 2003; Volume 2013, pp. 123–130. ISBN 9780203495728. [Google Scholar]
- Assaad, E.; Wang, Y.J.; Zhu, X.X.; Mateescu, M.A. Polyelectrolyte complex of carboxymethyl starch and chitosan as drug carrier for oral administration. Carbohydr. Polym. 2011, 84, 1399–1407. [Google Scholar] [CrossRef]
- Silverstain, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; ISBN 0-471-39362-2. [Google Scholar]
- Zdanowicz, M.; Spychaj, T. Viscosity Changes of Aqueous Carboxymethyl Starch by Partial Crosslinking and Montmorillonite Addition. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Pereira, S.; França, D.; Fonseca, C.; Roselena, S. Chitosan-Sugarcane Bagasse Microspheres as Fertilizer Delivery: On/Off Water Availability System. J. Polym. Environ. 2020, 28, 2977–2987. [Google Scholar] [CrossRef]
- Griffiths, P.R. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules. Vib. Spectrosc. 1992, 4, 121. [Google Scholar] [CrossRef]
- Saikia, C.; Hussain, A.; Ramteke, A.; Sharma, H.K.; Maji, T.K. Carboxymethyl starch-chitosan-coated iron oxide magnetic nanoparticles for controlled delivery of isoniazid. J. Microencapsul. 2014, 32, 1–11. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, B.; Hu, X.; Jin, Z.; Xu, X.; Tian, Y. Preparation and characterization of carboxymethyl starch microgel with different crosslinking densities. Carbohydr. Polym. 2015, 124, 245–253. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Sun, H.; Fan, S.; Hu, D.; Lin, J. Superabsorbent conducting hydrogel from poly(acrylamide-aniline) with thermo-sensitivity and release properties. Carbohydr. Polym. 2008, 73, 473–481. [Google Scholar] [CrossRef]
- Suzuki, A.; Hara, T. Kinetics of one-dimensional swelling and shrinking of polymer gels under mechanical constraint. J. Chem. Phys. 2001, 114, 5012–5015. [Google Scholar] [CrossRef][Green Version]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural Superabsorbents Based on Starch-g-poly(acrylic acid): Modification, Synthesis and Application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, E.; Nowaczyk, J. Synthesis and Characterization Superabsorbent Polymers Made of Starch, Acrylic Acid, Acrylamide, Poly(Vinyl Alcohol), 2-Hydroxyethyl Methacrylate, 2-Acrylamido-2-methylpropane Sulfonic Acid. Int. J. Mol. Sci. 2021, 22, 4325. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
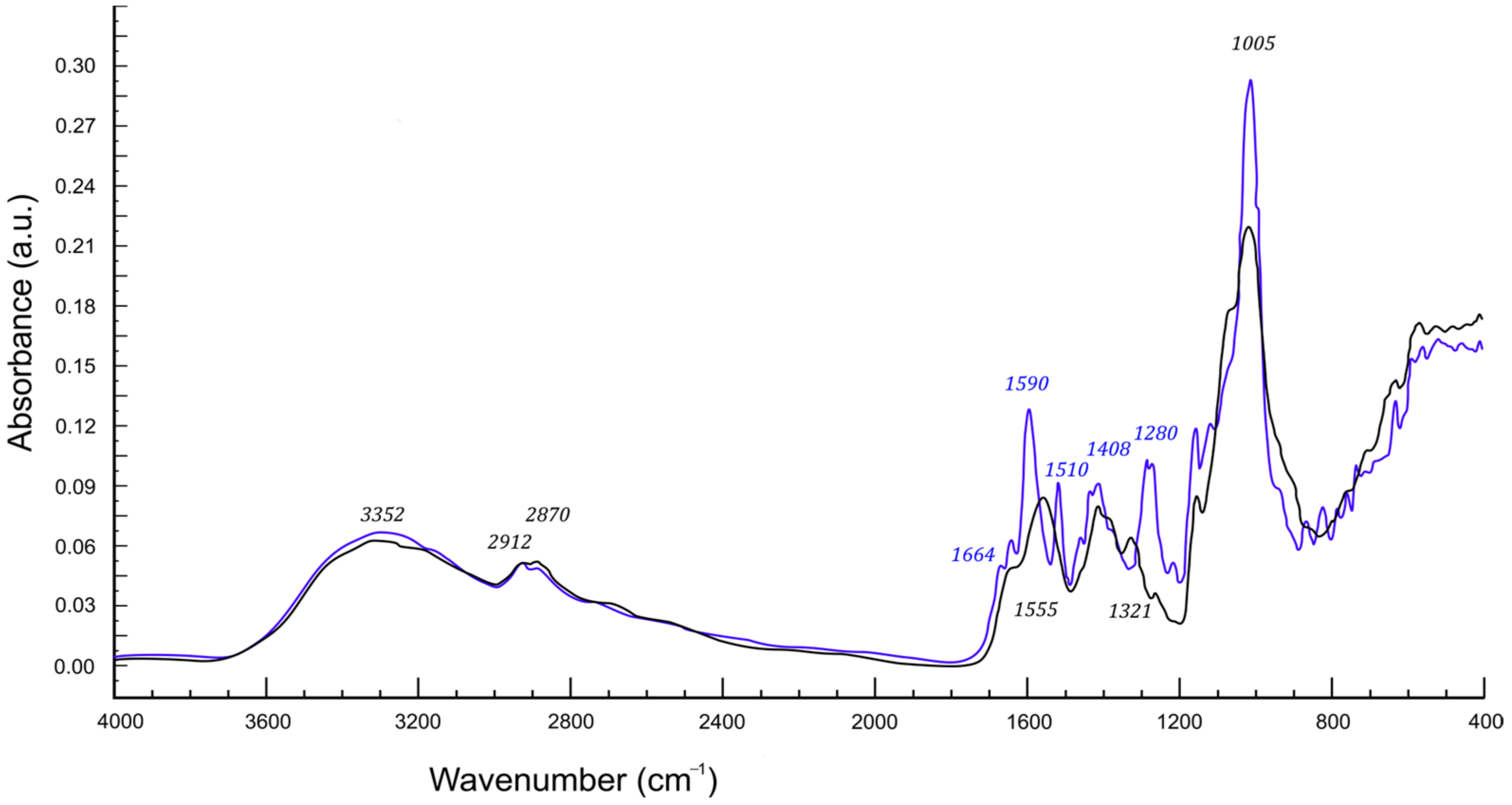

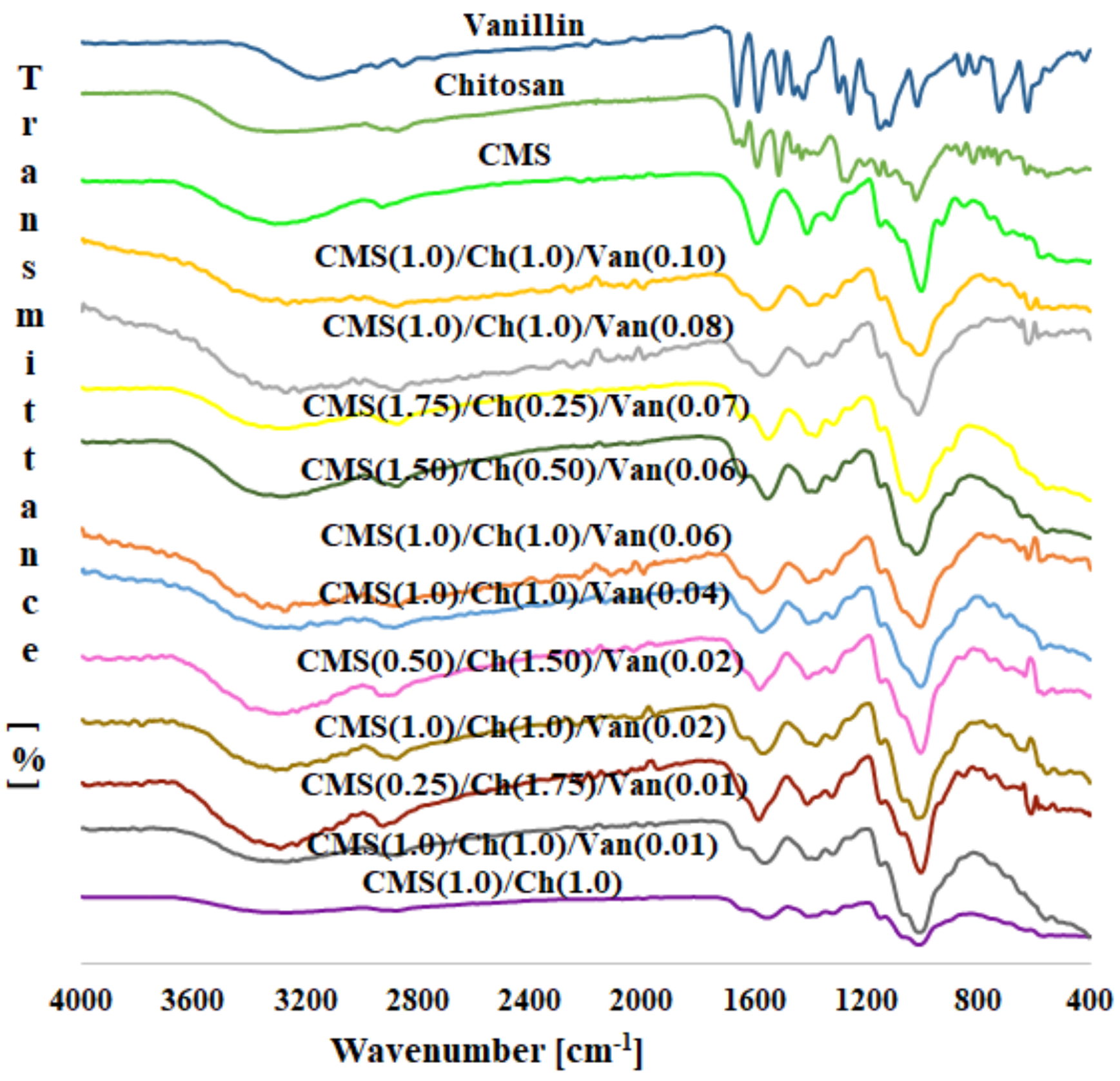
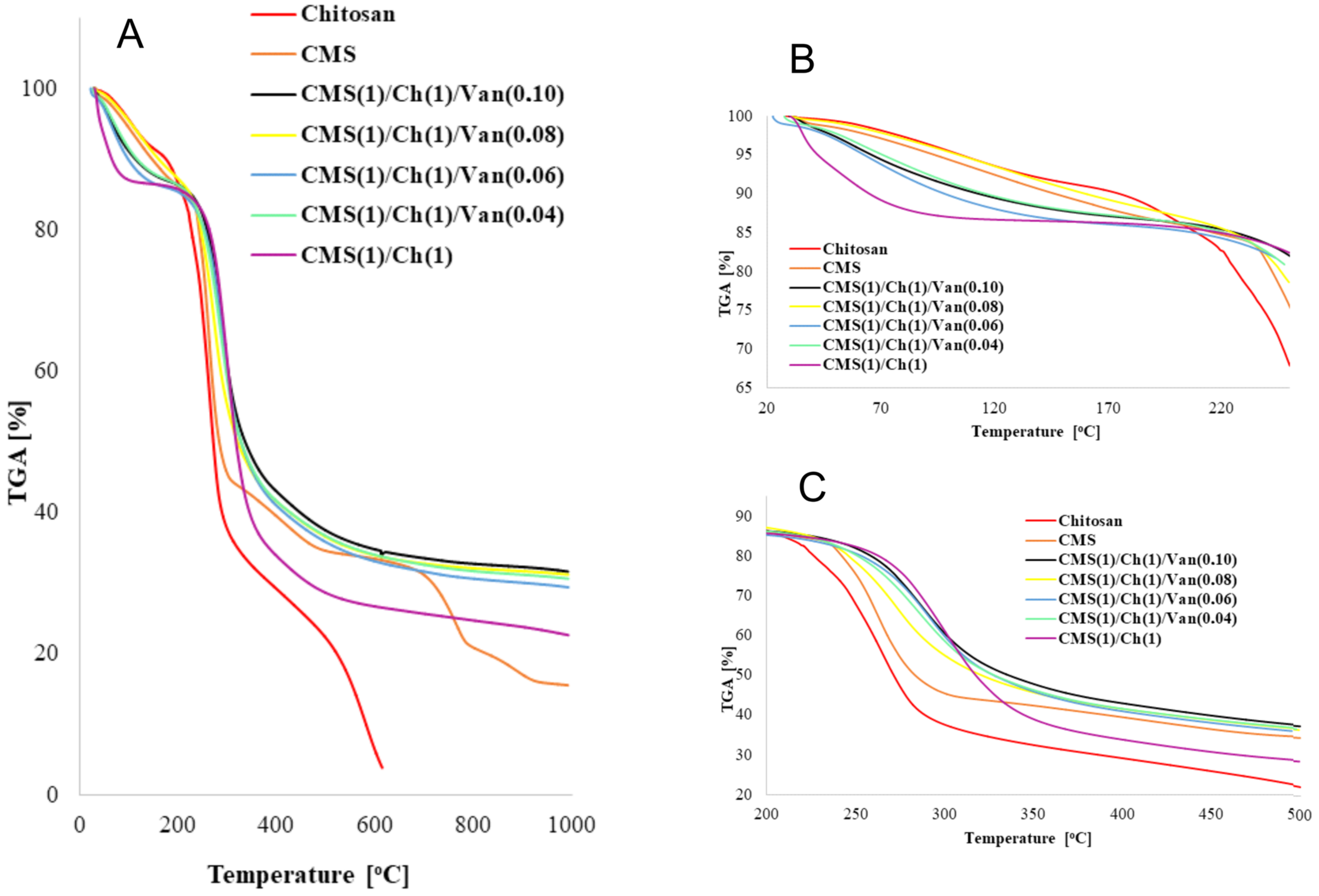
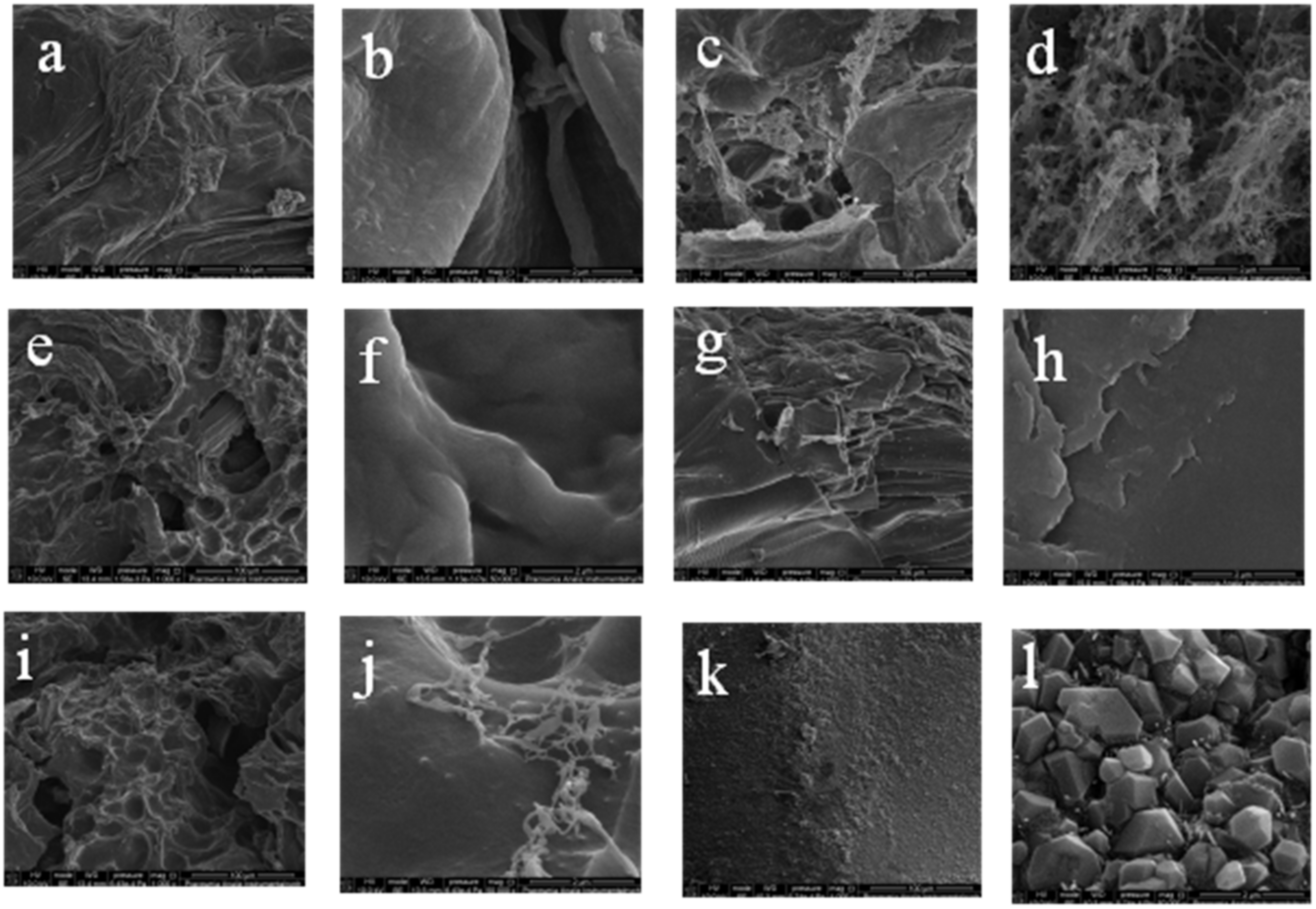




| Peak Position (cm−1) | Ch(1)/ CMS(1)/ Van(0) | Ch(1)/ CMS(1)/ Van(0.01) | Ch(1)/ CMS(1)/ Van(0.02) | Ch(1)/ CMS(1)/ Van(0.04) | Ch(1)/ CMS(1)/ Van(0.06) | Ch(1)/ CMS(1)/ Van(0.08) | Ch(1)/ CMS(1)/ Van(0.10) |
|---|---|---|---|---|---|---|---|
| 3352 | 0.2503 | 0.1948 | 0.3605 | 0.2992 | 0.7009 | 0.6933 | 0.3780 |
| 3011 | 0.0000 | 0.0016 | 0.0006 | 0.0034 | 0.0088 | 0.0086 | 0.0062 |
| 2912 | 0.0291 | 0.0322 | 0.0306 | 0.0306 | 0.0321 | 0.0292 | 0.0287 |
| 1639 | 0.0585 | 0.0432 | 0.0581 | 0.0592 | 0.0888 | 0.0864 | 0.0752 |
| 1558 | 0.0973 | 0.0701 | 0.0909 | 0.1172 | 0.1674 | 0.1603 | 0.1482 |
| 1406 | 0.0749 | 0.0525 | 0.0583 | 0.0818 | 0.1161 | 0.1159 | 0.1205 |
| 1375 | 0.0721 | 0.0558 | 0.0693 | 0.0780 | 0.1108 | 0.1154 | 0.1232 |
| 1317 | 0.0605 | 0.0469 | 0.0579 | 0.0738 | 0.0998 | 0.0924 | 0.0967 |
| 1259 | 0.0187 | 0.0148 | 0.0222 | 0.0194 | 0.0321 | 0.0432 | 0.0332 |
| 1150 | 0.0983 | 0.0767 | 0.0844 | 0.1164 | 0.1366 | 0.1408 | 0.1397 |
| 1064 | 0.2399 | 0.2700 | 0.3240 | 0.3524 | 0.4180 | 0.4440 | 0.5197 |
| 1005 | 0.3025 | 0.4207 | 0.5099 | 0.6001 | 0.6603 | 0.7202 | 0.7501 |
| Vanillin content | 0.0000 | 0.0100 | 0.0200 | 0.0400 | 0.0600 | 0.0800 | 0.1000 |
| 3352 | 3011 | 1639 | 1558 | 1406 | 1375 | 1317 | 1259 | 1150 | 1064 | 1005 | ||
| 3352 | 1 | |||||||||||
| 3011 | 0.774 | 1 | ||||||||||
| 1639 | 0.875 | 0.813 | 1 | |||||||||
| 1558 | 0.760 | 0.872 | 0.951 | 1 | ||||||||
| 1406 | 0.578 | 0.806 | 0.857 | 0.943 | 1 | |||||||
| 1375 | 0.573 | 0.779 | 0.843 | 0.902 | 0.966 | 1 | ||||||
| 1317 | 0.639 | 0.835 | 0.888 | 0.975 | 0.965 | 0.941 | 1 | |||||
| 1259 | 0.759 | 0.758 | 0.836 | 0.784 | 0.758 | 0.827 | 0.725 | 1 | ||||
| 1150 | 0.566 | 0.800 | 0.829 | 0.947 | 0.972 | 0.918 | 0.959 | 0.730 | 1 | |||
| 1064 | 0.391 | 0.686 | 0.594 | 0.702 | 0.762 | 0.862 | 0.792 | 0.680 | 0.748 | 1 | ||
| 1005 | 0.463 | 0.742 | 0.567 | 0.681 | 0.655 | 0.733 | 0.732 | 0.652 | 0.697 | 0.925 | 1 | |
| Van | 0.399 | 0.726 | 0.606 | 0.726 | 0.806 | 0.885 | 0.803 | 0.724 | 0.799 | 0.983 | 0.911 | 1 |
| Name Sample | Weight Change [%] at | Temperature [°C] at 50% Weight Change | Temperature of Complete Decomposition [°C] | |
|---|---|---|---|---|
| 120 °C (w 120 °C) | 275 °C (w 275 °C) | |||
| Chitosan | 6.86 | 52.95 | 271.27 | 264.95 |
| CMS | 7.56 | 45.58 | 283.27 | 262.59 |
| CMS(1)/Ch(1)/Van(0.10) | 10.53 | 26.15 | 335.69 | 286.85 |
| CMS(1)/Ch(1)/Van(0.08) | 12.91 | 28.67 | 321.13 | 288.46 |
| CMS(1)/Ch(1)/Van(0.06) | 11.95 | 26.81 | 327.35 | 291.34 |
| CMS(1)/Ch(1)/Van(0.04) | 10.32 | 28.82 | 327.70 | 285.05 |
| CMS(1)/Ch(1) | 13.35 | 23.90 | 317.34 | 302.12 |
| NaClaq | Sample | ||||
|---|---|---|---|---|---|
| Concentration | CMS(1)/Ch(1) | CMS(1)/Ch(1)/ Van(0.04) | CMS(1)/Ch(1)/ Van(0.06) | CMS(1)/Ch(1)/ Van(0.08) | CMS(1)/Ch(1)/ Van(0.10) |
| [%] | τ [s] | ||||
| 0.1 | 832 | 629 | 664 | 678 | 760 |
| 0.3 | 898 | 652 | 722 | 736 | 825 |
| 0.6 | 1783 | 685 | 746 | 765 | 906 |
| 0.9 | 1833 | 741 | 878 | 1049 | 1291 |
| CMS(1)/Ch(1) | CMS(1)/Ch(1)/Van(0.04) | CMS(1)/Ch(1)/Van(0.06) | CMS(1)/Ch(1)/Van(0.08) | CMS(1)/Ch(1)/Van(0.10) |
|---|---|---|---|---|
| Qeq [g·g−1] | ||||
| 75.99 | 183.87 | 174.51 | 171.72 | 167.47 |
| pH | Sample | ||||
|---|---|---|---|---|---|
| CMS(1)/Ch(1) | CMS(1)/Ch(1)/Van(0.04) | CMS(1)/Ch(1)/Van(0.06) | CMS(1)/Ch(1)/Van(0.08) | CMS(1)/Ch(1)/Van(0.10) | |
| τ [s] | |||||
| 1.48 | 214 | 168 | 174 | 177 | 185 |
| 3.00 | 413 | 47 | 131 | 178 | 286 |
| 4.30 | 512 | 151 | 252 | 351 | 470 |
| 7.00 | 512 | 122 | 252 | 351 | 470 |
| 9.70 | 364 | 152 | 166 | 293 | 347 |
| 12.40 | 375 | 142 | 213 | 246 | 258 |
| OZONE CHAMBER | ||||||
|---|---|---|---|---|---|---|
| Before Aging | After Aging | |||||
| C=O | C–H | Carbonyl Index | C=O | C–H | Carbonyl Index | |
| Chitosan | 0.06087 | 0.05633 | 1.08052 | 0.03252 | 0.06346 | 0.51242 |
| CMS | 0.02833 | 0.00724 | 3.91533 | 0.07305 | 0.06840 | 1.06792 |
| CMS(1)/Ch(1) | 0.01796 | 0.01694 | 1.06011 | 0.10436 | 0.09256 | 1.12758 |
| CMS(1)/Ch(1)/Van(0.04) | 0.08157 | 0.07953 | 1.02573 | 0.10666 | 0.09098 | 1.17229 |
| CMS(1)/Ch(1)/Van(0.08) | 0.04315 | 0.04693 | 0.91940 | 0.08700 | 0.08220 | 1.05834 |
| Time [min] | Chitosan | CMS | CMS(1)/Ch(1) | CMS(1)/Ch(1)/Van(0.04) | CMS(1)/Ch(1)/Van(0.08) |
|---|---|---|---|---|---|
| 0 | 0.0265 | 0.0458 | 0.1071 | 0.0130 | 0.0301 |
| 15 | 0.0269 | 0.0477 | 0.1097 | 0.0130 | 0.0309 |
| 30 | 0.0270 | 0.0489 | 0.1110 | 0.0131 | 0.0310 |
| 45 | 0.0279 | 0.0496 | 0.1119 | 0.0134 | 0.0314 |
| 60 | 0.0290 | 0.0498 | 0.1125 | 0.0135 | 0.0315 |
| 75 | 0.0301 | 0.0507 | 0.1147 | 0.0137 | 0.0318 |
| 90 | 0.0305 | 0.0513 | 0.1150 | 0.0137 | 0.0320 |
| Time [h] | CMS(1)/Ch(1) | CMS(1)/Ch(1)/Van(0.04) | CMS(1)/Ch(1)/Van(0.08) |
|---|---|---|---|
| 0 | 0.0280 | 0.0280 | 0.0353 |
| 3 | 0.9761 | 0.4289 | 0.3656 |
| 24 | 2.7959 | 1.0257 | 0.4936 |
| 48 | 4.2158 | 1.7213 | 0.4115 |
| Factor | 151 | 62 | 16 |
| Name of Sample | |||||||
|---|---|---|---|---|---|---|---|
| Time [days] | Native Sample | CMS(1)/Ch(1)/ Van(0.04) | CMS(1)/Ch(1)/ Van(0.08) | CMS(1)/Ch(1) | Chitosan | CMS | |
| 0 | Quantity | - | - | - | - | - | - |
| Length [cm] | - | - | - | - | - | - | |
| Σ length [cm] | - | - | - | - | - | - | |
| 3 | Quantity | 4 | - | 4 | 2 | 4 | 4 |
| Length [cm] | 2.5; 3.5; 2.2; 3.0 | - | 3.0; 2.4; 2.0; 1.5 | 0.7; 0.5 | 2.8; 2.0; 1.2; 1.0 | 1.0; 1.6; 1.5; 2.0 | |
| Σ length [cm] | 02.9 | - | 2.23 | 0.6 | 1.75 | 1.53 | |
| 5 | Quantity | 4 | 1 | 4 | 3 | 4 | 4 |
| Length [cm] | 5.3; 4.3; 4.0; 4.3 | little sprout | 5.0; 4.0; 4.7; 3.2 | 1.9; 1.0; 1.2 | 5.8; 3.0; 4.3; 2.5 | 2.5; 3.8; 3.5; 4.1 | |
| Σ length [cm] | 4.48 | little sprout | 4.23 | 1.37 | 3.9 | 3.48 | |
| 7 | Quantity | 4 | 4 | 4 | 4 | 5 | 5 |
| Length [cm] | 10.2; 11.3; 11.1; 9.5 | 0.4; 0.9; 0.6; 1.3 | 12.4; 11.0; 11.2; 9.9 | 1.9; 5.5; 5.5; 8.0 | 10.4; 8.7; 11.2; 7.2; 2.0 | 0.4; 10.2; 9.9; 9.8; 8.4 | |
| Σ length [cm] | 10.53 | 0.8 | 11.13 | 5.23 | 7.9 | 7.74 | |
| 8 | Quantity | 4 | 6 | 4 | 4 | 5 | 5 |
| Length [cm] | 13.0; 14.7; 13.1; 11.7 | 2.7; 3.0; 3.2; 4.0; 0.6; 1.2 | 15.9; 14.0; 15.0; 14.6 | 12.0; 8.9; 9.7; 6.5 | 12.1; 10.5; 3.1; 7.1; 12.2 | 12.4; 12.0; 13.0; 13.0; 0.4 | |
| Σ length [cm] | 13.13 | 2.45 | 14.88 | 9.28 | 9.0 | 10.16 | |
| 10 | Quantity | 4 | 5 | 4 | 4 | 5 | 5 |
| Length [cm] | 15.5; 15.0; 15.6; 19.0 | 8.1; 7.6; 11.6; 2.0; 6.0 | 18.4; 18.1; 18.3; 20.1 | 15.1; 13.0; 12.2; 15.3 | 16.3; 15.5; 14.4; 9.0; 7.1 | 16.4; 16.3; 16.1; 16.9; 3.3 | |
| Σ length [cm] | 16.28 | 7.06 | 18.73 | 13.9 | 12.46 | 13.8 | |
| 15 | Quantity | 4 | 6 | 4 | 4 | 5 | 5 |
| Length [cm] | 15.6; 16.3; 19.5; 16.4 | 13.1; 10.2;13.0;14.5; 15.8;0.1 | 19.0; 18.5; 18.7; 24.0 | 15.5; 15.3; 16.3; 17.8 | 18.7; 15.4; 11.0; 17.4; 11.1 | 19.1; 6.1; 17.2; 17.6; 17.0 | |
| Σ length [cm] | 16.95 | 11.12 | 20.05 | 16.23 | 14.72 | 15.4 | |
| 19 | Quantity | 4 | 6 | 4 | 4 | 5 | 5 |
| Length [cm] | 15.7; 20.5; 17.4; 16.0 | 0.5; 15.6; 20.0; 21.8; 14.0; 10.5 | 19.8; 18.9; 18.8; 19.5 | 24.0; 19.0; 20.1; 21.0 | 18.9; 11.0; 15.2; 20.6; 21.2 | 6.4; 19.3; 19.0; 21.0; 20,.5 | |
| Σ length [cm] | 17.4 | 13.73 | 19.25 | 21.03 | 17.38 | 17.24 | |
| 27 | Quantity | 4 | 6 | 4 | 4 | 5 | 5 |
| Length [cm] | 24.5; 17.2; 21.7; 19.0 | 24.2; 11.0; 0.5; 22.9; 21.8; 22.0 | 23.9; 19.9; 22.3; 21.6 | 21.8; 26.0; 21.2; 21.0 | 24.1; 23.1; 20.2; 17.0; 10.2 | 22.2; 23.5; 18.2; 21.8; 7.0 | |
| Σ length [cm] | 20.6 | 17.07 | 21.93 | 22.5 | 18.92 | 18.54 | |
| Element | mg/kg Dry Substance | Element | mg/kg Dry Substance |
|---|---|---|---|
| Zn | 150 | Cr | 50 |
| Cu | 50 | Mo | 1 |
| Ni | 25 | Se | 0.75 |
| Cd | 0.5 | As | 5 |
| Pb | 50 | F | 100 |
| Hg | 0.5 |
| Native Sample | |||||||
|---|---|---|---|---|---|---|---|
| Fe | Zn | Mn | Ti | Pd | Zr | Cu | Cr |
| 33.411 | 3.017 | 3.095 | <LOD | 13.426 | 3.135 | 2.704 | 0.367 |
| 30.975 | 2.819 | 3.485 | 2.141 | 16.305 | <LOD | 0.911 | <LOD |
| 29.536 | 2.916 | 4.260 | 2.436 | 12.245 | <LOD | 0.405 | <LOD |
| 29.582 | 2.513 | 3.793 | 1.666 | 14.726 | 2.469 | 0.966 | <LOD |
| CMS | |||||||
| Fe | Zn | Mn | Ti | Pd | Zr | Cu | Cr |
| 32.482 | 4.063 | 3.510 | 3.023 | <LOD | <LOD | <LOD | <LOD |
| 31.116 | 2.699 | 3.087 | 4.354 | 13.159 | 1.917 | 0.357 | <LOD |
| 29.425 | 3.142 | 3.885 | 4.341 | <LOD | 2.256 | <LOD | 0.593 |
| 31.47 | 3.275 | 4.061 | <LOD | 14.357 | <LOD | 2.713 | 0.838 |
| 32.528 | 2.422 | 2.919 | 3.510 | 15.450 | <LOD | 1.024 | <LOD |
| CMS(1)/Ch(1)/Van(0.04) | |||||||
| Fe | Zn | Mn | Ti | Pd | Zr | Cu | Cr |
| 30.356 | 2.963 | 3.680 | <LOD | 16.707 | 2.394 | 2.215 | 1.151 |
| 32.078 | 2.552 | 3.674 | <LOD | 12.297 | 2.160 | 0.960 | <LOD |
| 32.100 | 3.613 | <LOD | 2.136 | 15.910 | 2.423 | 1.845 | 0.862 |
| 27.475 | 2.450 | 4.665 | 2.489 | 12.512 | 1.879 | 0.753 | <LOD |
| Zn | Mn | Ni | Cu |
|---|---|---|---|
| Native Sample | |||
| 6.736 | 3.442 | 2.04 | <LOD |
| 6.037 | 4.209 | <LOD | 2.993 |
| 6.463 | 3.43 | 1.612 | <LOD |
| CMS | |||
| 6.924 | 3.554 | 1.446 | <LOD |
| 6.261 | 2.268 | 1.511 | <LOD |
| CMS(1)/Ch(1)/Van(0.04) | |||
| 7.322 | 6.478 | 1.405 | <LOD |
| 7.877 | 6.091 | 1.279 | <LOD |
| 7.118 | 6.249 | 2.060 | <LOD |
| 5.612 | 4.800 | <LOD | <LOD |
| Sample Code | Description |
|---|---|
| Ch(1)/CMS(1)/Van(0) | Chitosan, carboxymethyl starch, without the addition of vanillin |
| Ch(1)/CMS(1)/Van(0.01) | Chitosan, carboxymethyl starch with the addition of 0.01 g of vanillin |
| Ch(1)/CMS(1)/Van(0.02) | Chitosan, carboxymethyl starch with the addition of 0.02 g of vanillin |
| Ch(1)/CMS(1)/Van(0.04) | Chitosan, carboxymethyl starch with the addition of 0.04 g of vanillin |
| Ch(1)/CMS(1)/Van(0.06) | Chitosan, carboxymethyl starch with the addition of 0.06 g of vanillin |
| Ch(1)/CMS(1)/Van(0.08) | Chitosan, carboxymethyl starch with the addition of 0.08 g of vanillin |
| Ch(1)/CMS(1)/Van(0.10) | Chitosan, carboxymethyl starch with the addition of 0.10 g of vanillin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka, E.; Nowaczyk, J.; Prochoń, M.; Masek, A. Nanoarchitectonics for Biodegradable Superabsorbent Based on Carboxymethyl Starch and Chitosan Cross-Linked with Vanillin. Int. J. Mol. Sci. 2022, 23, 5386. https://doi.org/10.3390/ijms23105386
Czarnecka E, Nowaczyk J, Prochoń M, Masek A. Nanoarchitectonics for Biodegradable Superabsorbent Based on Carboxymethyl Starch and Chitosan Cross-Linked with Vanillin. International Journal of Molecular Sciences. 2022; 23(10):5386. https://doi.org/10.3390/ijms23105386
Chicago/Turabian StyleCzarnecka, Elżbieta, Jacek Nowaczyk, Mirosława Prochoń, and Anna Masek. 2022. "Nanoarchitectonics for Biodegradable Superabsorbent Based on Carboxymethyl Starch and Chitosan Cross-Linked with Vanillin" International Journal of Molecular Sciences 23, no. 10: 5386. https://doi.org/10.3390/ijms23105386
APA StyleCzarnecka, E., Nowaczyk, J., Prochoń, M., & Masek, A. (2022). Nanoarchitectonics for Biodegradable Superabsorbent Based on Carboxymethyl Starch and Chitosan Cross-Linked with Vanillin. International Journal of Molecular Sciences, 23(10), 5386. https://doi.org/10.3390/ijms23105386









