The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons
Abstract
1. Introduction
2. Results
2.1. Gene Expression
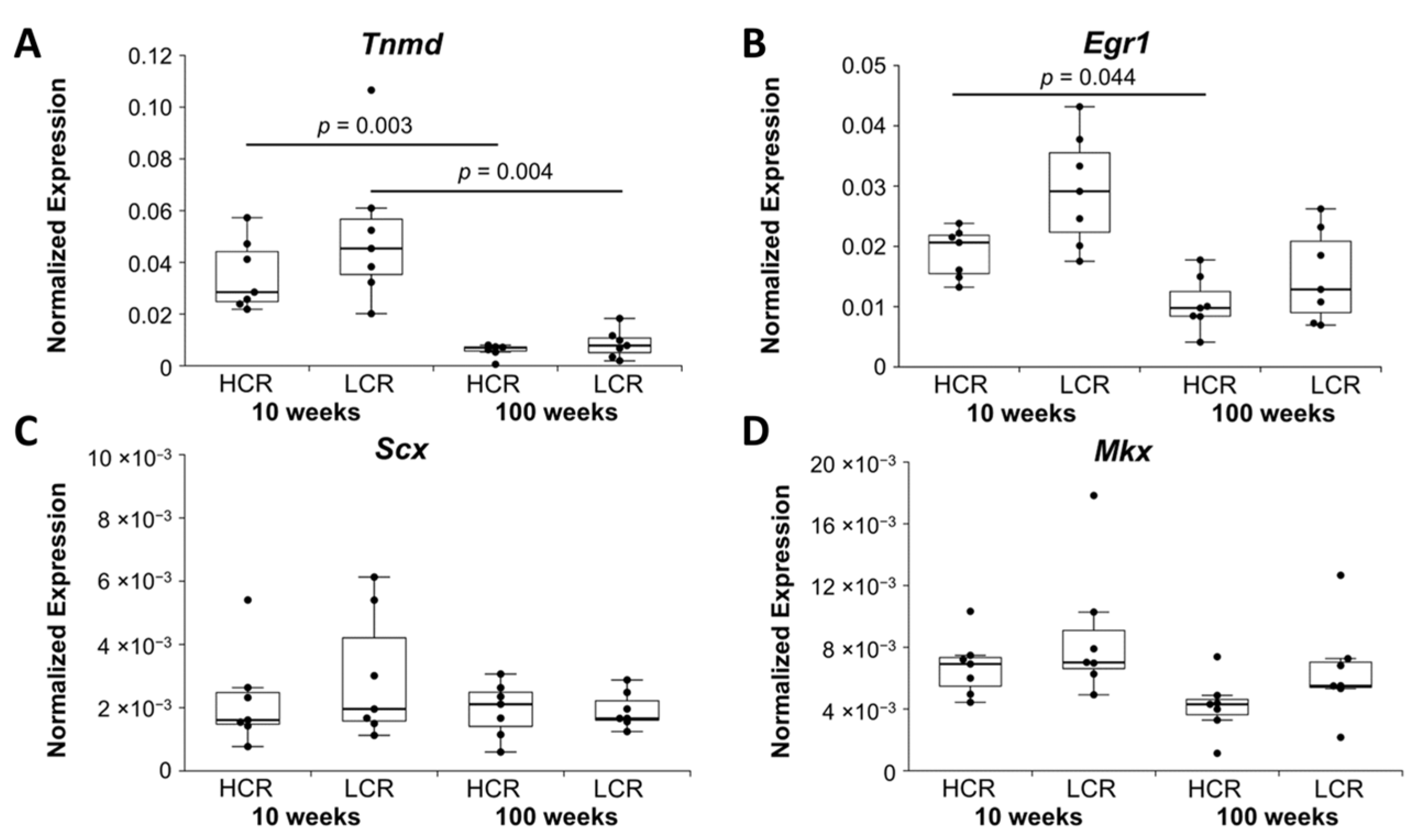
2.1.1. Tenocyte Markers
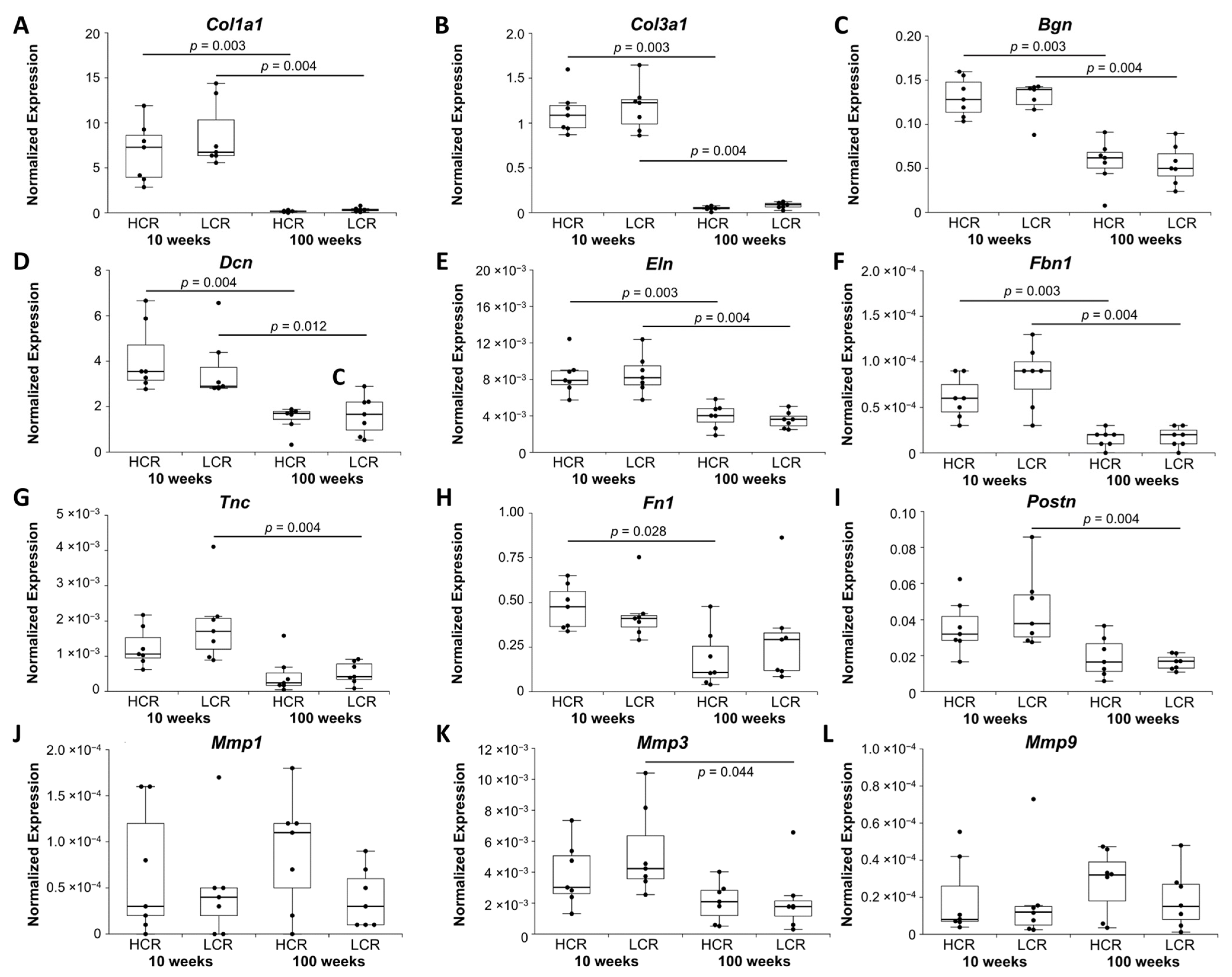
2.1.2. Markers of Modeling and Remodeling of ECM
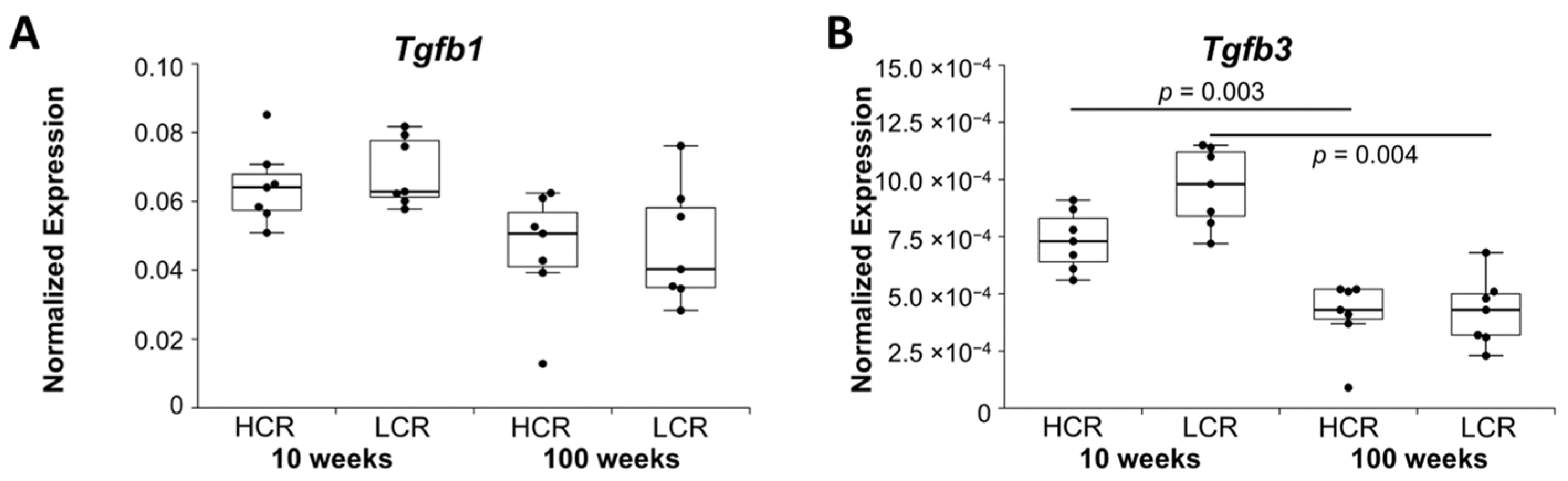
2.1.3. Growth Factors
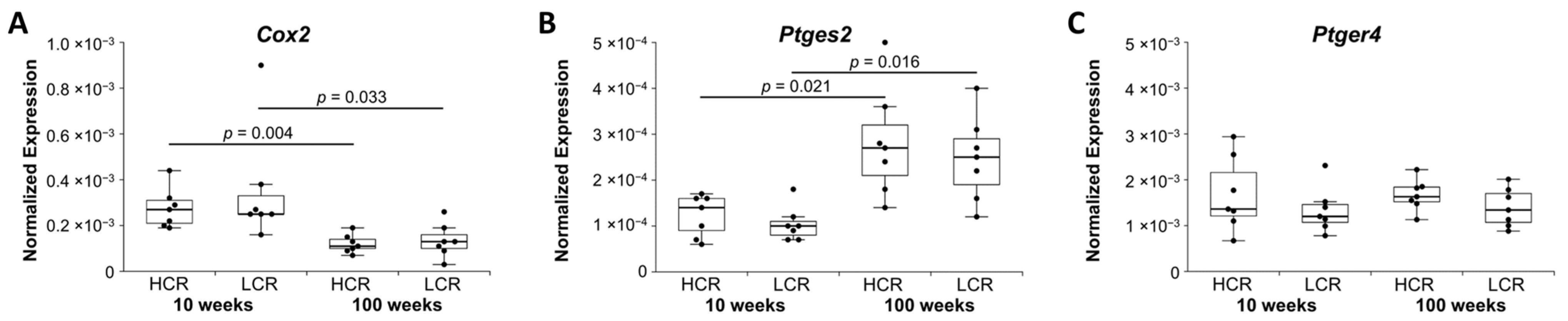
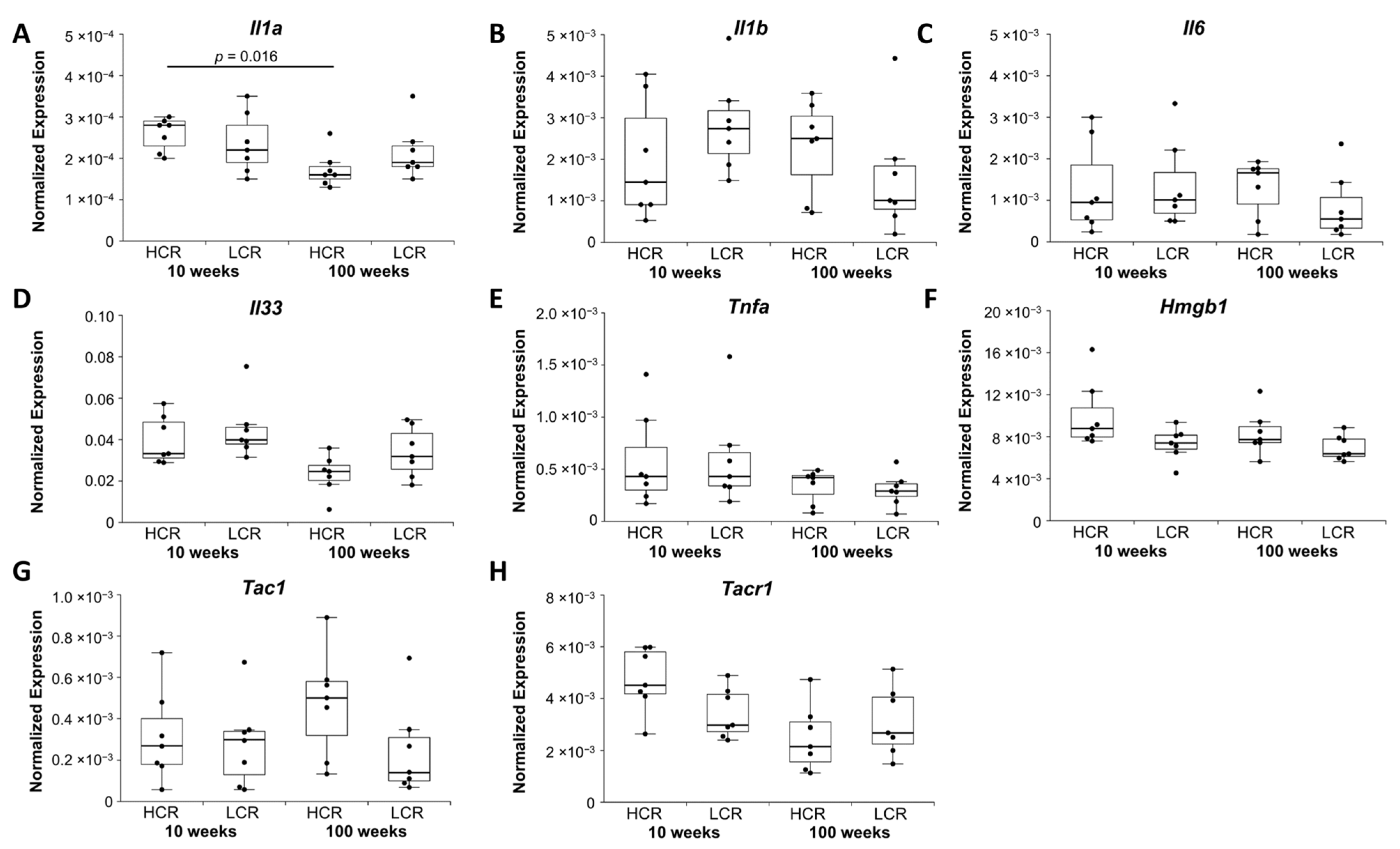
2.1.4. Inflammatory and Neuroinflammatory Markers
2.1.5. Summary of Gene Expression Results
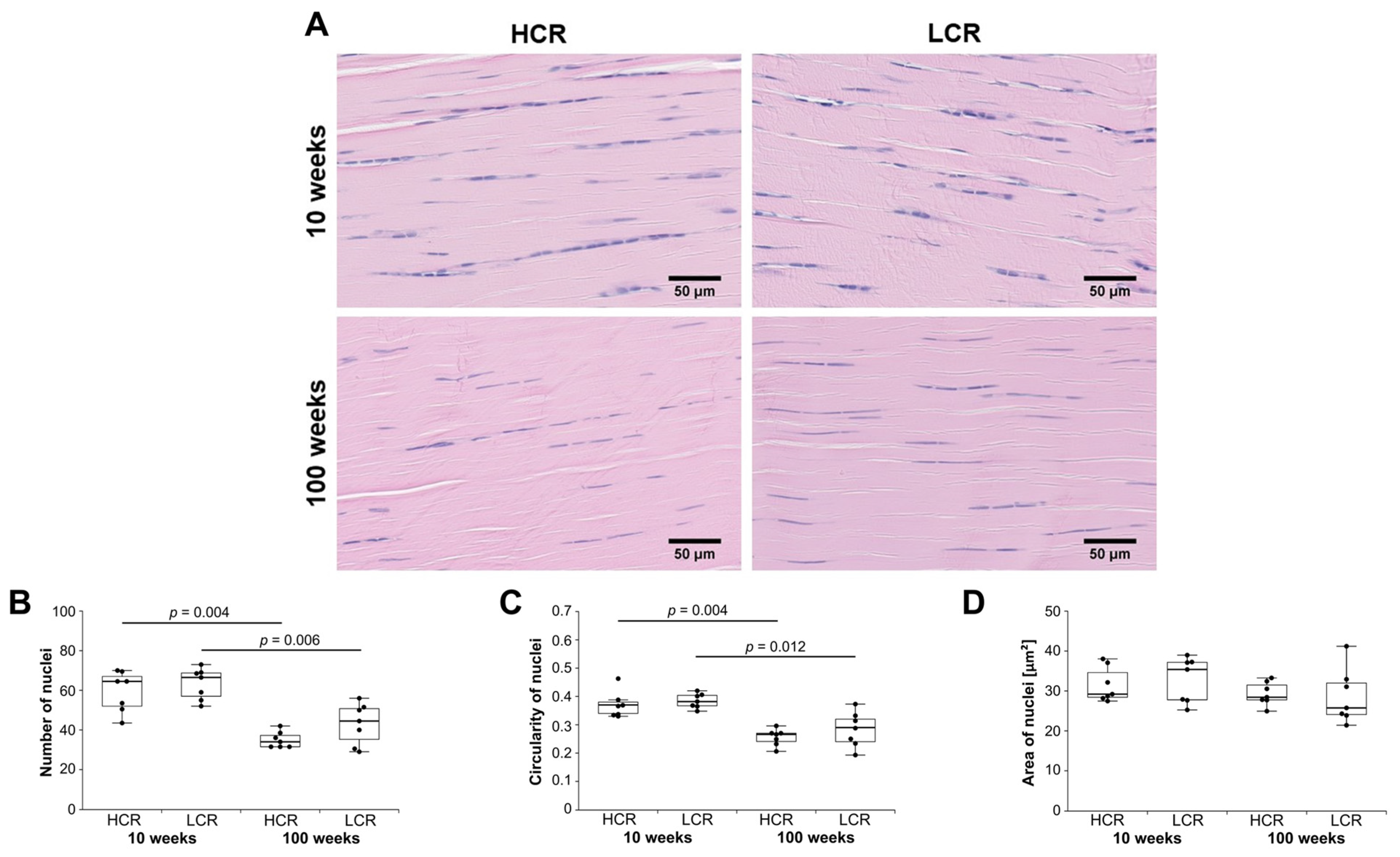
2.2. Histology of the Achilles Tendon
Histological Score
3. Discussion
Limitations
4. Materials and Methods
4.1. Animals
4.2. Sample Collection
4.3. qPCR
4.4. Histological Preparation
4.5. Immunohistochemistry
4.6. Semi-Automated Image Analysis
4.7. Histological Score
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kujala, U.M.; Sarna, S.; Kaprio, J. Cumulative Incidence of Achilles Tendon Rupture and Tendinopathy in Male Former Elite Athletes. Clin. J. Sport Med. 2005, 15, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Waldecker, U.; Hofmann, G.; Drewitz, S. Epidemiologic investigation of 1394 feet: Coincidence of hindfoot malalignment and Achilles tendon disorders. Foot Ankle Surg. 2012, 18, 119–123. [Google Scholar] [CrossRef]
- Riel, H.; Lindstrøm, C.F.; Rathleff, M.S.; Jensen, M.B.; Olesen, J.L. Prevalence and incidence rate of lower-extremity tendinopathies in a Danish general practice: A registry-based study. BMC Musculoskelet. Disord. 2019, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N. Overuse tendon conditions: Time to change a confusing terminology. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 840–843. [Google Scholar] [CrossRef]
- Scott, A.; Cook, J.L.; Hart, D.A.; Walker, D.C.; Duronio, V.; Khan, K.M. Tenocyte responses to mechanical loading in vivo: A role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007, 56, 871–881. [Google Scholar] [CrossRef]
- Rees, J.D.; Maffulli, N.; Cook, J. Management of Tendinopathy. Am. J. Sports Med. 2009, 37, 1855–1867. [Google Scholar] [CrossRef]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Kaux, J.-F.; Forthomme, B.; Le Goff, C.; Crielaard, J.-M.; Croisier, J.-L. Current Opinions on Tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar]
- O’Neill, S.; Watson, P.J.; Barry, S. A delphi study of risk factors for achilles tendinopathy- opinions of world tendon experts. Int. J. Sports Phys. Ther. 2021, 11, 684–697. [Google Scholar]
- Järvinen, T.A.; Kannus, P.; Maffulli, N.; Khan, K.M. Achilles Tendon Disorders: Etiology and Epidemiology. Foot Ankle Clin. 2005, 10, 255–266. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V.; Andia, I. Occurrence of tendon pathologies in metabolic disorders. Rheumatol. 2013, 52, 599–608. [Google Scholar] [CrossRef]
- Abate, M.; Gravare-Silbernagel, K.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef]
- Fredberg, U.; Stengaard-Pedersen, K. Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand. J. Med. Sci. Sports 2008, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surg. 2017, 15, 297–302. [Google Scholar] [CrossRef]
- Rees, J.D.; Stride, M.; Scott, A. Tendons–time to revisit inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef]
- Gracey, E.; Burssens, A.; Cambré, I.; Schett, G.; Lories, R.; McInnes, I.B.; Asahara, H.; Elewaut, D. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 193–207. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Naito, K.; Goto, K.; Kojima, Y.; Furuhata, A.; Igarashi, M.; Nagaoka, I.; Kaneko, K. Effect of aging on the tendon structure and tendon-associated gene expression in mouse foot flexor tendon. Biomed. Rep. 2019, 10, 238–244. [Google Scholar] [CrossRef]
- Lin, D.; Alberton, P.; Caceres, M.D.; Volkmer, E.; Schieker, M.; Docheva, D. Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing. Cell Death Dis. 2017, 8, e3116. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Minkwitz, S.; Schmock, A.; Bormann, N.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B. Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics. Int. J. Mol. Sci. 2018, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.; Harrall, R.; Curry, V.; Holloway, G.; Hackney, R.; Hazleman, B.; Riley, G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001, 20, 159–169. [Google Scholar] [CrossRef]
- Kostrominova, T.Y.; Brooks, S.V. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. AGE 2013, 35, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Magra, M. Matrix metalloproteases: A role in overuse tendinopathies. Br. J. Sports Med. 2005, 39, 789–791. [Google Scholar] [CrossRef]
- Fu, S.C.; Chan, B.P.; Wang, W.; Pau, H.M.; Chan, K.M.; Rolf, C.G. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop. Scand. 2002, 73, 658–662. [Google Scholar] [CrossRef]
- Alfredson, H.; Lorentzon, M.; Bäckman, S.; Bäckman, A.; Lerner, U. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic achilles tendinosis. J. Orthop. Res. 2003, 21, 970–975. [Google Scholar] [CrossRef]
- Thampatty, B.P.; Li, H.; Im, H.-J.; Wang, J.H.-C. EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1β treatment. Gene 2007, 386, 154–161. [Google Scholar] [CrossRef]
- Tsuzaki, M.; Guyton, G.; Garrett, W.; Archambault, J.M.; Herzog, W.; Almekinders, L.; Bynum, D.; Yang, X.; Banes, A.J. IL-1β induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. J. Orthop. Res. 2003, 21, 256–264. [Google Scholar] [CrossRef]
- Bergqvist, F.; Carr, A.J.; Wheway, K.; Watkins, B.; Oppermann, U.; Jakobsson, P.-J.; Dakin, S.G. Divergent roles of prostacyclin and PGE2 in human tendinopathy. Arthritis Res. 2019, 21, 74. [Google Scholar] [CrossRef]
- Dakin, S.; Dudhia, J.; Werling, N.; Werling, D.; Abayasekara, D.R.E.; Smith, R.K.W. Inflamm-Aging and Arachadonic Acid Metabolite Differences with Stage of Tendon Disease. PLoS ONE 2012, 7, e48978. [Google Scholar] [CrossRef] [PubMed]
- Sullo, A.; Maffulli, N.; Capasso, G.; Testa, V. The effects of prolonged peritendinous administration of PGE1 to the rat Achilles tendon: A possible animal model of chronic Achilles tendinopathy. J. Orthop. Sci. 2001, 6, 349–357. [Google Scholar] [CrossRef]
- Khan, M.H.; Li, Z.; Wang, J.H.-C. Repeated Exposure of Tendon to Prostaglandin-E2 Leads to Localized Tendon Degeneration. Clin. J. Sport Med. 2005, 15, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Movin, T.; Gad, A.; Reinholt, F.P.; Rolf, C. Tendon pathology in long-standing achillodynia: Biopsy findings in 40 patients. Acta Orthop. Scand. 1997, 68, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.G.; Britton, S.L. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genom. 2001, 5, 45–52. [Google Scholar] [CrossRef]
- Noland, R.C.; Thyfault, J.; Henes, S.T.; Whitfield, B.R.; Woodlief, T.; Evans, J.R.; Lust, J.A.; Britton, S.L.; Koch, L.G.; Dudek, R.W.; et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am. J. Physiol. Metab. 2007, 293, E31–E41. [Google Scholar] [CrossRef] [PubMed]
- Wisløff, U.; Najjar, S.M.; Ellingsen, Ø.; Haram, P.M.; Swoap, S.; Al-Share, Q.; Fernstro, M.; Rezaei, K.; Lee, S.J.; Koch, L.G.; et al. Cardiovascular Risk Factors Emerge After Artificial Selection for Low Aerobic Capacity. Science 2005, 307, 418–420. [Google Scholar] [CrossRef]
- Schwarzer, M.; Britton, S.L.; Koch, L.G.; Wisloff, U.; Doenst, T. Low intrinsic aerobic exercise capacity and systemic insulin resistance are not associated with changes in myocardial substrate oxidation or insulin sensitivity. Basic Res. Cardiol. 2010, 105, 357–364. [Google Scholar] [CrossRef][Green Version]
- Koch, L.G.; Kemi, O.J.; Qi, N.; Leng, S.X.; Bijma, P.; Gilligan, L.J.; Wilkinson, J.E.; Wisløff, H.; Høydal, M.A.; Rolim, N.; et al. Intrinsic Aerobic Capacity Sets a Divide for Aging and Longevity. Circ. Res. 2011, 109, 1162–1172. [Google Scholar] [CrossRef]
- Longo, U.G.; Berton, A.; Stelitano, G.; Madaudo, C.; Perna, M.; Ciuffreda, M.; Guarnieri, A.; Papalia, R.; Maffulli, N.; Denaro, V. 2017 Marathon of Rome. Clin. J. Sport Med. 2018, 31, e15–e20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhou, Y.; Tang, K. An overview of structure, mechanical properties, and treatment for age-related tendinopathy. J. Nutr. Heal. Aging 2014, 18, 441–448. [Google Scholar] [CrossRef]
- Abate, M.; Salini, V. Mid-portion Achilles tendinopathy in runners with metabolic disorders. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Speed, C. Inflammation in Tendon Disorders. Advances in Experimental Medicine and Biology 2016, 920, 209–220. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Pang, J.-H.S.; Wu, K.P.-H.; Chen, M.J.-L.; Chen, C.-H.; Tsai, W.-C. Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet. Disord. 2013, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Pingel, J.; Fredberg, U.; Qvortrup, K.; Larsen, J.O.; Schjerling, P.; Heinemeier, K.; Kjaer, M.; Langberg, H. Local biochemical and morphological differences in human Achilles tendinopathy: A case control study. BMC Musculoskelet. Disord. 2012, 13, 53. [Google Scholar] [CrossRef]
- Corps, A.N.; Robinson, A.H.N.; Movin, T.; Costa, M.L.; Hazleman, B.L.; Riley, G.P. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatol. 2006, 45, 291–294. [Google Scholar] [CrossRef]
- Minkwitz, S.; Schmock, A.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B.; Klatte-Schulz, F. Time-Dependent Alterations of MMPs, TIMPs and Tendon Structure in Human Achilles Tendons after Acute Rupture. Int. J. Mol. Sci. 2017, 18, 2199. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef]
- Koskinen, S.O.A.; Heinemeier, K.M.; Olesen, J.L.; Langberg, H.; Kjaer, M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J. Appl. Physiol. 2004, 96, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, S.O.A.; Hoyhtya, M.; Turpeenniemi-Hujanen, T.; Martikkala, V.; Makinen, T.T.; Oksa, J.; Rintamaki, H.; Lofberg, M.; Somer, H.; Takala, T.E.S. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand. J. Med. Sci. Sports 2001, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Alberton, P.; Dex, S.; Popov, C.; Shukunami, C.; Schieker, M.; Docheva, D. Loss of Tenomodulin Results in Reduced Self-Renewal and Augmented Senescence of Tendon Stem/Progenitor Cells. Stem Cells Dev. 2015, 24, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Dex, S.; Alberton, P.; Willkomm, L.; Söllradl, T.; Bago, S.; Milz, S.; Shakibaei, M.; Ignatius, A.; Bloch, W.; Clausen-Schaumann, H.; et al. Tenomodulin is Required for Tendon Endurance Running and Collagen I Fibril Adaptation to Mechanical Load. EBioMedicine 2017, 20, 240–254. [Google Scholar] [CrossRef]
- Guerquin, M.-J.; Charvet, B.; Nourissat, G.; Havis, E.; Ronsin, O.; Bonnin, M.-A.; Ruggiu, M.; Olivera-Martinez, I.; Robert, N.; Lu, Y.; et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J. Clin. Investig. 2013, 123, 3564–3576. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.C.; Shah, S.A.; Golman, M.; Song, L.; Li, X.; Kurtaliaj, I.; Akbar, M.; Millar, N.L.; Abu-Amer, Y.; Galatz, L.M.; et al. Targeting the NF-κB signaling pathway in chronic tendon disease. Sci. Transl. Med. 2019, 11, 4319. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin Modulates Nuclear Factor κB (NF-κB)-mediated Inflammation in Human Tenocytes in Vitro. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.C.; Wang, W.; Pau, H.M.; Wong, Y.P.; Chan, K.M.; Rolf, C.G. Increased Expression of Transforming Growth Factor-??1 in Patellar Tendinosis. Clin. Orthop. Relat. Res. 2002, 400, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Legerlotz, K.; Jones, E.R.; Screen, H.; Riley, G. Increased expression of IL-6 family members in tendon pathology. Rheumatol. 2012, 51, 1161–1165. [Google Scholar] [CrossRef]
- Murakami, M.; Nakatani, Y.; Tanioka, T.; Kudo, I. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 2002, 68–69, 383–399. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H.-C. Production of PGE2increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J. Orthop. Res. 2009, 28, 198–203. [Google Scholar] [CrossRef]
- Han, S.-H.; Choi, W.; Song, J.; Kim, J.; Lee, S.; Choi, Y.; Byun, S.-E.; Ahn, T.; Ahn, H.; Ding, C.; et al. The Implication of Substance P in the Development of Tendinopathy: A Case Control Study. Int. J. Mol. Sci. 2017, 18, 1241. [Google Scholar] [CrossRef]
- Andersson, G.; Backman, L.J.; Scott, A.; Lorentzon, R.; Forsgren, S.; Danielson, P. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br. J. Sports Med. 2011, 45, 1017–1022. [Google Scholar] [CrossRef]
- Backman, L.J.; Fong, G.; Andersson, G.; Scott, A.; Danielson, P. Substance P Is a Mechanoresponsive, Autocrine Regulator of Human Tenocyte Proliferation. PLoS ONE 2011, 6, e27209. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kim, D.K.; Han, S.H.; Lee, H.H.; Jeong, Y.; Baek, M.; Kim, H.; Ahn, W.; Lee, S. Sustained Exposure of Substance P Causes Tendinopathy. Int. J. Mol. Sci. 2020, 21, 8633. [Google Scholar] [CrossRef] [PubMed]
- Scott, A. Neuropeptides in tendinopathy. Front. Biosci. 2009, 33, 2203–2211. [Google Scholar] [CrossRef]
- Andersson, G.; Danielson, P.; Alfredson, H.; Forsgren, S. Presence of substance P and the neurokinin-1 receptor in tenocytes of the human Achilles tendon. Regul. Pept. 2008, 150, 81–87. [Google Scholar] [CrossRef]
- Molloy, T.; Wang, Y.; Murrell, G.A.C. The Roles of Growth Factors in Tendon and Ligament Healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.T.; Malmgaard-Clausen, N.M.; Puggaard, R.S.; Svensson, R.B.; Nybing, J.D.; Hansen, P.; Schjerling, P.; Zinglersen, A.H.; Couppé, C.; Boesen, M.; et al. Early development of tendinopathy in humans: Sequence of pathological changes in structure and tissue turnover signaling. FASEB J. 2020, 34, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Goodier, H.C.J.; Carr, A.J.; Snelling, S.J.B.; Roche, L.; Wheway, K.; Watkins, B.; Dakin, S.G. Comparison of transforming growth factor beta expression in healthy and diseased human tendon. Arthritis Res. 2016, 18, 48. [Google Scholar] [CrossRef]
- Meller, R.; Schiborra, F.; Brandes, G.; Knobloch, K.; Tschernig, T.; Hankemeier, S.; Haasper, C.; Schmiedl, A.; Jagodzinski, M.; Krettek, C.; et al. Postnatal maturation of tendon, cruciate ligament, meniscus and articular cartilage: A histological study in sheep. Ann. Anat.-Anat. Anz. 2009, 191, 575–585. [Google Scholar] [CrossRef]
- Almekinders, L.C.; Deol, G. The Effects of Aging, Antiinflammatory Drugs, and Ultrasound on the In Vitro Response of Tendon Tissue. Am. J. Sports Med. 1999, 27, 417–421. [Google Scholar] [CrossRef]
- Svensson, R.B.; Heinemeier, K.M.; Couppé, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. 2016, 121, 1353–1362. [Google Scholar] [CrossRef]
- Bell, R.; Gendron, N.R.; Anderson, M.; Flatow, E.L.; Andarawis-Puri, N. A potential new role for myofibroblasts in remodeling of sub-rupture fatigue tendon injuries by exercise. Sci. Rep. 2018, 8, 8933. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Cossermelli, W.; Brentani, R. Differential Staining of Collagens Type I, II and III by Sirius Red and Polarization Microscopy. Arch. Histol. Jpn. 1978, 41, 267–274. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, V.; Klatte-Schulz, F.; Minkwitz, S.; Schmock, A.; Rummler, M.; Seliger, A.; Willie, B.M.; Wildemann, B. In Vivo and In Vitro Mechanical Loading of Mouse Achilles Tendons and Tenocytes—A Pilot Study. Int. J. Mol. Sci. 2020, 21, 1313. [Google Scholar] [CrossRef]
- Montes, G.S.; Junqueira, L.C.U. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Memórias do Instituto Oswaldo Cruz 1991, 86, 1–11. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Dirks, R.C.; Galley, M.R.; Childress, P.J.; Fearon, A.M.; Scott, A.; Koch, L.G.; Britton, S.L.; Warden, S.J. Uphill running does not exacerbate collagenase-induced pathological changes in the Achilles tendon of rats selectively bred for high-capacity running. Connect. Tissue Res. 2013, 54, 386–393. [Google Scholar] [CrossRef]
- Kohn, D.F.; Clifford, C.B. Biology and Diseases of Rats; Elsevier: Amsterdam, The Netherlands, 2002; pp. 121–165. [Google Scholar]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co. Ltd.: London, UK, 1959. [Google Scholar]
- Deschner, J.; Rath-Deschner, B.; Agarwal, S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthr. Cartil. 2006, 14, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformation 2003, 19, 1439–1440. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]

| Young HCR | Old HCR | Young LCR | Old LCR | |
|---|---|---|---|---|
| Age in Weeks | 10.7 (10.2–10.9) | 98.6 (97.8–99.1) | 10.4 (10.1–10.6) | 99.4 (97.8–99.9) |
| Body weight [g] | 177.9 (168.3–185.0) # | 258.0 (244.5–291.8) †,## | 216.2 (196.1–230.6) | 307.6 (198.0–323.5) ‡ |
| Achilles tendon weight [mg] | 18.0 (15.5–20.0) | 30.0 (26.5–37.5) † | 18.0 (18.0–23.0) | 32.0 (30.5–33.5) ‡ |
| RNA per tendon ration [μg/mg] | 0.34 (0.29–0.36) | 0.14 (0.11–0.14) † | 0.40 (0.38–0.41) | 0.11 (0.09–0.14) ‡ |
| Regulation | Function | HCR | LCR | No Changes |
|---|---|---|---|---|
| 100 vs. 10 Weeks | 100 vs. 10 Weeks | |||
| Down | Tenocyte markers | Tnmd | Scx, Mkx | |
| Egr1 | ||||
| ECM modelling & remodelling | Col1a1, Col3a1, Bgn, Dcn, Eln, Fbn1 | Mmp1, Mmp9 | ||
| Fn1 | Mmp3, Postn, Tnc | |||
| Growth factors | Tgfb3 | Tgfb1 | ||
| Inflammation & Neuroinflammation marker | Cox2 | Il1b, Il6, Il33, Tnfa, Ptger4, Hmgb1, Tacr1, Tac1 | ||
| Il1a | ||||
| Up | Inflammation marker | Ptges2 | ||
| Gene | Accession Number | Primer Sequence | Amplicon Size (bp) |
|---|---|---|---|
| Bgn | NM_017087.1 | forward: 5′gattgagaatgggagcctga3′ reverse: 5′ccttggtgatgttgttggag3′ | 143 |
| Col1a1 | NM_053304.1 | forward: 5′tgactggaagagcggagagt3′ reverse: 5′gatagcgacatcggcaggat3′ | 250 |
| Col3a1 | NM_032085.1 | forward: 5′tgggatccaatgagggaga3′ reverse: 5′tcatggccttgcgtgttt3′ | 135 |
| Cox2 | U03389.1 | forward: 5′agggagtctggaacattgtga3′ reverse: 5′attgtaagttggtgggctgt3′ | 107 |
| Dcn | NM_024129.1 | forward: 5′gcagggaatgaagggtctc3′ reverse: 5′tccacaacggtgatgctatt3′ | 195 |
| Egr1 | NM_012551 | forward:5′cacctgaccacagagtcctttt3′ reverse: 5′aaagtgttgccactgttggg3′ | 152 |
| Eln | NM_012722.1 | forward: 5′gtgtcggtcttccaggtgta3′ reverse: 5′gaaccttggccttgactcct3′ | 117 |
| Fbn1 | NM_031825.1 | forward: 5′gtgtgaactgagcgcgaac3′ reverse: 5‘cactggccaccatcacagata3′ | 288 |
| Fn1 | NM_019143.2 | forward: 5′tcccacgatccgatgatgt3′ reverse: 5′tccacacggtatccagtcac3′ | 118 |
| Hmgb1 | NM_012963.2 | forward: 5′aaggggagaccaaaaagaagtt3′ reverse: 5′acaagaagaaggccgaagga3′ | 69 |
| Il1a | NM_017019.1 | forward: 5′atccaacccagatcagcac3′ reverse: 5′actcctgcttgacgatcc3′ | 76 |
| Il1b | NM_031512.2 | forward: 5′cccattagacagctgcact3′ reverse: 5′ccattgaggtggagagctt3′ | 95 |
| Il33 | NM_001014166.1 | forward: 5′gtggatgggaagaagctga3′ reverse: 5′tgaagaacaaagaaggcctga3′ | 140 |
| Il6 | M26744.1 | forward: 5′tgccttcttgggactgatg3′ reverse: 5′tggtctgttgtgggtggt3′ | 97 |
| Mkx | XM_017600733.1 | forward: 5′gctctaggctcgcagatgac3′ reverse: 5′gcgttgccctgaacatactt3′ | 143 |
| Mmp1 | NM_001134530.1 | forward: 5′gggtttttgaggaggaaggtg3′ reverse: 5′gcctggtgggaatgtgtga3′ | 113 |
| Mmp3 | NM_133523.3 | forward: 5′cggtggcttcagtaccttt3′ reverse: 5′tcacctcctcccagacctt3′ | 143 |
| Mmp9 [81] | NM_031055.1 | forward: 5′tgctcctggctctaggctac3′ reverse: 5′ttggaggttttcaggtctcg3′ | 88 |
| Ptger4 | NM_032076.3 | forward: 5′gcgcaaggagcagaaggaga3′ reverse: 5′ccagcccacataccagagt3′ | 50 |
| Ptges2 | NM_001107832.1 | forward: 5′aggacggaggagatgaagt3′ reverse: 5′cgttgggagagatgagatgc3′ | 67 |
| Postn | NM_001108550 | forward: 5′tagggtgtgagggagacagc3′ reverse: 5′caggtccgtgaaagtggttt3′ | 170 |
| Rps18 | NM_213557.1 | forward: 5′tgtggtgttgaggaaagcag3′ reverse: 5′cctctatgggctcggatttt3′ | 240 |
| Scx | NM_001130508.1 | Qiagen (QT01596028), no primer sequence provided | |
| Tnc | NM_053861.1 | forward: 5′atgttccaaagagccagcaa3′ reverse: 5′aggctgtagttgaggcggta3′ | 247 |
| Tac1 | NM_012666.2 | forward: 5′caatgcagaactacgaaagaagg3′ reverse: 5′gcggacacagatggagatga3′ | 73 |
| Tac1r | NM_012667.2 | forward: 5′caaacgcaaggtggtcaaaa3′ reverse: 5′gatgtagggcaggaggaagaa3′ | 94 |
| Tnfa | NM_012675.3 | forward: 5′gcctcttctcattcctgct3′ reverse: 5′aacttctcctccttgttggg3′ | 97 |
| Tgfb1 | NM_021578.2 | forward: 5′aactgtggagcaacacgtagaa3′ reverse: 5′tattccgtctccttggttcag3′ | 157 |
| Tgfb3 | NM_013174.2 | forward: 5′gagggtggaagccattagg3′ reverse: 5′gcagactgccagttcattgtg3′ | 256 |
| Tnmd | NM_022290.1 | forward:5′ggcccgaggtatccaagaag3′ reverse: 5′agatgccagtgtatccgttttt3′ | 177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinitz, R.; Heyne, E.; Koch, L.G.; Britton, S.L.; Thierbach, M.; Wildemann, B. The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons. Int. J. Mol. Sci. 2022, 23, 79. https://doi.org/10.3390/ijms23010079
Kinitz R, Heyne E, Koch LG, Britton SL, Thierbach M, Wildemann B. The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons. International Journal of Molecular Sciences. 2022; 23(1):79. https://doi.org/10.3390/ijms23010079
Chicago/Turabian StyleKinitz, Runa, Estelle Heyne, Lauren G. Koch, Steven L. Britton, Manuela Thierbach, and Britt Wildemann. 2022. "The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons" International Journal of Molecular Sciences 23, no. 1: 79. https://doi.org/10.3390/ijms23010079
APA StyleKinitz, R., Heyne, E., Koch, L. G., Britton, S. L., Thierbach, M., & Wildemann, B. (2022). The Effect of Age and Intrinsic Aerobic Exercise Capacity on the Expression of Inflammation and Remodeling Markers in Rat Achilles Tendons. International Journal of Molecular Sciences, 23(1), 79. https://doi.org/10.3390/ijms23010079







