Remodeling Osteoarthritic Articular Cartilage under Hypoxic Conditions
Abstract
1. Introduction
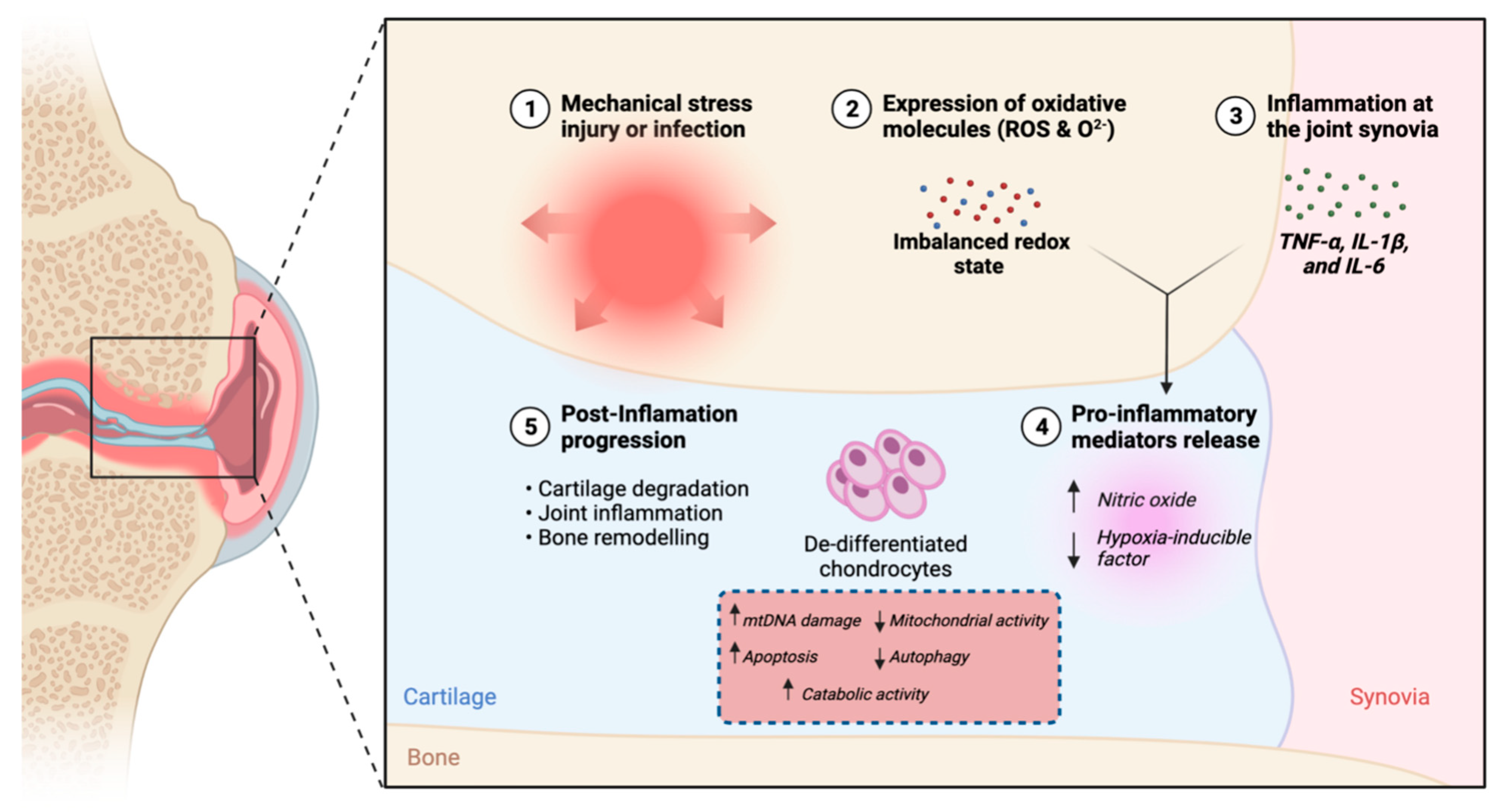
2. Pathophysiology of Osteoarthritis
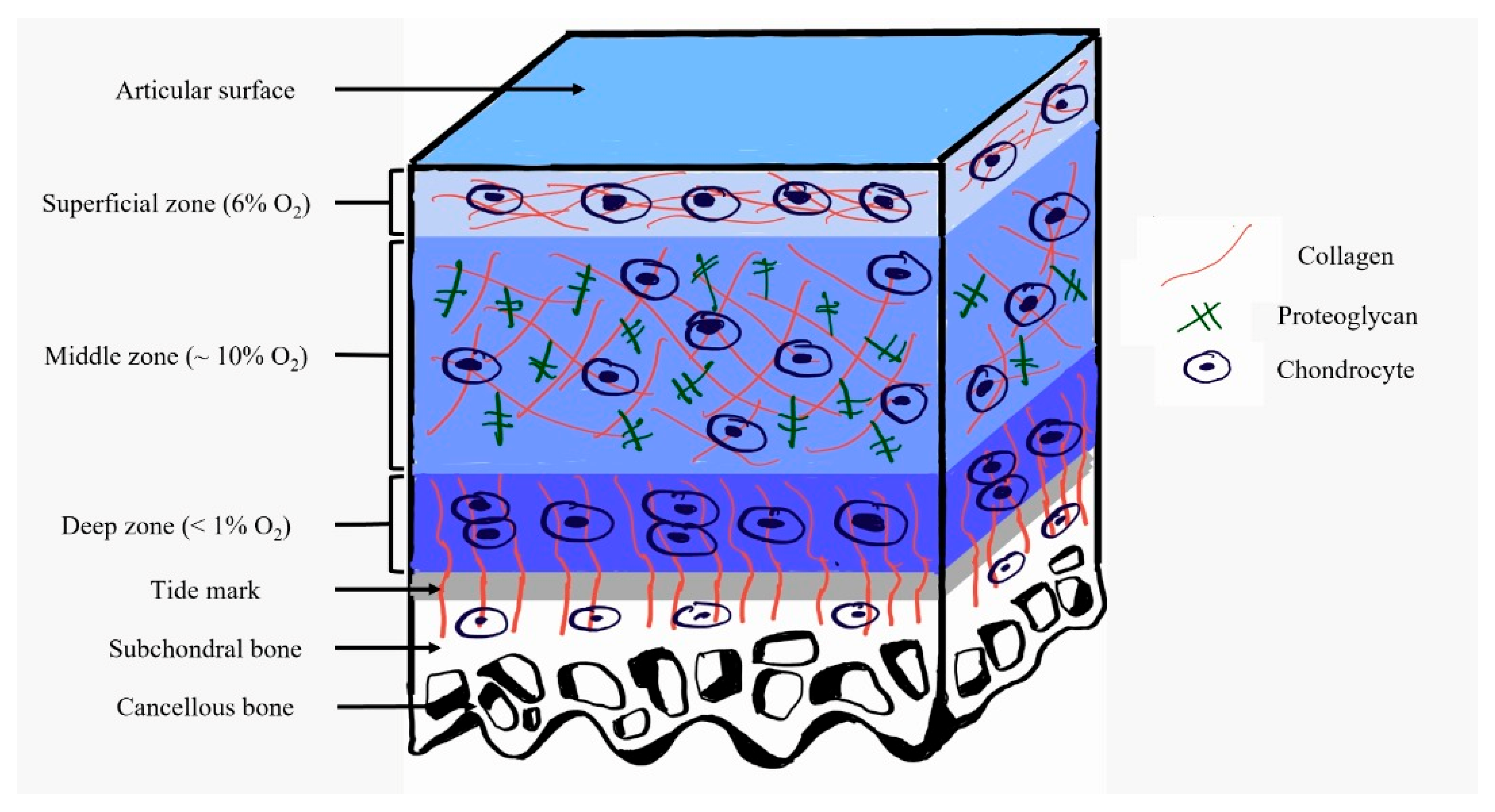
3. The Role of Hypoxia in Defeating Osteoarthritis
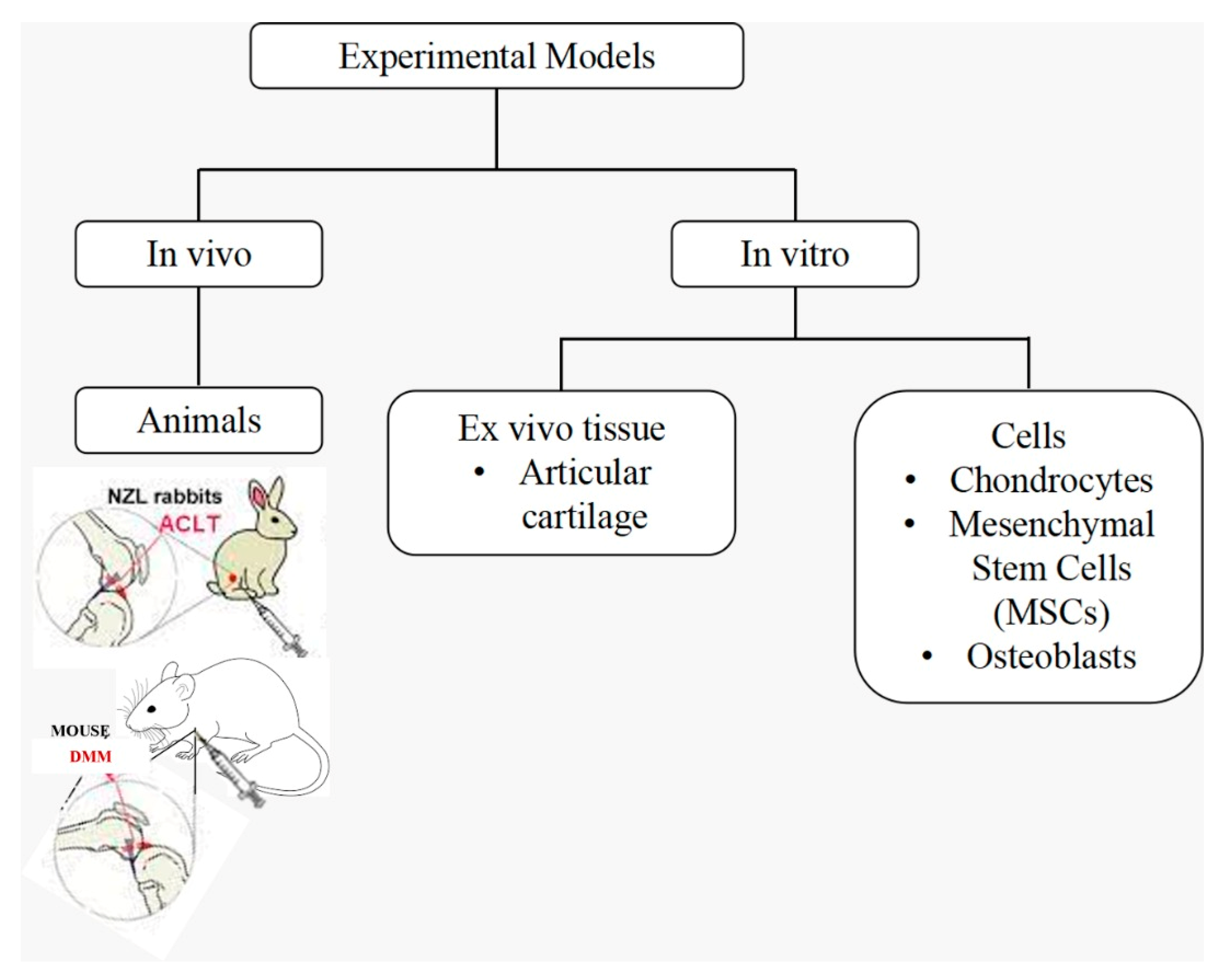
4. Current Experimental Models Addressing Osteoarthritis Treatment by Hypoxia
4.1. In Vitro Models
4.1.1. Cells
4.1.2. Ex Vivo Tissue
4.2. In Vivo Models
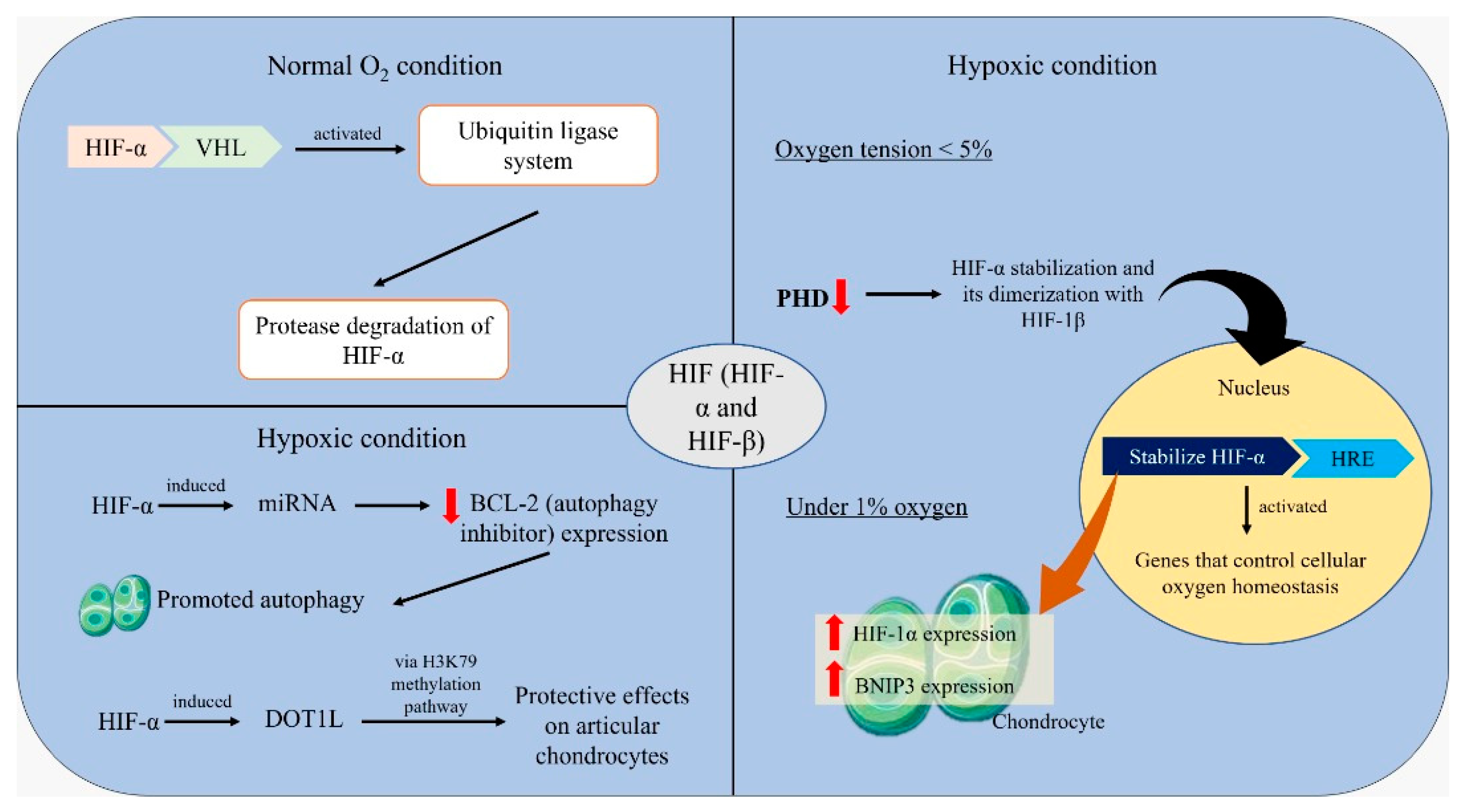
5. Mechanisms of Hypoxic Regulation
6. Concluding Remarks and Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Organization Osteoarthritis. 2012. Available online: https://www.who.int/medicines/areas/priority_medicines/Ch6_12Osteo.pdf (accessed on 16 February 2022).
- Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion D of PH. Osteoarthritis (OA). 2020. Available online: https://www.cdc.gov/arthritis/basics/osteoarthritis.htm (accessed on 16 February 2022).
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef] [PubMed]
- Thysen, S.; Luyten, F.P.; Lories, R.J.U. Targets, models and challenges in osteoarthritis research. DMM Dis. Models Mech. 2015, 8, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Giordano, T.; Kevil, C.G. Nitrite and nitric oxide metabolism in peripheral artery disease. Nitric Oxide Biol. Chem. 2012, 26, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Rieder, B.; Weihs, A.M.; Weidinger, A.; Szwarc, D.; Nürnberger, S.; Redl, H.; Rünzler, D.; Huber-Gries, C.; Teuschl, A.H. Hydrostatic pressure-generated reactive oxygen species induce osteoarthritic conditions in cartilage pellet cultures. Sci. Rep. 2018, 8, 17010. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Jovanovic, D.; Fernandes, J.C.; Manning, P.; Connor, J.R.; Currie, M.G.; Di Battista, J.A.; Martel-Pelletier, J. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum 1998, 41, 1275–1286. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Nojiri, H.; Ozawa, Y.; Watanabe, K.; Muramatsu, Y.; Kaneko, H.; Morikawa, D.; Kobayashi, K.; Saita, Y.; Sasho, T.; et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015, 5, srep11722. [Google Scholar] [CrossRef]
- Ross, A.D.; Banda, N.K.; Muggli, M.; Arend, W.P. Enhancement of collagen-induced arthritis in mice genetically deficient in extracellular superoxide dismutase. Arthritis Rheum 2004, 50, 3702–3711. [Google Scholar] [CrossRef]
- Fermor, B.; Weinberg, J.B.; Pisetsky, D.S.; Guilak, F. The influence of oxygen tension on the induction of nitric oxide and prostaglandin E2 by mechanical stress in articular cartilage. Osteoarthr. Cart. 2005, 13, 935–941. [Google Scholar] [CrossRef][Green Version]
- Fernández-Torres, J.; Martínez-Nava, G.A.; Gutiérrez-Ruíz, M.C.; Gomez-Quiroz, L.E.; Gutiérrez, M. Role of HIF-1α signaling pathway in osteoarthritis: A systematic review. Rev. Bras. Reumatol. 2017, 57, 162–173. [Google Scholar] [CrossRef]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Schoones, J.W.; Kloppenburg, M.; Ioan-Facsinay, A. Fatty acids and osteoarthritis: Different types, different effects. Jt. Bone Spine 2018, 86, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Kohsaka, H.; Liu, M.F.; Higashiyama, H.; Hirata, Y.; Kanno, K.; Saito, I.; Miyasaka, N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J. Clin. Investig. 1995, 96, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzì, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Van Der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; Van Der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Li, D.; Ni, S.; Miao, K.-S.; Zhuang, C. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones 2019, 24, 195–202. [Google Scholar] [CrossRef]
- Rego-Perez, I.; Fernandez-Moreno, M.; Soto-Hermida, A.; Fenandez-Lopez, C.; Oreiro, N.; Blanco, F.J. Mitochondrial genetics and osteoarthritis. Front. Biosci-Sch. 2013, S5, 360–368. [Google Scholar] [CrossRef]
- Grishko, V.; Ho, R.; Wilson, G.; Pearsall, A. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthr. Cart. 2009, 17, 107–113. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Xu, M.; Xo, R.; Mates, A.; Wilson, G.; Pearsall, A.; Grishko, V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr. Cartil. 2010, 18, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.; Nordin, A.; Kamal, H. Pathophysiological perspective of osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Fermor, B.; Christensen, S.E.; Youn, I.; Cernanec, J.M.; Davies, C.M.; Weinberg, J.B. Oxygen, nitric oxide and articular cartilage. Eur. Cells Mater. 2007, 13, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Zákány, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed interplay between autophagy and inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Valdes, A.; Rego-Pérez, I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018, 14, 327–340. [Google Scholar] [CrossRef]
- Cernanec, J.; Guilak, F.; Weinberg, J.B.; Pisetsky, D.S.; Fermor, B. Influence of hypoxia and reoxygenation on cytokine-induced production of proinflammatory mediators in articular cartilage. Arthritis Rheum. 2002, 46, 968–975. [Google Scholar] [CrossRef]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.-L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biology 2018, 71–72, 40–50. [Google Scholar] [CrossRef]
- Ströbel, S.; Loparic, M.; Wendt, D.; Schenk, A.D.; Candrian, C.; Lindberg, R.L.; Moldovan, F.; Barbero, A.; Martin, I. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res. Ther. 2010, 12, R34. [Google Scholar] [CrossRef] [PubMed]
- Lafont, J.E.; Talma, S.; Hopfgarten, C.; Murphy, C.L. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol Chem. 2008, 283, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Fermor, B.; Youn, I.; Christensen, S.E.; Guilak, F. The Influence of Oxygen Tension on Matrix Turnover and Physical Properties of Articular Cartilage. In Proceedings of the 51st Annual Meeting of the Orthopaedic Research Society, Durham, NC, USA, 11–13 September 2014; p. 299. [Google Scholar]
- Hashimoto, K.; Fukuda, K.; Yamazaki, K.; Yamamoto, N.; Matsushita, T.; Hayakawa, S.; Munakata, H.; Hamanishi, C. Hypoxia-induced hyaluronan synthesis by articular chondrocytes: The role of nitric oxide. Inflamm Res. 2006, 55, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Gong, X.; Dou, C.; Cao, Z.; Dong, S. Redox control of chondrocyte differentiation and chondrogenesis. Free. Radic. Biol. Med. 2019, 132, 83–89. [Google Scholar] [CrossRef]
- Heikal, M.Y.M.; Nazrun, S.A.; Chua, K.H.; Norzana, A.G. Stichopus chloronotus aqueous extract as a chondroprotective agent for human chondrocytes isolated from osteoarthitis articular cartilage in vitro. Cytotechnology 2019, 71, 521–537. [Google Scholar] [CrossRef]
- Mohd Heikal, M.Y.; Ahmad Nazrun, S.; Mohd Fauzi, B.; Chua, K.H.; Norzana, A.G.; Mohd, R.B. Pro-Chondrogenic Propensity of Sticopus Chloronotus Aqueous Extracts On Human Osteoarthritis Articular Chondrocytes In Vitro. Indian J. Med. Res. Pharm Sci. 2017, 4, 37–50. [Google Scholar]
- Zhang, F.; Wang, J.; Chu, J.; Yang, C.; Xiao, H.; Zhao, C.; Sun, Z.; Gao, X.; Chen, G.; Han, Z.; et al. MicroRNA-146a induced by hypoxia promotes chondrocyte autophagy through Bcl-2. Cell. Physiol. Biochem. 2015, 37, 1442–1453. [Google Scholar] [CrossRef]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Chérel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, C355–C364. [Google Scholar] [CrossRef]
- Betancourt, M.C.C.; Cailotto, F.; Kerkhof, H.J.; Cornelis, F.M.F.; Doherty, S.A.; Hart, D.J.; Hofman, A.; Luyten, F.P.; Maciewicz, R.A.; Mangino, M.; et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc. Natl Acad. Sci. USA 2012, 109, 8218–8223. [Google Scholar] [CrossRef]
- de Roover, A.; Núñez, A.E.; Cornelis, F.M.; Cherifi, C.; Casas-Fraile, L.; Sermon, A.; Cailotto, F.; Lories, R.J.; Monteagudo, S. Hypoxia induces DOT1L in articular cartilage to protect against osteoarthritis. JCI Insight 2021, 6, e150451. [Google Scholar] [CrossRef]
- Buckley, C.; Vinardell, T.; Kelly, D. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Thoms, B.L.; Dudek, K.A.; Lafont, J.E.; Murphy, C.L. Hypoxia Promotes the Production and Inhibits the Destruction of Human Articular Cartilage. Arthritis Rheum 2013, 65, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, C.; Ni, L.; Huang, C.; Chen, D.; Shi, K.; Jin, H.; Zhang, K.; Li, Y.; Xie, L.; et al. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020, 11, 481. [Google Scholar] [CrossRef]
- Coyle, C.H.; Izzo, N.; Chu, C.R. Sustained hypoxia enhances chondrocyte matrix synthesis. J. Orthop. Res. 2009, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.; Hu, J.; Athanasiou, K. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthr. Cartil. 2013, 21, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Chai, J.Y.; Foo, J.B.; Yahaya, N.H.M.; Yang, Y.; Ng, M.H.; Law, J.X. Potential of exosomes as cell-free therapy in articular cartilage regeneration: A review. Int. J. Nanomed. 2021, 16, 6749–6781. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.C.; Park, H.J.; Wang, S.Y.; Yang, H.R.; Lee, M.C.; Han, H.-S. Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater. Res. 2018, 22, 28. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; Hardingham, T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2α and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res. Ther 2007, 9, R55–R59. [Google Scholar] [CrossRef]
- Roy, S.; Tripathy, M.; Mathur, N.; Jain, A.; Mukhopadhyay, A. Hypoxia improves expansion potential of human cord blood-derived hematopoietic stem cells and marrow repopulation efficiency. Eur. J. Haematol. 2012, 88, 396–405. [Google Scholar] [CrossRef]
- Kim, D.S.; Ko, Y.J.; Lee, M.W.; Park, H.J.; Park, Y.J.; Kim, D.-I.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress Chaperones 2016, 21, 1089–1099. [Google Scholar] [CrossRef]
- Chiang, E.-R.; Ma, H.-L.; Wang, J.-P.; Liu, C.-L.; Chen, T.-H.; Hung, S.-C. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS ONE 2016, 11, e0149835. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, B.; Abed, E.; Yéléhé-Okouma, M.; Bianchi, A.; Mainard, D.; Netter, P.; Jouzeau, J.-Y.; Lajeunesse, D.; Reboul, P. Hypoxia and vitamin D differently contribute to leptin and dickkopf-related protein 2 production in human osteoarthritic subchondral bone osteoblasts. Arthritis Res. Ther. 2014, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous toll-like receptor 2/toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum 2010, 62, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Burton-Wurster, N.; Glant, T.T.; Tashman, S.; Sumner, D.R.; Kamath, R.V.; Lust, G.; Kimura, J.H.; Cs-Szabo, G. Spontaneous and experimental osteoarthritis in dog: Similarities and differences in proteoglycan levels. J. Orthop. Res. 2003, 21, 730–737. [Google Scholar] [CrossRef]
- MacFadyen, M.A.; Daniel, Z.; Kelly, S.; Parr, T.; Brameld, J.M.; Murton, A.J.; Jones, S.W. The commercial pig as a model of spontaneously-occurring osteoarthritis. BMC Musculoskelet. Disord. 2019, 20, 70. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef]
- Johnson, K.; Svensson, C.; Van Etten, D.; Ghosh, S.S.; Murphy, A.N.; Powell, H.C.; Terkeltaub, R. Mediation of Spontaneous Knee Osteoarthritis by Progressive Chondrocyte ATP Depletion in Hartley Guinea Pigs. Arthritis Rheum 2004, 50, 1216–1225. [Google Scholar] [CrossRef]
- Okada, K.; Mori, D.; Makii, Y.; Nakamoto, H.; Murahashi, Y.; Yano, F.; Chang, S.H.; Taniguchi, Y.; Kobayashi, H.; Semba, H.; et al. Hypoxia-inducible factor-1 alpha maintains mouse articular cartilage through suppression of NF-κB signaling. Sci. Rep. 2020, 10, 5425. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bai, R.; Zhao, A.-Q.; Zhao, Z.-Q.; Liu, W.-L.; Jian, D.-M. MicroRNA-195 induced apoptosis in hypoxic chondrocytes by targeting hypoxia-inducible factor 1 alpha. Eur. Rev. Med. Pharm. Sci. 2015, 19, 545–551. [Google Scholar]
- Nguyen, A.T.; Zhang, Y. The diverse functions of Dot1 and H3K79 methylation. [Genes Dev. 2011]-PubMed-NCBI. Genes Dev. 2011, 25, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Fukai, A.; Mabuchi, A.; Ikeda, T.; Yano, F.; Ohba, S.; Nishida, N.; Akune, T.; Yoshimura, N.; Nakagawa, T.; et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 2010, 16, 678–686. [Google Scholar] [CrossRef]
- Yang, S.; Kim, J.; Ryu, J.-H.; Oh, H.; Chun, C.-H.; Kim, B.J.; Min, B.H.; Chun, J.-S. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef]
- Ito, Y.; Matsuzaki, T.; Ayabe, F.; Mokuda, S.; Kurimoto, R.; Matsushima, T.; Tabata, Y.; Inotsume, M.; Tsutsumi, H.; Liu, L.; et al. Both microRNA-455-5p and -3p repress hypoxia-inducible factor-2α expression and coordinately regulate cartilage homeostasis. Nat. Commun. 2021, 12, 4148. [Google Scholar] [CrossRef]
- Nakajima, M.; Shi, D.; Dai, J.; Tsezou, A.; Zheng, M.; Norman, P.E.; Takahashi, A.; Ikegawa, S.; Jiang, Q. Replication studies in various ethnic populations do not support the association of the HIF-2α SNP rs17039192 with knee osteoarthritis. Nat. Med. 2011, 17, 26–27. [Google Scholar] [CrossRef]
- Nakajima, M.; Shi, D.; Dai, J.; Tsezou, A.; Zheng, M.; Norman, P.E.; Chou, C.-H.; Lee, M.T.M.; Hwang, J.-Y.; Kim, N.-H.; et al. A large-scale replication study for the association of rs17039192 in HIF-2α with knee osteoarthritis. J. Orthop. Res. 2012, 30, 1244–1248. [Google Scholar] [CrossRef]
- Bohensky, J.; Terkhorn, S.P.; Freeman, T.A.; Adams, C.S.; Garcia, J.A.; Shapiro, I.M.; Srinivas, V. Regulation of autophagy in human and murine cartilage: Hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum 2009, 60, 1406–1415. [Google Scholar] [CrossRef]
- Thoms, B.L.; Murphy, C.L. Inhibition of Hypoxia-inducible Factor-targeting Prolyl Hydroxylase Domain-containing Protein 2 (PHD2) Enhances Matrix Synthesis by Human Chondrocytes. J. Biol. Chem. 2010, 285, 20472–20480. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Yunus, M.H.; Lee, Y.; Nordin, A.; Chua, K.H.; Bt Hj Idrus, R. Remodeling Osteoarthritic Articular Cartilage under Hypoxic Conditions. Int. J. Mol. Sci. 2022, 23, 5356. https://doi.org/10.3390/ijms23105356
Mohd Yunus MH, Lee Y, Nordin A, Chua KH, Bt Hj Idrus R. Remodeling Osteoarthritic Articular Cartilage under Hypoxic Conditions. International Journal of Molecular Sciences. 2022; 23(10):5356. https://doi.org/10.3390/ijms23105356
Chicago/Turabian StyleMohd Yunus, Mohd Heikal, Yemin Lee, Abid Nordin, Kien Hui Chua, and Ruszymah Bt Hj Idrus. 2022. "Remodeling Osteoarthritic Articular Cartilage under Hypoxic Conditions" International Journal of Molecular Sciences 23, no. 10: 5356. https://doi.org/10.3390/ijms23105356
APA StyleMohd Yunus, M. H., Lee, Y., Nordin, A., Chua, K. H., & Bt Hj Idrus, R. (2022). Remodeling Osteoarthritic Articular Cartilage under Hypoxic Conditions. International Journal of Molecular Sciences, 23(10), 5356. https://doi.org/10.3390/ijms23105356





